Recommendations on pre-hospital & early hospital management of acute heart failure: a consensus paper from the Heart Failure Association of the European Society of Cardiology, the European Society of Emergency Medicine and the Society of Academic Emergency Medicine
Abstract
Acute heart failure is a fatal syndrome. Emergency physicians, cardiologists, intensivists, nurses and other health care providers have to cooperate to provide optimal benefit. However, many treatment decisions are opinion-based and few are evidenced-based. This consensus paper provides guidance to practicing physicians and nurses to manage acute heart failure in the pre-hospital and hospital setting. Criteria of hospitalization and of discharge are described. Gaps in knowledge and perspectives in the management of acute heart failure are also detailed. This consensus paper on acute heart failure might help enable contiguous practice.
List of abbreviations
-
- AHF
-
- Acute heart failure
-
- CHF
-
- Chronic heart failure
-
- CCU
-
- Coronary care unit
-
- ICU
-
- Intensive care unit
-
- ED
-
- Emergency department
-
- CS
-
- Cardiogenic shock
-
- SBP
-
- Systolic blood pressure
-
- BP
-
- Blood pressure
-
- NIV
-
- Noninvasive ventilation
-
- IVC
-
- Inferior vena cava
-
- AMI
-
- Acute myocardial inferction
-
- STEMI
-
- ST elevation myocardial infarction
-
- PCT
-
- procalcitonin
-
- ECG
-
- Electrocardiogram
-
- BNP
-
- B type natriuretic peptide
-
- NT-proBNP
-
- N Terminal pro BNP
-
- MR-proANP
-
- mid-regional proatrial natriuretic peptide
-
- BUN
-
- Blood urea nitrogen
-
- SpO2
-
- Peripheral capillary oxygen saturation
-
- COPD
-
- Chronic obstructive pulmonary disease
-
- NIV
-
- Noninvasive ventilation
-
- FiO2
-
- Fraction of inspired oxygen
-
- APE
-
- Acute pulmonary oedema
-
- CPAP
-
- Continuous positive airway pressure
-
- PS-PEEP
-
- Pressure support positive end-expiratory pressure
-
- DOSE
-
- Diuretic optimization strategies evaluation trial
-
- RR
-
- Respiratory rate
-
- SvO2
-
- Mixed venous oxygen saturation
-
- GP
-
- General practitioner
-
- IABP
-
- Intra-aortic balloon pump
-
- LVAD
-
- Left ventricular assist device
-
- HR
-
- heart rate
-
- iv
-
- intravenous
Introduction
Despite several critical steps forward in the management of chronic HF (CHF), the area of acute heart failure (AHF) has remained relatively stagnant. As stated in the updated ESC HF guidelines, clinicians responsible for managing patients with AHF must frequently make treatment decisions without adequate evidence, usually on the basis of expert opinion consensus.1 Specifically, the treatment of acute HF remains largely opinion-based with little good evidence to guide therapy. As a confirmation of that, just one recommendation has been deemed level of evidence type A with all the other recommendations receiving a level of evidence B or more frequently C in the recent ESC/HFA guidelines on acute and chronic heart failure.1
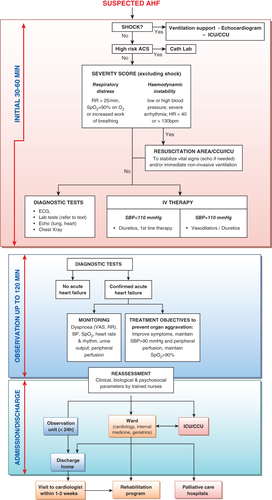
AHF is a syndrome in which emergency physicians, cardiologists, intensivists, nurses and other healthcare providers have to cooperate to provide “rapid” benefit to the patients. We hereby would like to underscore the wider experience grown in different settings of the area of intensive care on acute heart failure, actually larger and more composite than that got in specialized Care Units. The distillate of such different experiences is discussed and integrated in the present document. Hence, the authors of this consensus paper believe a common working definition of AHF covering all dimensions and modes of presentations has to be made, with the understanding that most AHF presentations are either acute decompensations of chronic underlying HF or the abrupt onset of dyspnea associated with significantly elevated blood pressure. Secondly, recent data show that, much like acute coronary syndrome, AHF might have a “time to therapy” concept. Accordingly, “prehospital” management is considered a critical component of care. Thirdly, most patients with AHF have normal or high blood pressure at presentation, and are admitted with symptoms and/or signs of congestion. This is in contradiction to the presentation where low cardiac output leads to symptomatic hypotension and signs/symptoms of hypoperfusion, a circumstance that is relatively rare, present in coronary care unit/intensive care unit (CCU/ICU) but associated with a particularly poor outcome. Hence, it is important to note that appropriate therapy requires appropriate identification of the specific AHF phenotype.2 The aim of the current paper is not to replace guidelines, but, to provide contemporary perspective for early hospital management within the context of the most recent data and to provide guidance, based on expert opinions, to practicing physicians and other healthcare professionals (Figure 1). We believe that the experience accrued in the different settings from the emergency department through to the ICU/CCU is collectively valuable in determining how best to manage the patients with AHF. Herein, a full version is provided. A summary of this consensus paper is published in European Heart Journal.
Definition and epidemiology of acute heart failure
- Acute heart failure (AHF) is the term used to describe the rapid onset of, or acute worsening of symptoms and signs of HF, associated with elevated plasma levels of natriuretic peptides. It is a life threatening condition that requires immediate medical attention and usually leads to urgent hospital admission.
- Most of the patients with AHF present with normal or high blood pressure and with symptoms and/or signs of congestion rather than low cardiac output.
Table 1 compares the characteristics among patients whose initial management was performed in cardiology/CCU, emergency department (ED) or prehospital setting. Clinical characteristics are somewhat different; AHF patients seen early, in the pre-hospital setting or in the ED not only have higher blood pressure but also are more frequently female and older 3.
| Admission site | Cardiac ICU/CCU | Emergency department | Prehospital setting | |||
|---|---|---|---|---|---|---|
| Euro-HF II N = 3580 | EFICA N = 599 | ADHERE N = 159168 | ATTEND N = 1100 | Ducros L et al N = 207 | Sporer KA et al. N = 319 | |
| Male (%) | 61 | 59 | 49 | 59 | 41 | 47 |
| Age (years) | 70 | 73 | 73 | 72 | 81 | 77 |
| SBP > 140 mmHg at admission (%) | 63 | 60 | 74 | 71 | 75 | 77 |
| CS or SBP < 90 mmHg (%) | 3.9 | 29 | 3 | NA | 1 | 3 |
| Initial SBP | 135 | 126 | 144 | 147 | 170 | 167 |
- Table legend: Of note, EFICA includes only ICU patients.
Prehospital and early management strategies in acute heart failure
- As for acute coronary syndromes, the “time-to-treatment” concept may be important in patients with AHF. Hence, all AHF patients should receive appropriate therapy as early as possible.
- In the pre-hospital setting, AHF patients should benefit from:
- ○ Noninvasive monitoring, including pulse oximetry, blood pressure, respiratory rate, and a continuous ECG, instituted within minutes of patient contact and in the ambulance if possible.
- ○ Oxygen therapy given based on clinical judgment unless oxygen saturation < 90% in which case oxygen therapy should be routinely administered.
- ○ Non-invasive ventilation, in patients with respiratory distress
- ○ Medical treatment should be initiated based on blood pressure and/or the degree of congestion using vasodilators and/or diuretics (i.e., furosemide)
- Rapid transfer to the nearest hospital should be pursued, preferably to a site with a cardiology department and/or CCU/ICU.
- On arrival in the ED/CCU/ICU, initial clinical examination, investigations and treatment should be started immediately and concomitantly
One way to achieve early treatment in AHF is to initiate acute management in the pre-hospital setting. Indeed many diagnostic and therapeutic tools are now available in the prehospital setting before admission to the ED (i.e, in the ambulance) in some countries8. This includes natriuretic peptides measured using a point-of-care device, if the diagnosis of AHF is uncertain9, non-invasive ventilation (NIV) that has been shown to decrease intubation rates and improve early outcome in acute cardiogenic pulmonary edema8 and any recommended intravenous treatment of AHF, especially nitrates and furosemide.10 Pre-hospital management should not delay the rapid transfer of AHF patients to the most appropriate medical environment.11
Data derived from registries indicate that early therapy is critical in the management of patients presenting with AHF, it is therefore rational to initiate treatment as soon as possible along with relevant investigations 5, 12. Furthermore, when the clinical diagnosis of AHF is unequivocal, based on clinical examination, treatment should begin promptly rather than waiting for further testing.
Initial clinical evaluation and investigations at arrival in the emergency department/coronary care unit/ intensive care unit (Figure 1)
-
- ○ Objective measurement of dyspnea severity, including the respiratory rate, intolerance of the supine position, effort of breathing and degree of hypoxia.
- ○ Systolic and diastolic blood pressure.
- ○ Heart rate and rhythm.
- ○ Objective determination of body temperature and signs/symptoms of hypoperfusion (cool extremities, narrow pulse pressure, mental status)
- The next step should include a search for congestion including peripheral oedema, audible rales (especially in absence of fever) and elevated jugular venous pressure,
- Additional testing that may be useful includes:
- ○ ECG, recognizing that in AHF this is rarely normal, and rarely diagnostic but necessary to exclude ST elevation myocardial infarction
- ○ Laboratory tests (see below)
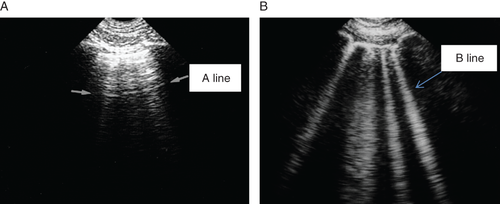
- ○ Bedside thoracic ultrasound for signs of interstitial oedema (Figure 2) and abdominal ultrasound for inferior vena cava diameter (and ascites) if expertise is available
- ○ Chest X-ray to rule-out alternative causes of dyspnoea, though, in nearly 20% of patients, it may be normal, limiting overall sensitivity.
- Immediate echocardiography is not needed during the initial evaluation in most cases except when hemodynamic instability is present. However, echocardiography is needed after stabilization, especially with de novo disease.
- Urinary catheterization should be avoided unless the benefits outweigh the risks of infection and longer –term complications related to continence
Determination of cardiopulmonary stability is the critical first step. Patients with respiratory failure or hemodynamic compromise should be triaged to a location where immediate respiratory and cardiovascular support can be provided (see Figure 1). Objective measurement of mental status using the mnemonic AVPU (alert, visual, pain, or unresponsive) 13 serves as an indicator of hypoperfusion.
Chest radiography is the one of the most widely used modalities in the evaluation of AHF with pulmonary venous congestion, pleural effusions, and interstitial or alveolar edema serving as the most specific indicators 14, 15. Chest X-ray is also useful to rule-out alternative causes of dyspnea (e.g., pneumonia, together with PCT). However, in nearly 20% of patients, it may be normal, limiting overall sensitivity 16. In reasonably expert hands the thoracic ultrasound maybe equally or more informative than the chest X-ray allowing also an important time saving. An ECG is also commonly obtained in those with suspected HF, and will often reveal some chronic baseline abnormality.
Immediate echocardiography is mandatory in all patients presenting with cardiogenic shock. In all others, though, it would be interesting to have echocardiography, it is not routinely available in most of the EDs throughout Europe, and hence, it may be performed later during hospitalization. Furthermore, echocardiography in nonexpert hands might potentially be misleading. If expertise available, bedside thoracic ultrasound can provide additional information by enabling direct visualization of interstitial oedema 17, 18 (Figure 2), providing a rough estimation of cardiac function, 19 and rapidly identifying pericardial effusion or other causes of hemodynamic compromise in the setting of dyspnea20.
Laboratory tests at presentation
- Upon presentation to the ED or CCU/ICU, a plasma natriuretic peptide level (BNP, NT-proBNP or MR-proANP) should be measured in all patients with acute dyspnoea and suspected AHF, ideally using a point-of-care assay, to help in the differentiation of AHF from non-cardiac causes of acute dyspnoea
- The following laboratory assessments should be performed at admission in the blood of all AHF patients: troponin, BUN (or urea), creatinine, electrolytes, glucose and complete blood count
- D-dimer is indicated in patients with suspicion of acute pulmonary embolism
- Routine arterial blood gas is not needed. However, arterial blood gas may be useful when a precise measurement of oxygen and carbon dioxide partial pressures is needed. Venous sample might acceptably indicate pH and CO2
Of note, the vast majority of patients with AHF will have an elevated troponin level with increasingly sensitivity assays making it hard to exclude an acute coronary syndrome unless the level is below the 99th percentile. As well as diagnosis, troponin measurement may be considered for prognostication 21 as elevated levels are associated with poorer outcomes 22. As shown in Figure 3, arterial blood gases are not routinely indicated and should be restricted to patients in whom oxygenation cannot be reliably assessed by pulse oximetry. In case of persistent respiratory distress, despite initial therapy with oxygen and/or non-invasive ventilation, venous blood gas analysis should be obtained to detect respiratory or metabolic acidosis23. We recommend measuring creatinine, BUN and electrolytes every 1–2 days while in the hospital, and creatinine, BUN, electrolytes and natriuretic peptides before discharge from the hospital. Of note, more frequent testing might be justified according to severity of the case.
Role of nursing management in acute heart failure
- Specific considerations of nursing management include:
- ○ Triage to appropriate environment for safe clinical care
- ○ Objective monitoring for change in signs and symptoms suggestive of response to treatment.
- ○ Discharge planning and referral to multidisciplinary disease management program.
- Anxiety of the patient should be addressed by promptly answering questions and providing clear information to the patient and family
- Relevant changes in clinical status should be promptly addressed and communicated to the physician.
- Effective and consistent communication should be maintained with the patient and/or family
A rapid nursing assessment should be undertaken to optimize the triage of patients to the appropriate level of care and inform the management plan. The ongoing monitoring for changes in signs and symptoms suggestive of a response to treatment should be no less than four hourly. This should include monitoring the hemodynamic, respiratory and mental status and fluid balance 24, 25. The side effects of treatment, such as electrolyte imbalance, should also be recorded. Significant changes should be communicated to the physician, ideally with an accompanying management plan. Unsatisfactory responses to treatment (persistent low saturation, low blood pressure, low diuresis) must be immediately communicated to the physician. The treatment should be carried out in an environment with safe staffing levels and by nurses with specialist knowledge and skills to limit the risk of adverse events 26. The patient and their family should be provided with consistent information on the management plan on a regular basis to improve overall satisfaction with care and patient outcome27.
Once the condition has stabilized a structured clinical, psychological and social assessment should be undertaken using objective and were possible validated tools, 28. This should underpin the discharge plan and referral to a multidisciplinary disease management program.
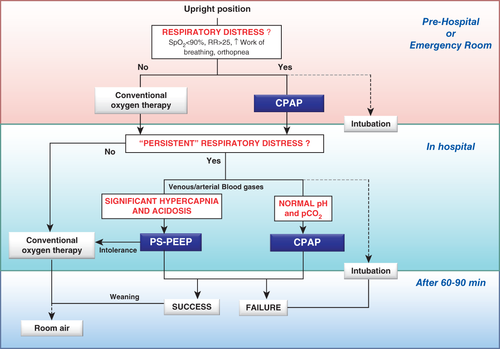
Oxygen therapy and/or ventilatory support (Figure 3)
- Oxygenation should be monitored with pulse-oximetry (SpO2) (Figure 3)
- Acid–base balance, complementing SpO2 monitoring, should be obtained on admission, especially in patients with acute pulmonary oedema (APE) or previous history of chronic obstructive pulmonary disease (COPD), using venous blood or especially in patients with cardiogenic shock through the arterial line
- Oxygen therapy should be considered in patients with AHF having SpO2 < 90%
- Non-invasive ventilation (NIV) is indicated in patients with respiratory distress and should be started as soon as possible. Non-invasive ventilation decreases respiratory distress and also reduces the rate of mechanical endotracheal intubation
Congestion affects lung function and increases intrapulmonary shunting, resulting in hypoxemia. A decrease in SpO2 may be detected even in mild forms of AHF 29. FiO2 should be increased up to 100% if necessary, according to SpO2, unless contra-indicated. Hyperoxia, however, should be avoided 30. Non-invasive ventilation (NIV) reduces respiratory distress and may decrease the intubation and mortality rates 31, although data regarding mortality are less conclusive 32, 33. Non-invasive ventilation should be started as soon as possible in patients with acute pulmonary edema (APE) showing respiratory distress (Figure 3). Continuous positive airway pressure (CPAP) is a feasible technique in the prehospital setting because it is simpler than pressure-support positive end-expiratory pressure (PS-PEEP) and requires minimal training or equipment. On hospital arrival, patients who still show signs of respiratory distress should continue with noninvasive ventilation, preferably PS-PEEP, in case of acidosis and hypercapnia, particularly in those with previous history of COPD or signs of fatigue (Figure 3).
Early administration of intravenous diuretics and vasodilators
- Initially, 20–40 mg intravenous furosemide can be considered in all AHF patients
- In cases of volume overload, intravenous diuretic dose should be tailored to the type of AHF (de novo with lower dose than exacerbation of CHF) (Table 2)
- When systolic BP is normal to high (≥110 mmHg)1, intravenous vasodilator therapy might be given for symptomatic relief as an initial therapy. Alternatively, sublingual nitrates may be considered.
| If new-onset HF or no maintenance diuretic therapy | Furosemide 40 mg intravenous |
| If established HF or on chronic oral diuretic therapy | Furosemide bolus at least equivalent to oral dose |
Intravenous loop diuretics, the most common AHF treatment, are used because few other interventions provide equivalent congestion relief. Unfortunately, data defining optimal diuretic timing and dose are incomplete. A relationship between time to diuretic and outcomes was reported in one registry 7, and others provide some suggestion as to appropriate dosing. In the “high-dose” arm of the Diuretic Strategies in Patients with Acute Decompensated Heart Failure (DOSE) trial, furosemide was administered at 2.5 times of the oral dose taken in the weeks preceding the index hospitalization. This resulted in improved diuresis and dyspnea relief but at a cost of transient worsening in renal function 34. However, in a registry study, mortality was higher when furosemide dosing exceeded 160 mg in the first 24 hours, though, it is disputed in the literature 35. Consequently, while early diuretic use is appropriate, the dose should be limited to the smallest amount necessary to provide adequate clinical effect 36. Hence, we recommend furosemide bolus at least equivalent to the oral dose in patients with chronic HF (as in the “low dose” arm of the DOSE trial), with a low dose when the AHF is new-onset (Table 2). It is reasonable to utilize diuretics concomitantly with vasodilator agents for symptomatic relief and decongestion.
Intravenous vasodilators are the second most used agent in AHF. Their use was shown to be associated with lower mortality, and a delay in administration was associated with a higher mortality.6 Randomized controlled trial (RCT) evidence comparing clinical outcomes of intravenous vasodilator, especially nitrates in AHF patients is limited and of relatively low methodological quality. A recently published review on vasodilators use in AHF patients by the Cochrane library only identified 4 randomized controlled trials comparing nitrates (isosorbide dinitrate and nitroglycerin) with alternative interventions over the last decades in adult population37. On other hand, nitroprusside, particularly in those with high arterial blood pressure, as an alternative to other nitrovasodilators could potentially be used in this setting, though, data are limited, and invasive monitoring is typically required38. However, there is a consensus that intravenous vasodilators are indicated in AHF with rather normal to high BP39 and are not indicated in patients with SBP is < 110 mm Hg 1. Of note, in patients with HF and atrial fibrillation, intravenous administration of a cardiac glycoside should be considered for rapid control of the ventricular rate, as recommended by guidelines. Furthermore, beta-blockers are considered as the preferred first-line treatment to control the ventricular rate in patients with HF and AF.
Drugs to be used cautiously in acute heart failure (excluding cardiogenic shock)
- Routine use of opioids in AHF patients is not recommended
- There is only a very limited place for sympathomimetics or vasopressors in patients with AHF excluding cardiogenic shock; they should be reserved for patients who have persistent signs of hypoperfusion despite adequate filling status.
In small studies, morphine was shown to reduce preload, afterload, heart rate and relieve dyspnea.40, 41 However, in The Acute Decompensated Heart Failure National Registry (ADHERE), morphine use was associated with higher rates of mechanical ventilation, ICU admission and death.42 Since, morphine has never been shown to improve outcomes, but may be associated with harm, its routine use cannot be recommended and hence the decision should be individualized.
As noted in the guidelines,1 there is no role for vasopressors if systolic BP > 110 mmHg or for routine use of sympathomimetics when signs of low cardiac output are absent. Further, there is no evidence that dobutamine should be given when pulmonary edema is associated with a normal or high systolic blood pressure.43
Management of Evidence Based Oral Therapies
- In case of decompensation of CHF, every attempt should be made to continue evidence-based, disease-modifying, oral therapies in patients with AHF (Table 3).
- In the case of de novo HF, every attempt should be made to initiate these therapies after hemodynamic stabilization.
| Normotension/Hypertension | Hypotension | Low heart rate | Potassium | Renal impairment | |||||
|---|---|---|---|---|---|---|---|---|---|
| 85–100 mmHg | <85 mmHg | <60 ≥50 bpm | <50 bpm | ≤3.5 mg/dl | >5.5 mg/dl | Cr<2.5, eGFR>30 |
Cr >2.5, eGFR<30 |
||
| ACE-I /ARB | review/ increase | reduce / stop | stop | No change | No change | review/ increase | stop | review | stop |
| Beta-blocker | No change | reduce / stop | stop | reduce | stop | No change | No change | No change | No change |
| MRA | No change | No change | stop | No change | No change | review/ increase | stop | reduce | stop |
| Diuretics | increase | reduce | stop | No change | No change | review/ No change | review/ increase | No change | review |
| Other vasodilators (Nitrates) | increase | reduce / stop | stop | No change | No change | No change | No change | No change | No change |
| Other heart rate slowing drugs (amiodarone, CCB, Ivabradine) | review | reduce / stop | stop | reduce / stop | stop | review/stop (*) | No change | No change | No change |
- Legends: CCB, Calcium channel blockers (mg/dl); Cr, creatinine blood level (mg/dl); eGFR, estimated glomerular filtration rate ml/min/1.73 m2; MRA, mineralocorticoid receptor antagonist; (*) amiodarone.
Oral disease-modifying heart failure therapy, should be continued on admission with AHF, except in case of hemodynamic instability (SBP < 85 mmHg; heart rate < 50 bpm), hyperkalemia (potassium > 5.5 mmol/L) or severely impaired renal function. In these cases, the daily dosage of oral therapy may, be reduced or stopped temporarily until the patient is stabilized (Table 3). In particular, beta blockers can be safely continued during AHF presentations except in cardiogenic shock 44.
Discharge from emergency department
- Clinical condition can change dramatically within a few hours of ED arrival. Hence, clinical response to initial treatment is an important indicator of likely disposition.
- Indicators of good response to initial therapy that might be considered in discharge include:
- ○ Patient-reported subjective improvement
- ○ Resting HR < 100 bpm
- ○ No hypotension when standing up
- ○ Adequate urine output
- ○ Oxygen saturation > 95% in room air
- ○ No or moderate worsening of renal function (chronic renal disease might be present)
- Fast track discharge from ED should be considered in hospitals with chronic disease management programs, once the trigger for decompensation has been identified and early management commenced
- Patients with de novo AHF should not be discharged home from ED
Of note, resting heart rate < 100 bpm should be associated with improvement in symptoms to indicate good response to therapy.
Mortality and readmission rates after discharge from an admission with AHF are high 45. Most of the studies have focused on identification of high risk patients with the assumption that better management of these patients could reduce readmission and mortality rates. Less is known about low risk AHF patients in the ED. A Canadian publication evaluated short and long term outcomes in elderly patients (≥65 years) presenting to the ED with AHF. The authors showed that patients that were not admitted had a higher rate of repeat ED visits but comparable rates of re-hospitalization at 30 days 46. The lack of well-established strategy for evaluation of AHF patients in the ED may lead the ED physicians to admit most patients 47. Approximately 80% of patients with AHF are admitted from the ED 4, although it is thought that up to 50% of patients could be safely discharged from the ED after a brief period of observation 48. This needs to be replicated and hence further explored to avoid unnecessary admissions and minimize readmissions.
Reducing hospitalization rate for AHF patients would probably reduce costs. A recently published risk score differentiated patients at various level of risk of death at 7-day after ED discharge 49. However the authors did not address risk of rehospitalization or longer term follow-up. One way to define low risk AHF patients is to consider the absence of any known high-risk feature (e.g., significantly elevated natriuretic peptide levels, low blood pressure, worsening renal failure, hyponatremia, positive troponin). Patients without these high risk features could be treated in the ED observation unit, where more time is available to evaluate the response to initial therapy. A good response to initial therapy should also be considered before discharge (see recommendations box above). Beyond this, ED physicians must consider comorbidities, psychological and social factors before discharge. Finally, an early follow-up should be organized - ideally contact with a physician or a nurse practitioner should take place within 72 hours of discharge 50. Patients with de novo AHF need further evaluation and should not be discharged from the ED or downgraded too quickly if hospitalized.
Criteria for hospitalization in ward vs. intensive care unit/ coronary care unit
- Patients with significant dyspnoea or hemodynamic instability should be triaged to a location where immediate resuscitative support can be provided if needed.
- Patients admitted to hospital with AHF should be looked after by doctors and nurses with specialist knowledge and expertise
- For high-risk patients, initial care should be provided in a high dependency setting (Coronary Care/Cardiac Care Unit). Patients with AHF and associated acute coronary syndrome should be referred to CCU.
- ○ Clinical risk algorithms developed to predict the in-hospital mortality of patients admitted with AHF can assist in determining which patients in the ED need the highest level of in-patient care
- ○ An ED specific algorithm may further improve risk assessment compared with prior methods developed in patients admitted with AHF
- ○ The criteria for triage at admission for ICU include RR > 25, SaO2 < 90%, use of accessory muscles for breathing, systolic BP < 90 mmHg,
- ○ Need for intubation (or already intubated) or signs of hypoperfusion: (oliguria, cold peripheries, altered mental status, lactate > 2 mmol/L, metabolic acidosis, SvO2 < 65%) are also indications for ICU referral
- For those who are admitted to the ICU/CCU, subsequent care should be on a cardiology ward if possible
- Hospitals should have an AHF pathway so all patients have access to cardiology advice
The level of care patients with AHF are triaged to (discharge, observation, ward, telemetry and intensive care unit [ICU]) has historically relied on clinical judgment. Clinical judgment often over estimates the risk of cardiac complications potentially resulting in over utilization of higher levels of care 51. Recently a decision model from Canada was developed utilizing administrative data for patients with AHF both discharged directly and admitted from the ED 49. Realistically, risk scores with and without biomarkers may assist in early differentiation of those AHF patients who will require admission to the hospital 52. The Acute Decompensated Heart Failure National Registry including more than 65,000 patients designated that high admission BUN (≥43 mg/dl), low systolic blood pressure (<115 mmHg) and high creatinine (≥2.75 mg/dl) can help identify high risk population (in-hospital mortality of 22%) that could potentially be directed to ICU environment.53 Triage to the ICU setting is generally best accomplished based on specific findings that require intensive monitoring and therapy for compromised respiratory or hemodynamic status. These factors include, but not restricted to, SaO2 < 90% despite supplemental oxygen, heart rate < 60 or >120 bpm; systolic blood pressure <90 mm Hg or evidence of right heart failure, particularly with evidence of organ hypoperfusion such as diminished urine output or altered mental status, though these factors are not individually validated in the literature.
Monitoring in the hospital
- Patient should be weighed daily and have an accurate fluid balance chart completed
- Standard noninvasive monitoring of pulse, respiratory rate and blood pressure should be performed
- Renal function and electrolytes should be measured daily
- Pre-discharge measurement of natriuretic peptides is useful for post-discharge planning
Patients should be weighed daily and an accurate fluid balance chart should be maintained. Renal function should preferably be monitored with daily measurement of urea, creatinine and electrolytes. Renal function is commonly impaired at admission, and may improve (or deteriorate) with diuresis. Routine monitoring of pulse, respiratory rate and blood pressure should continue. There is no study showing usefulness of invasive hemodynamic monitoring in patients with AHF excluding those with cardiogenic shock.
There is evidence that measuring natriuretic peptides during the hospital admission can help with discharge planning. Patients whose natriuretic peptide concentrations fall during admission have lower cardiovascular mortality and readmission rates at 6 months 54.
Criteria for discharge from the hospital and follow-up in high-risk period
- Patients admitted with AHF are medically fit for discharge:
- ○ when hemodynamically stable, euvolemic, established on evidence-based oral medication and with stable renal function for at least 24 h before discharge
- ○ once provided with tailored education and advice about self-care
- Patients should be:
- ○ enrolled in a disease management programs
- ○ seen by their general practitioner within 1 week of discharge
- ○ seen by the hospital cardiology team within two weeks of discharge if feasible
- Patients with chronic heart failure should be followed up within a multi-professional heart failure service
Following discharge from intensive care settings, patients should be cared for on Cardiology Wards by Cardiologists and Cardiology trained nurses. Patients might have better outcomes when this is achieved 55, 56. Patients should only be discharged when they have been hemodynamically stable, euvolaemic, have stable renal function and have been established on oral medication for at least 24 hours.
Follow-up plans must be in place prior to discharge and clearly communicated to the primary care team. Patients, ideally, should be seen by their general practitioners (GP) within 1 week of discharge and by the hospital based cardiology team within two weeks. Most recent ACCF/AHA guidelines recommend early follow-up visit within 2 weeks along with early telephone follow-up within 3 days of discharge38. All patients should be followed up by a multi-professional heart failure service. They also ensure continuation and up titration of disease modifying therapy for heart failure with reduced ejection fraction (HFREF), if appropriate.
Compliance is listed at the top of the most important precipitating factors for AHF38. Recognition of compliance problems along with other potential precipitating factors is critical step for optimal management of AHF. On the other hand, every patient following an attack of AHF should have an acceptably detailed evidence-based plan of care that ensures the achievement of optimal medical therapy and compliance with all the necessary measures.
Definition, initial management and monitoring of cardiogenic shock including device therapy
- Cardiogenic shock is defined as hypotension (SBP < 90 mmHg) despite adequate filling status and signs of hypoperfusion: (oliguria, cold peripheries, altered mental status, lactate > 2 mmol/L, metabolic acidosis, SvO2 < 65%)
- A patient with suspected cardiogenic shock (CS) should undergo immediate assessment
- ECG and echocardiography are required immediately in all patients with suspected CS
- Invasive monitoring with arterial line is needed
- There is no agreement on optimal method of hemodynamic monitoring in assessing and treating the patient in CS, including pulmonary artery catheter
- Fluid challenge (saline or ringer lactate, >200 ml/15-30 min) is recommended as the first line treatment if there is no sign of overt fluid overload
- Dobutamine may be used to increase cardiac output; levosimendan may be considered, especially in CHF patients on oral beta-blockade
- Vasopressors should only be used if there is a strict need to maintain systolic BP in the presence of persistent hypoperfusion; if needed, norepinephrine is recommended over dopamine
- All CS should be rapidly transferred to a tertiary care center which has a 24/7 service of cardiac catheterization, and a dedicated ICU with availability of short-term mechanical circulatory support
- IABP is not routinely recommended in CS
- Short-term mechanical circulatory support may be considered in refractory CS depending on patient age, comorbidities and neurological function
- Based on current evidence, we do not recommend one mode of short-term circulatory support over another
Cardiogenic shock (CS) is defined as state of low systolic blood pressure (<90 mmHg) > 30 minutes in spite of adequate volume status (fluid challenge) with signs of hypoperfusion (oliguria <0.5 ml/kg/h for at least 6 hours, altered mentation, cool extremities with livedo reticularis). Accumulation of blood lactate (>2-4 mmol/l) is additional evidence of organ hypoperfusion. Cardiogenic shock ranges from low-output advanced, end-stage chronic HF to acute-onset de-novo CS most often caused by STEMI but also by other mechanical causes such as acute valve problems. Pharmacologic therapy aims to improve organ perfusion by increasing cardiac output and blood pressure. After fluid challenge, pharmacologic management of CS consists of inotropic agent and vasopressor if needed 57. Treatment is guided by the continuous monitoring of organ perfusion and hemodynamics. Norepinephrine is the recommended vasopressor (over dopamine) when mean arterial pressure needs pharmacologic support 58. In case of low cardiac output, dobutamine might be used in patients with no beta-blockers. Levosimendan may be used as an alternative and is especially recommendable to patients on beta-blockers on admission, though it might not be available in all countries 1, 59. In this case high doses of dobutamine might be required, though, its use in patients under chronic carvedilol therapy remains controversial60, 61. However, rather than combining several inotropes, device therapy has to be considered when there is inadequate response. Currently, intra-aortic balloon pump (IABP) is the most widely used left ventricular assist device (LVAD) for hemodynamic support 62. Recently, the IABP-SHOCK II trial showed that use of an IABP did not improve outcomes in patients suffering from AMI and CS 63. Therefore, the routine use of IABP cannot be recommended based on the current evidence.
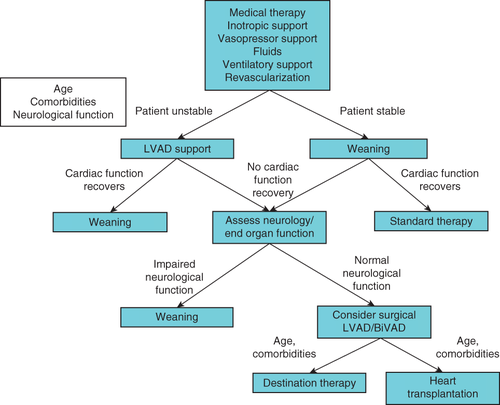
Percutaneous LVAD have been used in patients not responding to standard treatment including catecholamines, fluids and IABP and also as first-line treatment (Figure 4). However, the current experience and evidence is limited. A meta-analysis reported the results of 3 randomized trials comparing percutaneous LVADs versus IABP treatment (TandemHeart™, Impella® 2.5 with IABP). Patients treated with active LVADs demonstrated improved hemodynamics compared with IABP patients, though, outcomes were not different 64. Based on these results percutaneous LVADs cannot be recommended as first-line treatment in CS. Short-term mechanical support, including extracorporeal membrane oxygenation, can be used in CS patients who are failing maximal medical therapy.65
Gaps in knowledge and perspectives
In AHF, there are several areas which require further investigation. The use of biomarkers in risk stratification and to guide treatment, which are the most important signs of severity, and which are the best measures of efficacy need more extensive study. There is still a need to better delineate exactly what constitutes clinical improvement with acute therapy, types of rehospitalizations, and mortality (both short-term and long-term). There is also an appealing concept of “home visit” by “HF-teams” to avoid or to decrease ED visits and hospitalizations.
Recent phase III or IV investigations have offered future promise in clinical management of Acute Heart Failure. These include RELAX-AHF66 (serelaxin), ATOMIC-AHF trial 67(omecamtiv mecarbil), PRONTO 68(clevidipine), TRUE-AHF69 (Ularitide) (clinicaltrials.gov/ct2/show/NCT01661634?term=TRUE-AHF&rank=1), (clinicaltrials.gov/ct2/show/NCT01733134) ARTS-HF70 trials.
Conflict of interest: A.M. received speaker's honoraria from Alere, Bayer, Edwards Life Sciences, The Medicines Company, Novartis, Orion, Servier, Thermofisher, Vifor Pharma and also received fee as member of advisory board and/or Steering Committee from Bayer, Cardiorentis, The Medicine Company, Critical Diagnostics.
M.B.Y. received speaker's honoraria and research fee from Novartis and received fee as Steering Committee member of Cardiorentis, and is supported by TUBITAK.
P.L. received speaker's honoraria from Beckman Coulter and Novartis and also received fees as a member of advisory board and/or Steering Committee from Bayer, Cardiorentis, The Medicines Company, Cornerstone Therapeutics, Novartis, Otsuka, Janssen, Apex Innovations, Inte-Section Medical, and Trevena.
P.P. received speaker's honoraria from Bayer, Novartis, Servier, Vifor Pharma, Amgen, Pfizer, Cardiorentis, Merck-Serono, Abbott Vascular and Respicardia and also received fee as member of advisory board and/or Steering Committee from Bayer, Cardiorentis, Novartis, Vifor Pharma Ltd, Amgen, Servier, Abbott Vascular, Coridea and Respicardia.
W.F.P. received research grants from Abbott, Alere, Banyan, Cardiorentis, Portola, Roche, The Medicine's Company, served as a consultant for Alere,BG Medicine, Beckman, Boehringer-Ingelheim, Ardiorentis, Instrument Labs, Janssen, Prevencio, The Medicine'sCompany, ZS Pharma, and has ownership interests in Comprehensive Research Associates,
LLC, and Emergencies in Medicine, LLC.
S.L. received speaker's honoraria from Roche, and received fee as member of advisory board from Novartis.
A.R. received speaker's honoraria from Servier, Astra Zeneca, Boehringer-Ingelheim, Abbott, Teva, Richer Gedeon, and Merck-Serono and fee as a member of advisory board from Boehringer-Ingelheim and Merck-Serono.
E.L. received consultancy fee from Novartis.
J.M. received honoraria for speaker or advisor from Abbott, Novartis, Orion, Otsuka, and Sanofi and fee as a member of Steering Committee from Corthera, Novartis, and Cardiorentis.
J.R. received honoraria as a member of advisory board: Flora proactive and Novartis.
T.M. received honoraria from Novartis and Servier.
C.M. received research grants from the Swiss National Science Foundation and the Swiss Heart Foundation, the Cardiovascular Research Foundation Basel, 8sense, Abbott, ALERE, Brahms, Critical Diagnostics, Nanosphere, Roche, Siemens, and the University Hospital Basel, as well as speaker/consulting honoraria from Astra Zeneca, Abbott, ALERE, BG medicine, Biomerieux, Brahms, Cardiorentis, Lilly, Novartis, Pfizer, Roche, and Siemens.
C.D. received speakers honoraria from Roche Diagnostics, Critical Diagnostics and Radiometer, consulting honorarium/advisory board from Siemens Healthcare Diagnostics and Roche Diagnostics and research support from Roche Diagnostics and Alere.
V.P.H. received speaker's fee: Bayer, Orion, Resmed, Roche Diagnostics, Ratiopharm; consultation fees: Bayer, BMS, Boehringer-Ingelheim, Novartis, Pfizer, Roche Diagnostics, Servier.
H.A.T. received speaker's honoraria from Daiichi Sankyo, Lilly, Medicines Company, AstraZeneca, Boehinger Ingelheim. Advisory board for Maquet Cardiovascular. Institutional research support by Maquet Cardiovascular, Teleflex, Terumo, Lilly, The Medicine Company.
M.M. received honoraria as Committee member or co-chairman from Bayer, Novartis, Servier.
F.R. received consultant agreement with Cardiorentis, speaker honoraria Servier.
A.L.M. received speaker's honoraria from Astra Zeneca.
S.D.A. consultant for Bosch GmbH, Impulse Dynamics, BioVentrix, CardioMems, Thermo Fischer GmbH, Vifor International (clinical events committee), Servier (Steering Committee), Jansen (Steering Committee), Medical Sensible, Novartis (Steering Committee), Cardiorentis (Steering Committee), BG Medicine (Steering Committee), Psioxus (Steering Committee), Bayer (Steering Committee); speaker for Servier and Vifor International.
G.F. has received research grants and/or has served as Committee member or Cochair of studies sponsored by Bayer, Novartis, Cardiorentis, Vifor Pharma, and the European Union.




