Effect of phosphodiesterase-5 inhibition on SystEmic Right VEntricular size and function. A multicentre, double-blind, randomized, placebo-controlled trial: SERVE
Abstract
Aims
In adults with congenital heart disease and systemic right ventricles, progressive right ventricular systolic dysfunction is common and is associated with adverse outcomes. Our aim was to assess the impact of the phosphodiesterase-5-inhibitor tadalafil on right ventricular systolic function.
Methods and results
This was a double-blind, randomized, placebo-controlled, multicentre superiority trial (NCT03049540) involving 100 adults with systemic right ventricles (33 women, mean age: 40.7 ± 10.7 years), comparing tadalafil 20 mg once daily versus placebo (1:1 ratio). The primary endpoint was the change in right ventricular end-systolic volume after 3 years of therapy. Secondary endpoints were changes in right ventricular ejection fraction, exercise capacity and N-terminal pro-B-type natriuretic peptide concentration. Primary endpoint assessment by intention to treat analysis at 3 years of follow-up was possible in 83 patients (42 patients in the tadalafil group and 41 patients in the placebo group). No significant changes over time in right ventricular end-systolic volumes were observed in the tadalafil and the placebo group, and no significant differences between treatment groups (3.4 ml, 95% confidence interval −4.3 to 11.0, p = 0.39). No significant changes over time were observed for the pre-specified secondary endpoints for the entire study population, without differences between the tadalafil and the placebo group.
Conclusions
In this trial in adults with systemic right ventricles, right ventricular systolic function, exercise capacity and neuro-hormonal activation remained stable over a 3-year follow-up period. No significant treatment effect of tadalafil was observed. Further research is needed to find effective treatment for improvement of ventricular function in adults with systemic right ventricles.
Graphical Abstract
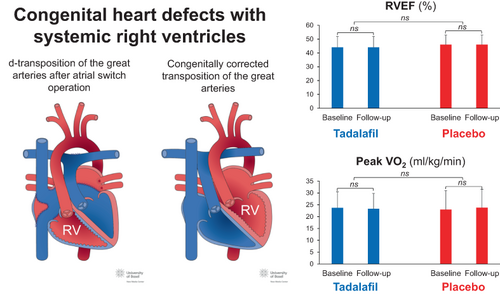
Promising effects of phosphodiesterase-5 inhibition on function of hypertrophied right ventricles in experimental and animal models did not translate into a clinical benefit in this randomized, placebo-controlled trial in adults with congenital heart disease and systemic right ventricles. Tadalafil had no impact on right ventricular function, exercise capacity or neuro-hormonal activation. Further prospective studies on the effectiveness of novel treatment options for these patients are urgently needed. RV, right ventricle; RVEF, right ventricular ejection fraction; VO2, oxygen uptake.
Introduction
Patients with systemic (subaortic) right ventricles (RVs) in a biventricular circulation comprise up to 10% of adults with complex congenital heart disease, followed at specialized centres.1 These include patients with complete transposition of the great arteries (d-TGA) after atrial switch operations and patients with congenitally corrected transposition of the great arteries (ccTGA). Although midterm outcome is favourable, systemic ventricular dysfunction is common during long-term follow-up. It is associated with clinical heart failure, arrhythmias and premature death.2-6 The optimal management of patients with systemic RVs is unknown. Medical heart failure therapy with angiotensin-converting enzyme inhibitors, angiotensin II receptor blockers, beta-blockers, and aldosterone antagonists has been shown to improve left ventricular function and survival in patients with acquired heart disease.7 However, several studies failed to show a similar clinical benefit of these drugs in adults with a failing systemic RV.8-17
Previous studies found that in RVs facing an increased afterload, as present in an RV in systemic position, hypertrophic myocardial cells express a fetal gene pattern with an increase in phosphodiesterase-5 (PDE-5) expression, which is not seen in the normal RV.18 PDE-5 inhibitors have been shown to increase contractility in experimental models of right ventricular hypertrophy, but not in the normal RV.18 In a rat model of fixed right ventricular pressure load after pulmonary artery banding, PDE-5 inhibition with sildenafil had a positive effect on right ventricular diastolic function and attenuated interstitial fibrosis in animals with established right ventricular dysfunction, independent of afterload.19
The aim of our investigator-initiated study was, therefore, to assess the effect of the PDE-5 inhibition on systolic right ventricular function in adults with systemic RVs.
Methods
Design
Details of the study design have been published previously.20 In brief, we conducted a parallel group, double-blind, randomized, placebo-controlled, multicentre superiority trial comparing tadalafil versus placebo in a 1:1 ratio. Both, tadalafil and the placebo were manufactured by TEVA/MEPHA Schweiz AG. Tadalafil was chosen over sildenafil for its higher selectivity for PDE-5 receptors and its longer half-live, allowing a single daily dose. The study complies with the Declaration of Helsinki, the responsible ethics committees and the competent authorities approved the protocol. All participants gave written informed consent prior to participation in the study. The study was registered at ClinicalTrials.gov (Identifier: NCT03049540).
Study population
Eligible participants were recruited from participating study centres. We included clinically stable adults (≥18 years) with d-TGA or adults with ccTGA. Patients with a life expectancy <6 months, severe renal dysfunction (creatinine clearance ≤30 ml/m2), severe hepatic insufficiency and known contraindications to the study drug were excluded. Sample size calculation was based on the expected change in right ventricular end-systolic volume (RV-ESV) between study groups. A sample size of 78 patients at 3-year follow-up was determined to obtain an 80% power to detect the expected difference in RV-ESV between the two treatment groups. Considering a possible dropout of 20%, the goal was to recruit 98 study participants (49 patients for each group).
Interventions
At baseline, all patients underwent clinical examination, standardized transthoracic echocardiography, cardiovascular magnetic resonance (CMR) imaging or cardiac multirow detector computed tomography (CMDCT) in patients with contraindications for CMR, symptom-limited cardiopulmonary exercise testing and blood sampling for analysis of neuro-hormonal activity. After completion of baseline examinations, participants were started on tadalafil 20 mg or placebo once daily without any titration period.
Randomization and masking
The randomized allocation lists (tadalafil versus placebo in 1:1 ratio) were generated by an independent statistician using a random seed with the help of a computer programme, and lists were generated stratified according to the presence or absence of a pacemaker (PM) or automated implantable cardioverter-defibrillator (AICD) at baseline (with randomly varying block sizes of 2, 4, or 6 patients). Lists were deposited password protected in a dedicated folder on a central GCP-compliant server. The randomized allocation lists were implemented in the electronic data capturing system and were concealed from all study personnel. As soon as the patient fulfilled the inclusion and exclusion criteria, the patient was randomized according to the concealed list, the list appropriate to the site and presence/absence of PM at baseline. All study personnel, including CMR or CMDCT assessors, trial statisticians and central data monitors remained blinded after the assignment of the treatments. Packages containing the drugs (tadalafil pills or similarly looking placebo pills) contained identifiers to allow emergency unblinding.
Outcome measurements
The primary objective was to assess the effect of tadalafil on systemic RV-ESV after 3 years of therapy or earlier, in case of permanent discontinuation of the study drug, measured by CMR imaging or CMDCT in patients with contraindications for CMR. Measurement of RV-ESV was chosen as the primary endpoint over right ventricular ejection fraction (RVEF), as it is likely more sensitive to detect changes in right ventricular systolic function. This is based on the notion that in patients with pulmonary arterial hypertension the RV adapts to its chronically increased afterload first by myocardial remodelling with hypertrophy and increased contractility. If these compensatory mechanisms fail, the RV begins to dilate, followed by a decrease in RVEF. Accordingly, an increase in right ventricular volumes precedes the decrease in RVEF. We assumed that the RV in systemic position may behave similarly. With progressive right ventricular failure and dilatation, there is also an increase in secondary tricuspid regurgitation. The increased amount of tricuspid regurgitation may falsely improve RVEF, whereas RV-ESV will nevertheless be increased as a sign of progressive right ventricular failure. Pre-specified secondary objectives were the effects of tadalafil on systemic RVEF measured by CMR or CMDCT at 3 years of follow-up, its impact on exercise capacity, quality of life and serum neuro-hormonal activation at 3 years of follow-up.
Cardiovascular magnetic resonance and cardiac multirow detector computed tomography
Imaging studies followed a pre-specified protocol and were performed at baseline, after 1 and 3 years of follow-up. In patients who had to discontinue the study medication prematurely, RV-ESV was re-measured within 4 weeks of the study drug withdrawal and this measurement was then used for the primary endpoint analysis unless the treatment period was <3 months. Analyses were performed in core labs by experienced observers, blinded to the sequence of imaging studies and blinded to treatment groups (for CMR: ‘swissCVIcorelab’ at the Centre Hospitalier Universitaire Vaudois, CHUV, Switzerland; for CMDCT: University Hospital Zurich, Switzerland).
Cardiopulmonary exercise testing
Cardiopulmonary exercise testing was performed at baseline and at 3 years of follow-up or earlier in case of permanent discontinuation of the study drug. Data analysis was performed at a core-lab (University Hospital Inselspital Bern) and measurements were reported following the recommendations of the European Association of Cardiovascular Prevention and Rehabilitation.21 Reported measurements included maximum workload in Watt, peak oxygen consumption (VO2), both in absolute and relative values based on Jones and Wasserman references, minute ventilation/carbon dioxide production (VE/VCO2) slope, end-tidal carbon dioxide, oxygen pulse trajectory, ΔVO2/ΔW trajectory, heart rate kinetics and heart rate recovery.21, 22
Neurohormonal activation
Neurohormonal activation was measured in all participants at baseline, at 1 year and at study end. All samples were analysed in a single core-lab with expertise in biomarker research (Cardiovascular Research Institute Basel, CRIB). Measurements included concentrations of the neurohormones B-type natriuretic peptide (BNP), N-terminal pro-B-type natriuretic peptide (NT-proBNP), and high-sensitivity cardiac troponin T (hs-cTnT).
Quality of life
Quality of life was assessed by the use of three self-administered questionnaires or scales at baseline, at 12 months, and at study end. These include a linear analogue scale (LAS), the Satisfaction with Life Scale (SWL) and the General Self-Efficacy Scale (GSE).23-25
Adverse events and safety endpoints
Adverse events were continuously reported as required by medical authorities. As pre-specified per protocol, serious adverse events were reviewed by an independent Data Safety Monitoring Board in March 2019 (after inclusion of all patients) and in March 2020.
Subgroup analysis
Subgroup analyses for assessment of differences in treatment effects were performed for gender, d-TGA versus ccTGA, symptomatic versus asymptomatic patients, patients with or without PM/AICD and patient groups stratified for NT-proBNP and hs-cTnT levels.
Statistical analysis
The primary endpoint was analysed using ANCOVA (analysis of covariance), with RV-ESV as the response variable, randomized treatment as main independent variable, baseline RV-ESV and time between baseline and follow-up right ventricular volume measurement (in months since baseline) as covariates. Secondary endpoints were analysed using ANCOVA (analysis of covariance), with the secondary endpoint (RVEF, peak VO2, NT-proBNP, hs-cTnT) as the response variable, randomized treatment as main variable, baseline measurement (RVEF, peak VO2, NT-proBNP, hs-cTnT, respectively) and time between baseline and follow-up measurement (in months since baseline) as covariates. All primary and secondary endpoints were analysed by intention-to-treat, using two-sided superiority testing with alpha set at 5%.
Results
Study population
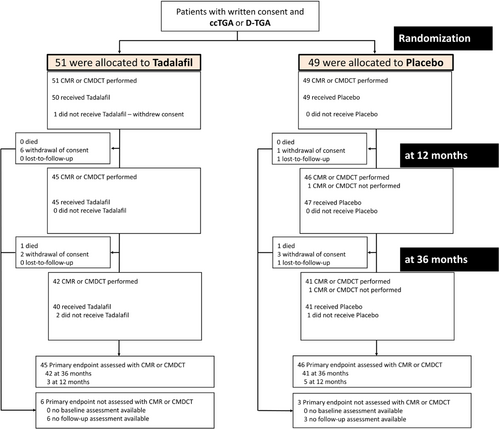
From the seven participating study centres, a total of 100 consecutive patients were enrolled, 75 (75%) with d-TGA and 25 (25%) with ccTGA. Of these patients, 51 were randomly assigned to the tadalafil group and 49 to the placebo group. Enrolment occurred between 18 October 2017 and 6 August 2018. Submission of last study data from 3-year follow-up to core-labs occurred on 15 October 2021. Baseline characteristics of study participants are displayed in Table 1. As illustrated in the study flow-chart (Figure 1), primary endpoint assessment by intention-to-treat analysis at 1 and 3 years of follow-up was possible in 91 and 83 patients, respectively (45 and 42 patients in the tadalafil group and 46 and 41 patients in the placebo group). Patients on beta-blockers more commonly had a history of atrial tachy-arrhythmias (19/34, 56% vs. 15/66, 23%, p = 0.001) and had a lower peak heart rate during exercise testing compared to patients without beta-blockers (137 ± 25 bpm vs. 163 ± 19 bpm, p < 0.001).
| Variable | Tadalafil (n = 51) | Placebo (n = 49) |
|---|---|---|
| Age, years, mean (SD) | 41.3 (10.2) | 40.1 (11.4) |
| Male sex | 37 (73%) | 30 (61%) |
| Female sex | 14 (27%) | 19 (39%) |
| Cardiac anatomy | ||
| ccTGA | 13 (25%) | 12 (24%) |
| d-TGA after Mustard operation | 7 (14%) | 16 (33%) |
| d-TGA after Senning operation | 31 (61%) | 21 (43%) |
| Additional lesions | 22 (43%) | 18 (37%) |
| Ventricular septal defect | 8 (16%) | 3 (6%) |
| Pulmonary stenosis | 4 (8%) | 4 (8%) |
| Ventricular septal defect and pulmonary stenosis | 4 (8%) | 6 (12%) |
| Other | 6 (12%) | 5 (10%) |
| Previous pacemaker or AICD implantation | 11 (22%) | 10 (20%) |
| History of atrial tachy-arrhythmias | 19 (37%) | 15 (31%) |
| Moderate or severe tricuspid regurgitation | 15 (30%) | 19 (39%) |
| NYHA class ≥II | 8 (16%) | 9 (18%) |
| Regular cardiac medication | ||
| Beta-blocker | 20 (39%) | 14 (29%) |
| ACE inhibitor or ARB | 26 (51%) | 20 (41%) |
| Diuretics | 10 (20%) | 5 (10%) |
| Antiarrhythmic drugs | 4 (8%) | 2 (4%) |
- Data expressed as n (%), mean (SD).
- ACE, angiotensin-converting enzyme; AICD, automated implantable cardioverter-defibrillator; ARB, angiotensin II receptor blocker; ccTGA, congenitally corrected transposition of the great arteries; d-TGA, complete transposition of the great arteries after atrial switch operation (Senning or Mustard operation); NYHA, New York Heart Association; SD, standard deviation.
Primary endpoint
As outlined in Table 2, mean RV-ESV did not change during follow-up in the tadalafil group (145 ± 57 vs. 148 ± 56, p = 0.27) or in the placebo group (123 ± 49 vs. 121 ± 46, p = 0.67). There was no significant difference between the tadalafil and the placebo group (3.37, 95% confidence interval: −4.29 to 11.03, p = 0.39).
| Variable | Tadalafil | Placebo | Tadalafil versus placebo | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Baseline | n | Follow-up | Change | p-value | n | Baseline | n | Follow-up | Change | p-value | Mean difference of the changea (95% CI) | p-value | |
| CMR/CMDCTb | ||||||||||||||
| RV-ESV, mlc | 51 | 145 (57) | 45 | 148 (56) | 3.2 (19.5) | 0.27 | 49 | 123 (49) | 46 | 121 (46) | 1.2 (16.7) | 0.67 | 3.37 (−4.29 to 11.03) | 0.39 |
| RV-EDV, ml | 51 | 253 (65) | 45 | 258 (65) | 5.0 (23.1) | 0.14 | 49 | 224 (61) | 46 | 222 (58) | 1.3 (26.1) | 0.80 | 6.08 (−4.38 to 16.54) | 0.26 |
| RVEF, %d | 51 | 44 (8) | 45 | 44 (8) | 0.1 (3.5) | 0.64 | 49 | 46 (8) | 46 | 46 (7) | −0.2 (3.3) | 0.67 | −0.21 (−1.53 to 1.11) | 0.76 |
| RV mass, g | 38 | 94 (21) | 34 | 97 (21) | 3.5 (12.0) | 0.09 | 40 | 87 (22) | 37 | 88 (22) | 2.7 (5.8) | 0.005 | 1.25 (−3.2 to 5.7) | 0.58 |
| LVEF, % | 51 | 62 (8) | 45 | 63 (7) | 0.1 (3.5) | 0.74 | 49 | 63 (9) | 46 | 64 (7) | 0.3 (3.9) | 0.56 | −0.43 (−1.83 to 0.97) | 0.55 |
| LV mass, g | 38 | 74 (13) | 34 | 77 (16) | 3.0 (7.4) | 0.02 | 40 | 71 (16) | 37 | 72 (18) | 1.7 (6.4) | 0.11 | 1.13 (−2.16 to 4.43) | 0.50 |
| CPET | ||||||||||||||
| Maximum load, W | 50 | 154 (50) | 38 | 159 (56) | 3.3 (23.4) | 0.35 | 49 | 155 (58) | 39 | 156 (54) | −1.5 (22.7) | 0.72 | 4.60 (−5.65 to 14.85) | 0.38 |
| Maximum load % predicted, % | 50 | 77 (19 | 38 | 79 (22) | 2.5 (12.3) | 0.22 | 49 | 83 (21) | 39 | 84 (19) | 2.9 (12.9) | 0.21 | −1.05 (−6.68 to 4.57) | 0.71 |
| Peak VO2, ml/min/kg | 50 | 23.7 (6.8) | 38 | 23.3 (6.5) | −1.2 (3.7) | 0.07 | 49 | 25.0 (8.0) | 41 | 23.8 (7·7) | −1.1 (4.7) | 0.13 | −0.13 (−1.89 to 1.64) | 0.89 |
| Peak VO2% predicted, % | 50 | 75 (16) | 38 | 75 (16) | −0.1 (12.9) | 0.78 | 49 | 77 (18) | 41 | 76 (14) | 0.7 (12.6) | 0.97 | −1.21 (−6.37 to 3.95) | 0.65 |
| VO2 workload slope | 50 | 9.6 (1.6) | 38 | 9.1 (2.0) | −0.5 (2.0) | 0.11 | 49 | 9.2 (1.9) | 37 | 9.1 (1.7) | −0.4 (1.5) | 0.28 | −0.09 (−0.86 to 0.67) | 0.81 |
| O2/heart rate, ml/beat | 50 | 12.9 (4.4) | 38 | 13.0 (4.2) | −0.3 (1.8) | 0.43 | 49 | 12.2 (4.2) | 40 | 12.4 (3.6) | −0.1 (2.4) | 0.96 | 0.03 (−0.78 to 0.84) | 0.94 |
| VE/VCO2 workload slope | 50 | 28.5 (6.2) | 38 | 30.1 (5.4) | 0.5 (5.6) | 0.23 | 49 | 28.9 (6.2) | 41 | 30.9 (8.3) | 1.9 (5.3) | 0.02 | −1.32 (−3.67 to 1.04) | 0.27 |
| Heart rate recovery at 1 min, beats | 50 | 21 (12) | 39 | 19 (14) | −3 (12) | 0.22 | 49 | 22 (12) | 41 | 20 (11) | 0 (11) | 0.66 | −2 (−7 to 2) | 0.30 |
| Neuro-hormones | ||||||||||||||
| NT-proBNP, pg/ml | 50 | 497 (852) | 38 | 496 (621) | −45 (653) | 0.77 | 48 | 398 (584) | 39 | 342 (336) | 43 (182) | 0.19 | 42 (−115 to 200) | 0.60 |
| hs-cTnT, ng/l | 50 | 9.2 (6.4) | 38 | 10.4 (5.7) | 1.9 (2.5) | <0.001 | 48 | 7.8 (6.1) | 39 | 8.4 (4.3) | 1.9 (2.9) | <0.001 | 0.34 (−0.81 to 1.49) | 0.57 |
| Quality of life | ||||||||||||||
| LAS | 51 | 75 (15) | 41 | 76 (17) | 0.6 (12.4) | 0.89 | 49 | 83 (11) | 42 | 83 (12) | −1.9 (13.7) | 0.73 | −0.80 (−6.50 to 4.89) | 0.78 |
| Satisfaction scale | 51 | 27 (6 | 41 | 28 (5) | 1.3 (4.2) | 0.06 | 49 | 29 (4) | 42 | 29 (4) | −0.9 (3.4) | 0.14 | 0.98 (−0.56 to 2.52) | 0.21 |
| General self-efficacy | 51 | 31 (5) | 41 | 32 (4) | 0.4 (2.7) | 0.27 | 49 | 33 (4) | 42 | 32 (5) | −1.1 (4.6) | 0.18 | 1.04 (−0.55 to 2.64) | 0.20 |
- Data expressed as n (%), mean (standard deviation), or median (25% to 75% interquartile range).
- CI, confidence interval; CPET, cardiopulmonary exercise test; CRM, cardiovascular magnetic resonance; hs-cTnT, high-sensitivity cardiac troponin T; LAS, linear analogue scale; LV, left ventricular; LVEF, left ventricular ejection fraction; CMDCT, cardiac multirow detector computed tomography; NT-proBNP, N-terminal pro-B-type natriuretic peptide; RV, right ventricular; RV-EDV, right ventricular end-diastolic volume; RVEF, right ventricular ejection fraction; RV-ESV, right ventricular end-systolic volume; VE/VCO2, minute ventilation to carbon dioxide; VO2, oxygen consumption.
- a Estimates from ANCOVA with the follow-up assessment as response, baseline assessment and time between follow-up assessment and baseline assessment (months) as covariates.
- b Thirty six-month CMR/CMDCT measurements used, except if not available (e.g. patient died or withdrew consent or discontinued investigational medical product), in which case the 12-month CMR/CMDCT study was used (n = 3 in the tadalafil group, n = 5 in the placebo group).
- c Primary outcome as change at 36 months or last assessment versus baseline.
- d Secondary endpoint as change at 36 months or last assessment versus baseline.
Secondary endpoints
Analyses of secondary endpoints are summarized in Table 2. There were no significant changes in end-diastolic volumes and right and left ventricular ejection fraction within the tadalafil and the placebo group and no significant differences between the tadalafil and the placebo group. A small change was observed for right ventricular mass in the placebo group but no significant differences between the tadalafil and the placebo group.
Measures of exercise capacity remained stable over the 3-year follow-up period, both within the tadalafil and the placebo group, without differences between groups.
Among neuro-hormones, no significant changes in NT-proBNP-levels were observed during the 3-year follow-up period or between the tadalafil and the placebo group. In contrast, a significant increase of hs-cTnT was observed, both within the tadalafil and the placebo group (p < 0.001 for both comparisons) without significant differences between the tadalafil and the placebo group (p = 0.57). Measures of quality of life remained stable during follow-up, without differences between the tadalafil and the placebo group.
Subgroup analyses
No treatment effect of tadalafil versus placebo was found when analysis was specified for symptomatic patients (New York Heart Association [NYHA] functional class ≥II, n = 17) versus asymptomatic patients (NYHA functional class I, n = 83). No significant treatment effects were found when patients were stratified for previous PM/AICD implantation, ccTGA versus d-TGA, endpoint assessment with CMDCT versus CMR, male versus female, NT-proBNP ≥233 pg/ml or <233 pg/ml (median of NT-proBNP-measurements) and hs-cTnT ≥7 ng/L versus <7 ng/L (median of hs-cTnT measurements), moderate or severe tricuspid regurgitation at baseline and d-TGA after Mustard versus Senning operation (online supplementary Table S1).
Adverse events and safety endpoints
A higher rate of drug-specific adverse events (headache, nausea and dyspepsia) was observed in the tadalafil group but there was no indication of a safety concern. An overview of adverse events in the entire study population is summarized in Table 3. Results were unchanged when analysis of adverse events was performed in the safety population (only considering patients while on study drug). In both the tadalafil and the placebo group one patient died. Death in the treatment arm was not related to the study drug (post-interventional death after percutaneous mitral valve repair). There was no significant difference in drug adherence between patients in the tadalafil and placebo group (excluding patients with permanent discontinuation of the study drug).
| Tadalafil (n = 51) | Placebo (n = 49) | Rates tadalafil (95% CI) | Rates placebo (95% CI) | Zero-inflated Poisson regression | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Adverse event | No. of patients with event | Total number of events | % of this event IMP (temporarily) stopped | No. of patients with event | Total number of events | % of this event IMP (temporarily) stopped | Rate ratio (95% CI) | p-value | ||
| Person-years at risk | 136.3 | 138.1 | ||||||||
| Serious adverse events | ||||||||||
| Death | 1 (2%) | 1 | 0 (0%) | 1 (2%) | 1 | 0 (0%) | 0.007 (0.000–6.2e + 07) | 0.007 (0.000–7.2e + 07) | 0.95 (0.03–30.88) | 1.0 |
| Worsening heart failure | 2 (4%) | 2 | 0 (0%) | 3 (6%) | 3 | 1 (33%) | 0.015 (0.000–1.5e + 05) | 0.022 (0.000–1.3e+04) | 0.68 (0.11–4.04) | 0.67 |
| Vascular eventa | 0 (0%) | 0 | 0 (0%) | 0 (0%) | 0 | |||||
| New or worsening arrhythmias | 7 (14%) | 9 | 1 (11%) | 6 (12%) | 10 | 1 (10%) | 0.066 (0.000–134.763) | 0.072 (0.000–105.256) | 0.69 (0.23–2.12) | 0.35 |
| Renal function impairment | 1 (2%) | 1 | 1 (100%) | 0 (0%) | 0 | 0.007 (0.000–6.2e + 07) | 1.0 | |||
| Other SAE leading to discontinuation of study drug | 0 (0%) | 0 | 0 (0%) | 1 (2%) | 1 | 1 (100%) | 0.007 (0.000–7.2e + 07) | 0.49 | ||
| SAE other than any of the above | 7 (14%) | 11 | 2 (18%) | 6 (12%) | 10 | 1 (10%) | 0.081 (0.000–79.548) | 0.072 (0.000–105.256) | 1.06 (0.36–3.09) | 1.0 |
| Adverse events | ||||||||||
| Headache | 11 (22%) | 13 | 3 (23%) | 4 (8%) | 5 | 1 (20%) | 0.096 (0.000–54.089) | 0.036 (0.000–1074.746) | 2.79 (0.90–8.68) | 0.16 |
| Renal function impairment | 0 (0%) | 0 | 0 (0%) | 1 (2%) | 1 | 0 (0%) | 0.007 (0.000–7.2e + 07) | 0.49 | ||
| Allergic reaction | 1 (2%) | 1 | 0 (0%) | 0 (0%) | 0 | 0.007 (0.000–6.2e + 07) | 1.0 | |||
| Epipharyngitis | 1 (2%) | 1 | 0 (0%) | 0 (0%) | 0 | 0.007 (0.000–6.2e+07) | 1.0 | |||
| Nausea and dyspepsia | 4 (8%) | 4 | 0 (0%) | 1 (2%) | 1 | 0 (0%) | 0.029 (0.000–2704.737) | 0.007 (0.000–7.2e + 07) | 4.05 (0.45–36.27) | 0.36 |
| Symptomatic arterial hypotension | 0 (0%) | 0 | 0 (0%) | 1 (2%) | 1 | 0 (0%) | 0.007 (0·000–7.2e + 07) | 0.49 | ||
| Other AE leading to discontinuation of study drug | 3 (6%) | 3 | 2 (67%) | 1 (2%) | 1 | 1 (100%) | 0.022 (0.000–1.2e+04) | 0.007 (0.000–7.2e + 07) | 6.72 (0.36–127.03) | 0.62 |
| New or worsening arrhythmias | 6 (12%) | 10 | 0 (0%) | 2 (4%) | 2 | 0 (0%) | 0.074 (0.000–101.340) | 0.014 (0.000–1.7e + 05) | 5.26 (0.96–28.65) | 0.55 |
| AE other than any of the above | 13 (26%) | 18 | 2 (11%) | 5 (10%) | 5 | 1 (20%) | 0.132 (0.001–28.964) | 0.036 (0.000–1074.746) | 3.76 (1.31–10.80) | 0.12 |
- Data are expressed as number of patients with event (% of all patients) and total number of events reported.
- AE, adverse event; SAE, serious adverse event.
- P-value from Poisson regression comparing the event rates, with time to last assessment as offset (i.e. days between baseline and 3-year follow-up or last contact).
- a Stroke, transient ischaemic attack, myocardial infarction, peripheral embolism, other vascular events.
Discussion
In this prospective, multicentre, randomized, placebo-controlled trial with long-term follow-up, we investigated a novel pharmacologic treatment concept for improvement of ventricular function in adults with systemic RVs. Promising results from concepts in basic research and animal models did not translate into favourable clinical results. As compared to placebo, tadalafil, a PDE-5 inhibitor, did not improve systemic right ventricular function and had no impact on other clinical endpoints, such as exercise capacity, neuro-hormonal activation or quality of life.
Scope of the problem
Among all adults with congenital heart disease and end-stage heart failure undergoing orthotopic heart transplantation, those with systemic RVs currently comprise about one third of all patients.26 Given the evolution and demographical characteristics of these patient cohorts, a further increase in the number of adults with systemic RVs and end-stage heart failure is expected over the upcoming years and decades.27 This contrasts with the very limited number of donor organs, limiting the availability of timely heart transplantation for all patients in need.26 There is thus an urgent need for novel treatment alternatives in adults with systemic RVs and heart failure.8
Current evidence and interpretation of study results
Most treatment recommendations for adults with failing systemic RV are based on data from retrospective studies and expert opinion.28 Prospective randomized trials are scarce in the field of adult congenital heart disease. To the best of our knowledge, all small prospective trials investigating conventional heart failure medication for patients with systemic RVs failed to demonstrate favourable treatment effects.
Our trial indicates that tadalafil does not improve systemic ventricular function in adults with systemic RVs. There is also no signal that it has a beneficial effect on other clinical endpoints, such as exercise capacity or neuro-hormonal activation. At least, treatment with tadalafil was not associated with an increased risk for major adverse events (Graphical Abstract).
Study design and power calculations of this trial were partially based on results from a previously published, randomized controlled study, assessing the effects of valsartan in adults with systemic RVs.9 Compared to the patient cohort reported by van der Bom and colleagues, patients within our cohort were on average about 7 years older. Still, in the study by van der Bom, more patients were symptomatic at baseline (NYHA class ≥II in 32% vs. 17% within our cohort), and fewer were on conventional heart failure medication (renin–angiotensin–aldosterone system blockers 20% vs. 56%, beta-blockers 16% vs. 34% and diuretics 7% vs. 15%). While the study by van der Bom found a significant treatment effect for valsartan in patients with heart failure symptoms at baseline, no treatment effect was found for tadalafil in our study, when patients were stratified for symptoms at baseline. The frequency of adverse cardiac events over the 3-year study period was comparable between the two studies. While in the study by van der Bom et al., a significant increase in RV-ESV over the 3 years of follow-up was found in the placebo group, this was not observed in our study. Interestingly, a significant decrease in exercise capacity over 3 years of follow-up was observed in the study by van der Bom and colleagues for the valsartan and the placebo group, while in our cohort, average exercise capacity remained stable. We did not formally assess day-to-day physical activity of study participants nor changes in physical activity over the study period. Regular physical activity has, however, shown to improve exercise capacity in adults with congenital heart disease with systemic RVs and should therefore be encouraged in all patients.29, 30
There may be several possible explanations why conventional heart failure medication does not work in adults with systemic RVs. First, structure, architecture, and function of a morphological RV differs in many ways from a morphological left ventricle.8 Second, heart failure in patients with complex congenital heart disease may be promoted by the repair strategy. Stiff venous baffles, redirecting venous blood on atrial level may importantly limit ventricular preload, independent of the actual myocardial function. Extensive scar tissue from previous repair operations, the high prevalence of atrial arrhythmias, sinus node dysfunction and conduction abnormalities may interact with ventricular function and haemodynamics.
Since planning and designing of the current trial, several novel treatment options for patients with heart failure, such as sacubitril/valsartan and sodium–glucose cotransporter 2 (SGLT2) inhibitors have been introduced into clinical practice. Based on lack of evidence for similar efficacy of standard heart failure medication in adults with systemic RVs, it remains questionable whether these novel heart failure drugs will be effective in patients with systemic RVs. It is thus important that the efficacy of these drugs in adults with systemic RVs will be properly investigated in prospective trials. Our trial demonstrates that investigator-initiated prospective multicentre trials, meeting contemporary quality standards, are feasible. We thus hope that our efforts encourage other groups to conduct such trials to enhance the urgently needed evidence base for improved treatment of adults with congenital heart disease.
In a recent study, a substantial proportion of adults with systemic RVs, undergoing cardiac catheterization, was found to have pulmonary hypertension.31 Almost one third of patients were found to have pre-capillary or combined pre- and post-capillary pulmonary hypertension. Such patients may benefit from specific treatment with PDE-5 inhibitors. In our study, treatment with tadalafil was associated with expected side-effects of treatment with a PDE-5 inhibitor, such as headaches and dyspepsia, but showed no signal for an excess in cardiovascular complications.
Within our trial, a large amount of high-quality data were prospectively collected, including data from cardiac imaging, neuro-hormonal activation and exercise testing. Careful analysis of these data by means of pre-specified sub-analyses and sub-studies are planned and will likely improve our understanding of the disease trajectory in adults with systemic RVs and may help to facilitate prognostication in the future.
Limitations
Although our study was not powered to detect a reduction in hard clinical endpoints, given the results of our analysis, it is rather unlikely that such a benefit was to be expected in larger patient cohorts with longer follow-up duration. Although caused by chance, differences in baseline characteristics may have influenced study results. The lack of evidence of a treatment effect of tadalafil in any of the tested subgroups makes it unlikely that differences in baseline characteristics had an impact on outcomes of this trial. Whether the lack of efficacy of tadalafil is a class effect or specifically related to tadalafil cannot be answered by the current study. Similarly, our study cannot determine whether higher doses of tadalafil may have had a treatment effect. Given that there was no statistical trend toward a benefit of tadalafil on right ventricular systolic function, it is unlikely that larger study groups would have changed the results of our study. Arrhythmic events during follow-up may have had an impact on measures of ventricular volumes and function. Given that the rate of hospital admissions for worsening heart failure had been relatively low over the 3-year follow-up, this may indicate that patients with more advanced heart failure were not enrolled in this study. None of the patients underwent transplant assessment or were transplanted during the study period. In this study invasive haemodynamic measurements, particularly measurement of pulmonary artery pressures and pulmonary vascular resistance were not part of the study protocol. As pulmonary hypertension is common in adults with systemic RVs, as outlined above, careful non-invasive assessment to identify patients with pulmonary hypertension may be important. Pulmonary hypertension may be suspected from echocardiographic measurements or assessment of measurements from cardiopulmonary exercise testing (exercise oscillatory ventilation and VE/VCO2 slopes). Severity of tricuspid regurgitation may change during exercise. Routine use of exercise imaging may add to a better understanding of haemodynamics in patients with systemic RVs. Although the majority of patients within our study were asymptomatic, a substantial proportion were on treatment with angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers. Reasons for treatment with these medications in individual patients were not systematically assessed. While CMR has evolved as a reference method for measurement of right ventricular volumes and function, CMDCT is rarely used in clinical practice and is less well validated. To overcome some of these limitations, a strict imaging protocol was developed and all studies were analysed in a core-lab by experienced imagers, blinded to sequence of studies and allocation of study groups.
Conclusion
In conclusion, in this trial in adults with systemic RV, right ventricular systolic function, exercise capacity and neuro-hormonal activation remained stable over a 3-year follow-up period. No significant treatment effect of tadalafil was observed. Further research is needed to find effective treatment for improving systemic ventricular function in adults with systemic RVs.
Acknowledgement
Open access funding provided by Universitat Zurich.
Funding
The SERVE trial was funded by the Swiss National Foundations Schweizerischer Nationalfonds zur Förderung der wissenschaftlichen Forschung (31IC30_166855) and supported by the participating centers (University Hospital Basel, Inselspital Bern, University Hospital Geneva, University Hospital Lausanne, Kantonsspital St. Gallen, University Hospital Vienna (Austria), University Hospital Zurich).
Conflict of interest: J.S. receives an unrestricted grant of Bayer Healthcare Schweiz AG for the research activities of the Cardiac MR Center of the University Hospital Lausanne (CHUV). All other authors have nothing to disclose.
Appendix
David Herzig, Prisca Eser (CPET Core Laboratory University Hospital Bern).
Coralie Blanche (University Hospital Geneva).
Dominik Stambach, Niklas Ehl (Kantonsspital St. Gallen).
Irene M. Lang, Julia Mascherbauer, Matthias Schneider, Rochus Pokan (University Hospital Vienna).
Christine H. Attenhofer Jost, Bruno Santos Lopes, Francesca Bonassin Tempesta, Lukas Meier, Daniela Babic, Georgios Benetos (University Hospital Zurich, Switzerland).
David Jean Winkel (University Hospital of Basel, Basel, Switzerland).