Diagnostic pathways to wild-type transthyretin amyloid cardiomyopathy: a multicentre network study
Abstract
Aim
Epidemiology of wild-type transthyretin cardiac amyloidosis (ATTRwt-CA) remains poorly defined. A better characterization of pathways leading to ATTRwt-CA diagnosis is of key importance, and potentially informative of disease course and prognosis. The aim of this study was to describe the characteristics of contemporary pathways leading to ATTRwt-CA diagnosis, and their potential association with survival.
Methods and results
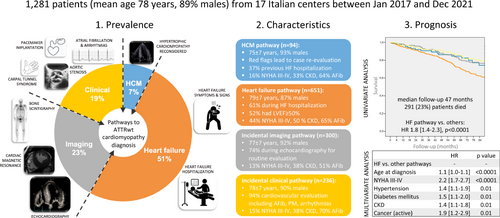
This was a retrospective study of patients diagnosed with ATTRwt-CA at 17 Italian referral centres for CA. Patients were categorized into different ‘pathways’ according to the medical reason that triggered the diagnosis of ATTRwt-CA (hypertrophic cardiomyopathy [HCM] pathway, heart failure [HF] pathway, incidental imaging or incidental clinical pathway). Prognosis was investigated with all-cause mortality as endpoint. Overall, 1281 ATTRwt-CA patients were included in the study. The diagnostic pathway leading to ATTRwt-CA diagnosis was HCM in 7% of patients, HF in 51%, incidental imaging in 23%, incidental clinical in 19%. Patients in the HF pathway, as compared to the others, were older and had a greater prevalence of New York Heart Association (NYHA) class III–IV and chronic kidney disease. Survival was significantly worse in the HF versus other pathways, but similar among the three others. In multivariate model, older age at diagnosis, NYHA class III–IV and some comorbidities but not the HF pathway were independently associated with worse survival.
Conclusions
Half of contemporary ATTRwt-CA diagnoses occur in a HF setting. These patients had worse clinical profile and outcome than those diagnosed either due to suspected HCM or incidentally, although prognosis remained primarily related to age, NYHA functional class and comorbidities rather than the diagnostic pathway itself.
Graphical Abstract
Introduction
Cardiac amyloidosis (CA) due to transthyretin (TTR) deposition within the cardiac tissue is increasingly recognized in its wild-type form (ATTRwt-CA) due to the possibility of performing, in the majority of cases, a non-invasive diagnosis combining scintigraphy with bone tracers, blood tests for ruling out a monoclonal gammopathy and genetic analysis.1-3 Although specific clinical conditions have been recognized where CA is prevalent, the epidemiology of ATTRwt-CA remains poorly defined.4, 5 As novel therapies for CA treatment are becoming available, a better characterization of pathways leading to ATTRwt-CA diagnoses is of key importance. Indeed, medications that slow TTR amyloid deposition have shown beneficial effects on survival in patients with mild heart failure (HF) symptoms and after a relatively long period of treatment.6, 7 Identification of scenarios in which ATTRwt-CA is diagnosed earlier in the disease course and with a low associated comorbidity burden may thus help optimize cost-effectiveness of specific treatments.4, 8 However, screening for TTR-related CA in selected clinical settings, such as HF with a preserved left ventricular ejection fraction (LVEF) and aortic stenosis undergoing intervention, has led to diagnoses in patients usually >80 years old and with several comorbidities, possibly missing subjects who might benefit most from earlier interventions.4, 5
We designed the present retrospective observational nationwide study with the aim of describing characteristics of contemporary pathways leading to ATTRwt-CA diagnosis, and their potential association with survival.
Methods
The DIagnostic pathways to transthyretin AMyloid cardiomyOpathy: a multicentre Network stuDy (DIAMOND) was a retrospective study of patients with a final diagnosis of ATTRwt-CA among 17 Italian referral centres for CA. Inclusion criteria were: (i) a definite diagnosis of ATTRwt-CA according to the Gillmore algorithm,1 (ii) first evaluation between January 2016 and December 2021, (iii) age at first evaluation ≥18 years, and (iv) written informed consent for the collection and treatment of anonymized clinical data for research purpose. Patients were enrolled regardless of their vital status at the time of data collection.
Patients fulfilling inclusion criteria were categorized into different ‘pathways’ according to the medical reason that triggered the diagnosis of ATTRwt-CA, based on previous literature4, 5, 9-11 and authors' experience. Pathways were mutually exclusive one with another and hierarchical. Exclusive means that patients could be categorized only in one pathway. Hierarchical means that the investigator collecting the data had the possibility to move from the first pathway to the next only if the condition of the first pathway was not fulfilled. The diagnostic pathway flowchart is described in online supplementary Figure Appendix S1. The first pathway to be confirmed or excluded was that of a previous diagnosis of hypertrophic cardiomyopathy (HCM) (HCM pathway). Patients were included in this pathway if they had a previous definite diagnosis of HCM and only after were re-evaluated and diagnosed with ATTRwt-CA. For this pathway, we collected information regarding ‘red flags’ triggering case re-evaluation and ATTRwt-CA diagnosis, and history of previous HF hospitalizations (HFH). If the HCM pathway was excluded, the following option was that of a medical evaluation due to HF (HF pathway). We collected information regarding the type of HF evaluation (HFH vs. outpatient); history of previous HF evaluations; LVEF at time of index HF evaluation. If the HF pathway was excluded, the following option was that of an ATTRwt-CA diagnosis triggered by incidental imaging testing findings (incidental imaging pathway). Only patients undergoing imaging testing without known or manifested cardiac disease or symptoms could be included in this pathway. For this pathway, we collected information regarding type of imaging testing (echocardiography, cardiac magnetic resonance [CMR], bone scintigraphy) and reason for testing. If the incidental imaging pathway was excluded, the last option was that of ATTRwt-CA diagnosis reached after a suspicion was raised during a medical evaluation due to causes other than HCM or HF (incidental clinical pathway). Only patients with new-onset medical conditions in whom, during clinical evaluation, specific findings triggered the diagnosis of ATTRwt-CA were included in this pathway. For this pathway, we collected information regarding reason for the medical evaluation.
Two independent investigators (G.T. and M.C.) reviewed pathway information and confronted with each centre in case of uncertainty regarding inclusion in a specific pathway.
For all pathway, we also collected information regarding the date of the index evaluation and the date of definite ATTRwt-CA diagnosis (which was established as the date of bone scintigraphy and/or biopsy); age at diagnosis; sex; clinical characteristics at the time of ATTRwt-CA diagnosis, including: arterial hypertension, diabetes mellitus, coronary artery disease (CAD), chronic kidney disease (CKD), chronic obstructive pulmonary disease (COPD), history of cancer (previous or active), known atrial fibrillation (AF), pacemaker implantation, implantable cardioverter defibrillator (ICD) implantation, New York Heart Association (NYHA) functional class at diagnosis; status at time of data collection (dead vs. alive; in the first case, date of death was collected). Prognosis was investigated with all-cause mortality as endpoint. Time to diagnosis was defined as the time from the date of the index evaluation (i.e. date of pathway inclusion: date of HCM diagnosis; date of index HF evaluation; date of imaging testing; date of clinical evaluation) and the date of ATTRwt-CA definite diagnosis.
Data were collected and managed using the Research Electronic Data Capture (REDCap) tool hosted at the Department of Internal Medicine of the University of Genoa, Italy.12 The Institutional Review Board of the Genova institution approved the study protocol and the collection of retrospective anonymized data on 24 November 2020. Data collection started on 11 October 2021 and completed on 10 January 2022. The database was locked in March 2022, with 1336 ATTRwt-CA patients initially included. After revision, 55 duplicate patients were excluded, leaving a final sample of 1281 patients, with 719 patients diagnosed at the three largest centres of Pavia (n = 347, 27%), Firenze (n = 221, 17%) and Bologna (n = 151, 12%), and the remaining at centres contributing with 1–6% of the study population each.
Continuous variables were reported as mean ± standard deviation or as median with interquartile range (IQR). The distribution of continuous variables across all pathways was compared with the ANOVA test, and differences between each pair of pathways with the Tukey–Kramer test. The standard chi-square test was used to compare proportions. A p-value ≤0.05 was considered statistically significant. Survival analyses were conducted with the Kaplan–Meier method, and univariate and multivariate Cox regression analysis. Multivariate analysis included all variables with a significant univariate association. A backward stepwise elimination method was used for variable selection in multivariate analysis, and only variables with p < 0.10 were included in the final model. Statistical analysis was performed using SAS 9.4 (SAS Corporation, Cary, NC, USA).
Results
The study population was of 1281 ATTRwt-CA individuals. Three-quarters were diagnosed after the year 2017, with a peak of diagnoses in the years 2018–2019 (2016–2017: n = 322, 25%; 2018–2019: n = 526, 41%; 2020–2021: n = 433, 34%). Overall characteristics of included patients are displayed in Table 1. Mean age at ATTRwt-CA diagnosis was 78 ± 7 years, and it significantly increased from the first to the last 2-year period (2016–2017: 76 ± 7 years; 2018–2019: 78 ± 7 years; 2020–2021: 79 ± 7 years, p < 0.0001). Nine out of ten patients were male, although the percentage of females displayed an increase with time (8% vs. 10% vs. 15%, p < 0.0001). At the time of diagnosis, 71% of patients were in NYHA functional class I–II (NYHA class I: 15%, NYHA class II: 56%, NYHA class III: 27%, NYHA class IV: 2%), 62% had AF, 15% were already implanted with a pacemaker and 6% with an ICD. Overall median time to diagnosis was 4 [1–9] months.
| Overall study cohort | HCM pathway (a) | HF pathway (b) | Incidental imaging pathway (c) | Incidental clinical pathway (d) | p-value | Significant pathway vs. pathway comparisons | |
|---|---|---|---|---|---|---|---|
| N (%) | 1281 | 94 (7) | 651 (51) | 300 (23) | 236 (19) | ||
| Sex | 0.10 | b vs. c* | |||||
| Male | 1143 (89) | 87 (93) | 568 (87) | 276 (92) | 212 (90) | ||
| Female | 138 (11) | 7 (7) | 83 (13) | 24 (8) | 24 (10) | ||
| Age (years) | 78 ± 7 | 75 ± 7 | 79 ± 7 | 77 ± 7 | 78 ± 7 | <0.0001 | a vs. b***, a vs. d*, b vs. c* |
| NYHA class | <0.0001 | a vs. b***, b vs. c***, b vs. d*** | |||||
| I/II | 907 (71) | 79 (84) | 366 (56) | 262 (87) | 200 (85) | ||
| III/IV | 374 (29) | 15 (16) | 285 (44) | 38 (13) | 36 (15) | ||
| Arterial hypertension | 947 (74) | 65 (69) | 476 (73) | 227 (76) | 179 (76) | 0.52 | - |
| Diabetes mellitus | 231 (18) | 15 (16) | 138 (21) | 42 (14) | 36 (15) | 0.03 | b vs. c* |
| CAD | 257 (20) | 15 (16) | 128 (20) | 58 (19) | 56 (24) | 0.37 | - |
| CKD | 564 (44) | 31 (33) | 328 (50) | 115 (38) | 90 (38) | <0.0001 | a vs. b***, b vs. c**, b vs. d** |
| COPD | 169 (13) | 14 (15) | 105 (16) | 30 (10) | 20 (9) | 0.01 | b vs. c*, b vs. d* |
| Cancer | <0.0001 | a vs. c**, b vs. c***, c vs. d*** | |||||
| Previous | 183 (14) | 11 (12) | 87 (13) | 58 (19) | 27 (11) | ||
| Active | 66 (5) | 3 (3) | 21 (3) | 36 (12) | 6 (3) | ||
| Atrial fibrillation | 799 (62) | 60 (64) | 423 (65) | 152 (51) | 164 (70) | <0.0001 | a vs. c*, b vs. c***, c vs. d*** |
| Pacemaker | 197 (15) | 14 (15) | 102 (16) | 33 (11) | 48 (20) | <0.0001 | c vs. d** |
| ICD | 72 (6) | 14 (15) | 31 (5) | 11 (4) | 16 (7) | <0.0001 | a vs. b***, a vs. c***, a vs. d*, c vs. d* |
| Time to diagnosis (months) | 4 [1–9] | 29 [8–56] | 3 [1–8] | 3 [1–7] | 4 [1–10] | <0.0001 | a vs. all others*** |
- Values are given as n (%), mean ± standard deviation, or median [interquartile range].
- CAD, coronary artery disease; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; HCM, hypertrophic cardiomyopathy; HF, heart failure; ICD, implantable cardioverter defibrillator; NYHA, New York Heart Association.
- * p < 0.05.
- ** p < 0.01.
- *** p < 0.001.
The diagnostic pathway leading to ATTRwt-CA diagnosis was that of a re-evaluation in the context of a previous diagnosis of HCM in 94 (7%) patients, of HF in 651 (51%), of incidental imaging findings in 300 (23%), and of a medical evaluation for a reason other than HCM or HF in 236 (19%) (Figure 1).

Description of pathways
Hypertrophic cardiomyopathy pathway
Mean age at diagnosis in the HCM pathway was 75 ± 7 years. The main reasons reported as a ‘red flag’ suggesting a diagnosis different from HCM and leading to case re-evaluation were: symmetric left ventricular hypertrophy and/or right ventricular hypertrophy (n = 72), history of carpal tunnel surgery (n = 34), unexpected CMR or electrocardiogram (ECG) findings (n = 32). Furthermore, 35 (37%) patients had experienced a HFH prior to ATTRwt-CA diagnosis.
Heart failure pathway
Mean age at diagnosis in the HF pathway was 79 ± 7 years. The HF event leading to ATTRwt-CA diagnosis was a HFH in 399 (61%) patients and an outpatient evaluation in 252 (39%) (Figure 1). In 276 (42%) cases, this was the last of >1 evaluations due to HF in the patient's medical history (in 160 hospitalized patients and in 116 outpatients). A total of 341 (52%) patients had HF with preserved (≥50%), 191 (29%) with mildly reduced (41–49%) and 119 (18%) with reduced (≤40%) LVEF. NYHA functional class III–IV was reported in 285 (44%) patients. Significant differences between HFH and outpatient individuals were noticed for NYHA class III–IV (47% vs. 39%, p = 0.03), diabetes mellitus (24% vs. 16%, p = 0.01), CAD (23% vs. 15%, p = 0.02), AF (68% vs. 60%, p = 0.03) and reduced LVEF (24% vs. 10%, p < 0.0001).
Incidental imaging pathway
Mean age at diagnosis in the incidental imaging pathway was 77 ± 7 years. The imaging testing was echocardiography in 221 (74%), CMR in 23 (7%), and bone scintigraphy in 56 (19%) of cases (Figure 1). Reasons for imaging testing leading to ATTRwt-CA diagnosis are reported in online supplementary Table Appendix S1.
Incidental clinical pathway
Mean age at diagnosis in the incidental clinical pathway was 78 ± 7 years. In 221 (94%) cases, the medical evaluation leading to ATTRwt-CA diagnosis was due to cardiovascular reasons, including new-onset AF (n = 50), pacemaker implantation (n = 32), chest pain (excluding coronary syndromes, n = 29), severe aortic stenosis (n = 27) and ischaemic stroke (n = 17). Amongst 15 (16%) evaluations due to non-cardiovascular reasons, the majority (n = 9) were advised after bilateral carpal tunnel syndrome surgery (Figure 1 and online supplementary Table Appendix S1). There were few significant differences between the diverse subgroups included in the incidental clinical pathway (online supplementary Table S2). Within the pathway, patients from diverse subgroups showed similar age at diagnosis, sex and NYHA functional class III–IV distribution, prevalence of arterial hypertension, diabetes mellitus, CKD, COPD and active cancer. CAD was more common in those receiving a diagnosis during severe aortic stenosis assessment and in those evaluated incidentally for other cardiovascular reasons (including acute coronary syndrome). Time to ATTRwt-CA diagnosis was longer in the carpal tunnel syndrome subgroup.
Differences between pathways
Differences between diagnostic pathways are shown in Table 1. Distribution of diagnostic pathways did not significantly change over time, except for fewer diagnoses in the HF pathway in the most recent years (2016–2017: 54%; 2018–2019: 53%; 2020–2021: 45%, p = 0.02; online supplementary Figure S2). In all pathways as in the overall population, age at ATTRwt-CA diagnosis increased over time (online supplementary Figure S3). Patients were the oldest in the HF pathway, and the youngest in the HCM pathway. There was a male predominance in all pathways; females were significantly more prevalent in the HF versus incidental imaging pathway. NYHA functional class III–IV, CKD and COPD were significantly more common in the HF versus all other pathways (except for COPD which had a similar prevalence in the HCM one). Diabetes mellitus was more common only in the HF versus the incidental imaging pathway. Known AF at diagnosis had a significantly lower prevalence in the incidental imaging versus all other pathways, while a history of cancer was significantly more frequent in the former. ICD implantation was significantly more frequent in the HCM versus all other pathways. Arterial hypertension, CAD and pacemaker implantation had a similar prevalence across pathways (except for pacemaker implantation at diagnosis which was less common in the incidental imaging vs. incidental clinical pathway). Time to diagnosis (i.e. from the date of pathway inclusion to the date of definitive diagnosis) was the longest in the HCM pathway, but similar in the other three pathways, although a wide distribution was noticed within each pathway (online supplementary Figure S4).
Influence of diagnostic pathways on mortality
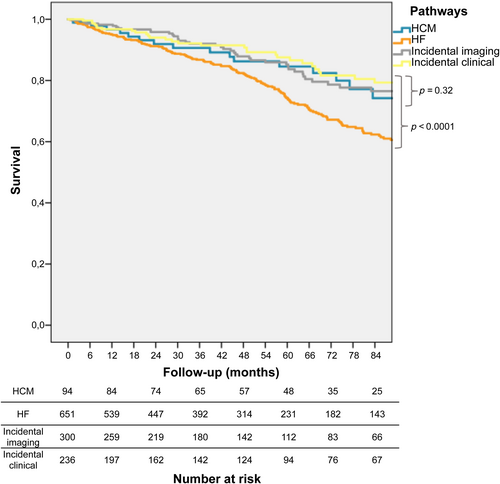
During a median follow-up of 47 (20–81) months, 291 (23%) patients died: 21 (22%) in the HCM pathway, 183 (28%) in the HF, 53 (18%) in the incidental imaging, and 34 (14%) in the incidental clinical (p < 0.0001). At Kaplan–Meier analysis, survival was significantly worse in the HF versus other pathways (p log < 0.0001), but was similar among the three others (p log = 0.32) (Figure 2). The HF pathway was significantly associated with increased mortality at univariate Cox regression analysis (hazard ratio 1.8 [1.4–2.3], p < 0.0001), together with age at diagnosis, NYHA functional class III–IV, arterial hypertension, diabetes mellitus, CAD, CKD, COPD and active cancer (Graphical Abstract). Age at diagnosis, NYHA functional class III–IV, arterial hypertension, diabetes mellitus, CKD and active cancer remained independently associated with increased mortality at multivariate analysis, whereas the HF pathway did not (Table 2). Age at diagnosis was significantly associated with increased mortality in all pathways (online supplementary Table S3), with no significant interaction between age at diagnosis and pathway (p = 0.29). Within the larger HF pathway, HFH (vs. outpatient evaluation) and reduced LVEF were also significantly associated with increased mortality together with age at diagnosis and NYHA functional class III–IV (online supplementary Table S3). A Kaplan–Meier analysis exploring survival differences between the diverse subgroups of patients included in the incidental clinical pathway showed similar mortality (p log = 0.72, online supplementary Figure S5).
| Univariate | Multivariate | |||
|---|---|---|---|---|
| Variable | HR (95% CI) | p-value | HR (95% CI) | p-value |
| HF vs. other pathways | 1.8 (1.4–2.3) | <0.0001 | – | – |
| Age at diagnosis | 1.1 (1.1–1.2) | <0.0001 | 1.1 (1.0–1.1) | <0.0001 |
| NYHA functional class III–IV | 2.8 (2.2–3.5) | <0.0001 | 2.2 (1.7–2.7) | <0.0001 |
| Arterial hypertension | 1.5 (1.1–2.0) | 0.01 | 1.4 (1.1–1.9) | 0.01 |
| Diabetes mellitus | 1.5 (1.1–2.0) | 0.003 | 1.5 (1.1–2.0) | 0.01 |
| CAD | 1.3 (1.0–1.7) | 0.05 | – | – |
| CKD | 1.7 (1.4–2.2) | <0.0001 | 1.4 (1.1–1.8) | 0.01 |
| COPD | 1.4 (1.1–1.9) | 0.02 | – | – |
| Cancer (active) | 1.8 (1.2–2.8) | 0.01 | 1.9 (1.2–2.9) | 0.01 |
| Atrial fibrillation | 1.1 (0.9–1.4) | 0.49 | – | – |
| Pacemaker carrier | 0.9 (0.7–1.3) | 0.62 | – | – |
| ICD carrier | 1.2 (0.8–1.8) | 0.48 | – | – |
| Diagnostic delay (months) | 1.0 (0.9–1.0) | 0.86 | – | – |
- CAD, coronary artery disease; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; HF, heart failure; HR, hazard ratio; ICD, implantable cardioverter defibrillator; NYHA, New York Heart Association.
Discussion
The DIAMOND study described epidemiology of ATTRwt and diagnostic pathways leading to its diagnosis in a large, real-world, nationwide, contemporary cohort. In our population, we found a mean age at diagnosis of 78 years and a high prevalence of NYHA functional class II patients and of AF. We also reported an increase in the number of ATTRwt-CA diagnoses over time, and a concurrent increase in the average age at diagnosis (from 76 years in 2016 to 79 years in 2021).
This is the first multicentre study specifically aimed at describing diagnostic pathways to ATTRwt-CA. It has been acknowledged that CA, and ATTRwt-CA in particular, may be concealed in specific ‘at-risk’ populations, among which are patients with HF with a preserved LVEF, aortic stenosis undergoing interventions and left ventricular hypertrophy (either unexplained or initially attributed to HCM). Yield of screening for TTR-related CA in these conditions ranged from 11% to 15%.4, 5 However, how patients reach an ATTRwt-CA diagnosis outside these scenarios is poorly investigated. In a previous cohort of 108 ATTRwt-CA patients from two referral centres (Bologna and Madrid), the clinical profile leading to diagnosis was HF in 68%, unexplained left ventricular hypertrophy in 14%, an incidental diagnosis in 11% and atrioventricular conduction disorder in 7% of cases.9 In a more recent single-centre French cohort including 122 ATTRwt-CA patients, symptoms of HF were identified as the first manifestation of the disease in 50% of cases, with most of the remaining patients being diagnosed in the absence of symptoms during routine follow-up for a known chronic condition such as arterial hypertension.13 Similarly, in our cohort, half of ATTRwt-CA diagnoses occurred in the HF pathway, about 40% in the incidental pathway (either imaging or clinical) and less than 10% in patients previously labelled as having HCM. We found that ATTRwt-CA patients in the HF pathway were older, with worse NYHA functional class and higher prevalence of CKD as compared to the other pathways. In about 60% of these patients the suspect of the disease was raised only at the time of an HFH. Moreover, half of patients in the HF pathway had a preserved LVEF. These two pieces of information are of great relevance when considering that this is a population increasingly admitted and managed in non-cardiological wards.14, 15 Besides HF, most of ATTRwt-CA diagnoses were done in the incidental pathways, in which prevailed patients undergoing echocardiography for routine examinations or bone scintigraphy not for CA diagnosis or patients with new-onset arrhythmic disorders (AF or pacemaker implantation). These findings strengthen those from previous smaller series.9, 13 The fact that the two large incidental pathways included patients in which ATTRwt-CA diagnoses were reached ‘by chance’, with a vast heterogeneity of presentation outside the more defined clinical setting of HF, is representative of the increasing awareness toward the disease in recent years. This ‘sensibilization’ has also been driven by the surfacing of novel treatments, which have transformed ATTRwt-CA from an incurable to a potentially treatable disease.2, 16 Our temporal trend analysis confirmed an exponential rise in the number of ATTRwt-CA diagnoses in most recent years,16-21 which were increasingly performed in the incidental pathways (from 38% in the years 2016–2017 to 47% in the years 2020–2021; online supplementary Figure S2). A recent large Italian screening initiative involving 5315 patients undergoing echocardiography for reasons other than CA found 381 (7%) examinations suggestive for CA, with 62 patients finally diagnosed with CA (1.2%), including 51 TTR-related CA and 11 light chain-CA. Notably, TTR-related CA patients were on average 79 years of age at diagnosis, with 35% having a history of HF and a mean LVEF of 55%.22 This approach would reasonably further enhance the opportunity to diagnose CA earlier than commonly performed with current symptom-driven approach.
Of note, this growing awareness toward ATTRwt-CA does not seem to directly translate into timely diagnoses. In our study, time to diagnosis was relatively short, with a median of 4 months, as it represented time from the index event raising the suspicion of ATTRwt-CA to the definite diagnosis. Previous studies instead investigated time from first appearance of symptoms or events potentially related to the disease (i.e. diagnostic delay) and reported longer periods, ranging from months to years.8, 13, 19, 20, 23, 24 Our data indicate that a previous event, similarly bearing the potentiality of suggesting ATTRwt-CA diagnosis, was missed in many cases from our cohort as well. For example, the number of patients receiving an ATTRwt-CA diagnosis after new-onset AF was relatively low (about one out of five cases in the incidental clinical pathway), yet AF was extremely common in the entire cohort. Thus, it cannot be excluded that in some cases, across all pathways, development of AF was overlooked and not investigated. Similarly, 42% of patients in the HF pathway already had at least one previous HF event in their clinical course, during which ATTRwt-CA was not suspected. Moreover, NYHA class III–IV was found in 13–15% of patients in the incidental pathways, meaning that in these cases a ‘concealed’ HF status was missed. The HCM pathway is the exemplification of this concept, as after the HCM diagnosis was established, it took a median of 29 months to be reconsidered. A recent study showed a 17% CA prevalence among patients ≥60 years of age with an initial diagnosis of HCM, which in most cases was TTR-related.10 Importantly, HCM is increasingly diagnosed at older ages,25 thus cardiologists should maintain a high level of suspicion for phenocopies in order to prevent delay in the diagnosis and treatment of specific diseases such as ATTRwt-CA.
A relevant result of our study is that differences between pathways were driven by the HF one. Indeed, the profile of pathways other than HF was relatively similar. Accordingly, these had comparable survival. One of the scopes of describing pathways leading to ATTRwt-CA diagnosis was to characterize patients and to potentially identify the fitter candidates for disease-modifying therapies. However, rather than the pathways themselves, in our study, older age at diagnosis, advanced NYHA functional class and comorbidities such as arterial hypertension and CKD appeared associated with increased mortality. These clinical variables are indeed more represented in the HF pathway, thus indicating that prognosis is influenced not by the pathway itself, but by the clinical characteristics of the patients included in it. Where and how to look for ATTRwt-CA patients with the greater probability to be prescribed and respond to disease-modifying treatments remains to be defined.4, 5 Our results may somehow help in a reverse manner, as the majority of diagnoses occurred in the HF pathway, where patients were the oldest and had the most comorbidities, representing a subgroup with an already reduced survival probability and hence likely to benefit less from disease-specific treatments.6 A more refined characterization of features that define frailty versus fitness of ATTRwt-CA patients, beside the inevitable toll of age, is strongly needed.26-28 Such an approach would be of value both in evaluating patients diagnosed in pathways other than the HF one, but also within this latter, being it the most represented, here and in previous literature.9, 13 Building on epidemiological data to improve characterization of ATTRwt-CA patients would be of particular importance not only to optimize patients' management, but also to identify the best timing and modality of treatments, which will necessarily increasingly take into account cost-effectiveness issues.29 These issues appear even more important if considering the trend of increasing age at diagnosis found in our cohort.
The present study has strengths and limitations that should be acknowledged. The large sample collected from multiple centres offered a unique opportunity to investigate diagnostic pathways to ATTRwt-CA diagnosis in an unbiased manner. As per study protocol, investigators were requested to include only patients with a definite ATTRwt-CA diagnosis according to the Gillmore algorithm.1 This is why we specifically chose to analyse only ATTRwt-CA patients diagnosed after January 2016. Nonetheless, there was no centralized validation of diagnoses. Detailed features at the time of diagnosis (including therapies at and after diagnosis, ECG, echocardiographic parameters and cardiac biomarkers) and prospective events other than death were not entered into the database to streamline data collection according to the original aims of the project. Our results, and in particular the associations found for prognosis, are thus limited by the absence of the aforementioned variables. A hierarchical approach was used for pathway identification to improve data consistency and to simplify the assignment of cases potentially fitting into different pathways, which nonetheless were centrally reviewed and discussed. This approach was specifically built for this study, thus, its generalizability may be limited and needs to be tested in cohorts from other regions. We were not able to perform detailed sub-pathway analysis and comparisons due to the low number of patients included in each of these sub-groups, especially in the incidental imaging and clinical pathways. Finally, as previously discussed, we dated time to diagnosis from date of first clinical suspicion rather than symptom onset. This means that the true diagnostic delay (i.e. from symptom onset) may be underestimated in our study.
Conclusion
In conclusion, our findings depict contemporary epidemiological and clinical characteristics of ATTRwt-CA patients and the pathways leading to their diagnosis. Our description of diagnostic pathways may serve as a benchmark for future characterization of features and course of the disease, overall and within each pathway. Half of ATTRwt-CA diagnoses occurred in the HF pathway; these patients had the worse clinical profile and outcome. However, despite some differences, none of the three other pathways comprised ‘fit’ patients, and age at diagnosis, NYHA functional class and some comorbidities appeared as the most important factors influencing prognosis overall and in each pathway. Future prospective initiatives aiming at identifying settings favouring early ATTRwt-CA diagnoses are warranted.
Acknowledgement
Open Access Funding provided by Universita degli Studi di Genova within the CRUI-CARE Agreement.
Funding
This work was supported by the unrestricted general research grant #64890405 by Pfizer, without any control on intellectual contents; and by the Italian Ministry of Health, RC-2022-2 773 270 project.
Conflict of interest: No conflicts of interest to declare for any author in relation to the submitted work. Outside the submitted work: P.M. received honoraria and advisory fees from Janssen-Cilag and Pfizer. A.G.C. received advisor fees from Pfizer. A.C. received a research grant from Pfizer. G.S. received fees from Biotronik, Boston Scientific, Astra Zeneca and Novartis. M.M. received fees from Pfizer, Novartis and Vifor Pharma, and an unrestricted general research grant on amyloidosis by Pfizer, without any control on intellectual contents. B.M. received advisor fees from Pfizer and Alnylam. E.B. received advisor fees from Amicus, Sanofi Genzyme, Takeda. G.P. received honoraria from Alexion, Argobio, Janssen, Protego, The Binding Site, Pfizer, Prothena, Sebia, Siemens, and research fundings from Gate bioscience and The Binding Site. M.C. received speaker and advisor fees from Akcea Therapeutics, Alnylam, Astrazeneca, Boehringer Ingelheim, Boston Scientific, Novartis, Pfizer, Sanofi e Sanofi Genzyme, and two investigator-initiated grants from Pfizer.