Length of hospital stay and 30-day readmission following heart failure hospitalization: insights from the EVEREST trial
Abstract
Aims
Previous reports have provided conflicting data regarding the relationship between length of stay (LOS) and subsequent readmission risk among patients hospitalized for heart failure (HF).
Methods and results
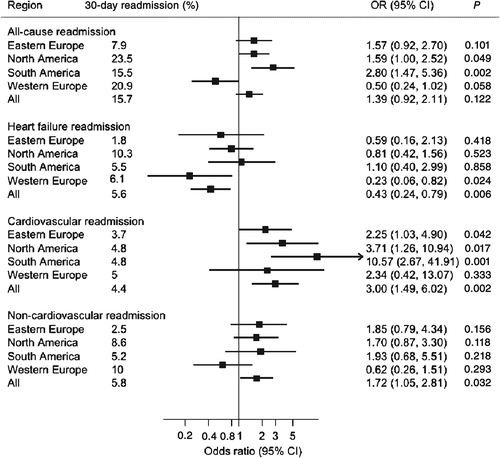
We performed a post-hoc analysis of the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study with Tolvaptan (EVEREST) trial to evaluate the differences in LOS overall and between geographic regions (North America, South America, Western Europe, and Eastern Europe) in association with all-cause and cause-specific [HF, cardiovascular (CV) non-HF, and non-CV] readmissions within 30 days of discharge after HF hospitalization. The present analysis included 4020 patients enrolled from 20 countries who were alive at discharge. Median [interquartile range (IQR)] LOS was 8 (4–11) days. The 30-day readmission rates were 15.7% [95% confidence interval (CI) 14.6–16.8] for all-cause; 5.6% (95% CI 4.9–6.3) for HF; 4.4% (95% CI 3.8–5.1) for CV non-HF; and 5.8% (95% CI 5.1–6.6) for non-CV readmissions. There was a positive correlation between LOS and all-cause readmissions (r = 0.09, 95% CI 0.06–0.12). The adjusted odds ratio for the top (≥14 days) vs. the bottom (≤3 days) quintile for LOS was 1.39 (95% CI 0. 92–2.11) for all-cause readmissions, 0.43 (95% CI 0.24–0.79) for HF, 2.99 (95% CI 1.49–6.02) for CV non-HF, and 1.72 (95% CI 1.05–2.81) for non-CV readmissions. With the exception of Western Europe, these findings remained largely consistent across geographic regions.
Conclusion
In this large multinational cohort of hospitalized HF patients, longer LOS was associated with a higher risk for all-cause, CV non-HF, and non-CV readmissions, but a lower risk of HF readmissions within 30 days of discharge. These results may inform strategies to reduce readmissions.
Introduction
There are more than 25 million people with a diagnosis of heart failure (HF) worldwide and over 1 million annual hospitalizations for HF in the USA alone.1 These patients are at high risk for subsequent hospitalization with a 20–25% readmission rate within 30 days of discharge.2, 3 Hospitalizations are responsible for the majority of costs of care related to HF, leading to the United States Center for Medicare and Medicaid Services (CMS) mandate to report hospital-level 30-day readmission rates among these patients and the corresponding financial penalties for higher readmission rates.4, 5
This focus on HF readmissions has evolved over a background of previous concerns concerning hospital length of stay (LOS). Reduction in LOS was an important goal after the Diagnosis Related Group payment model bundled fixed payments for particular disease states. To minimize financial loss and maximize profit margin in this environment, shortening LOS was imperative, but led to concerns that shortening LOS may increase risk of readmission. This may be particularly applicable to HF as persistent congestion is associated with a higher risk for readmissions,6, 7 and longer LOS may facilitate added inpatient decongestion and optimization of HF therapy. Conversely, longer LOS may expose patients to greater risk of nosocomial infections, venous thromboembolism, sleep deprivation, and deconditioning, which could all contribute to an increased risk of readmission. Longer LOS may also simply be a marker of ‘high-risk’ patients who, by definition, are at heightened risk of readmission. Data from previous studies regarding the association between LOS and readmission risk are mixed.8-11 These conflicting results may be related to suboptimal definitions of risk with administrative databases, inadequate adjustment for confounders, initiation of follow-up periods at ‘baseline’ rather than actual time of discharge, and heterogeneity in standard medical practices, financial incentives, and reasons for readmission across geographic regions.
In this study, we examined the overall and region specific relationship between LOS and overall vs. cause-specific readmission risk in the Efficacy of Vasopressin Antagonism in Heart Failure Outcome Study With Tolvaptan (EVEREST) trial. The EVEREST trial enrolled patients from four distinct regions of the globe, collected detailed baseline and discharge patient level data, and subsequently adjudicated cause-specific hospitalization information, thus providing an opportunity to examine the relationship between LOS and readmission risk in granular detail.
Methods
Data source
The design and primary results of EVEREST have been described previously.12, 13 A total of 4133 participants were randomized from 359 sites in 20 countries across North America, South America, and Europe between October 7 2003 and February 3 2006. This prospective, international, randomized, double-blind, placebo-controlled trial examined the efficacy and safety of tolvaptan, in addition to standard therapy, in patients hospitalized for worsening HF. Eligible patients were ≥18 years of age with left ventricular ejection fraction (EF) ≤40%, and hospitalized for HF with two or more signs/symptoms of fluid overload. Patients were randomized within 48 h of hospitalization to tolvaptan or matching placebo in addition to conventional therapy. Median follow-up was 9.9 months. The Institutional Review Board at each participating centre independently approved the trial protocol and written informed consent was obtained from all participants.
Global regions
Countries that participated in the EVEREST trial were grouped into four global regions: North America (Canada and USA), South America (Argentina and Brazil), Western Europe (Belgium, France, Germany, Italy, the Netherlands, Norway, Spain, Sweden, Switzerland and the UK), and Eastern Europe (Bulgaria, Czech Republic, Lithuania, Poland, Romania, and Russia).12, 14
Length of stay and hospitalizations
Modes of death and causes of readmission were adjudicated by an independent blinded events committee during the entire follow-up period for the trial. Hospitalization was defined as a stay >24 h from admission to discharge. Outcomes of interest for the present analysis were all-cause, HF, cardiovascular (CV) non-HF, and non-CV readmission within 30 days of discharge from the index hospitalization. Hospitalization for HF was defined as admission for worsening signs or symptoms of HF resulting in the augmentation of HF therapies. Cardiovascular non-HF hospitalizations included all CV hospitalizations except HF hospitalization, including those related to arrhythmias, ischaemia, and stroke. Non-CV hospitalizations included all other hospitalization that were not classified as either HF or CV non-HF hospitalization.15
Statistical analysis
There were no significant differences in the risk for mortality or rehospitalization (all-cause, HF, CV non-HF and non-CV) among patients randomized to placebo vs. tolvaptan in the EVEREST trial.12 Accordingly, the present analysis included patients from both study arms. Similar to publicly reported readmission rates for Medicare beneficiaries, we included all patients who died after discharge (including those who died within the first 30 days of discharge) but excluded those who died before discharge. Mean (standard deviation, SD), median (inter-quartile range, IQR), and proportions were used to describe the data where appropriate. The LOS was skewed and was therefore log transformed to achieve an approximately symmetrical distribution and to assess correlation between LOS and readmissions using partial correlation coefficient adjusted for age and gender.
To assess clinically meaningful associations between LOS and 30-day readmissions, untransformed LOS (in days) was divided into quintiles and entered as a categorical variable into logistic regression models. To account for the hierarchical nature of the data in which patients were clustered in sites within regions, we used a two-level mixed effects logistic regression model (i.e. region and patient) to evaluate the relationships between region-level LOS for the index admission and region-level readmission rates within 30 days after discharge. We included a random intercept accounting for the clustering effects of region in the analysis. Multivariable models were constructed and included the following pre-specified patient-level baseline variables: age, gender, treatment group (tolvaptan vs. placebo), race, history of diabetes mellitus or any CV disease, heart rate, respiratory rate, systolic blood pressure, serum albumin, sodium, natriuretic peptide levels, blood urea nitrogen (BUN) and serum creatinine. Additional models were constructed with: (i) the above covariates (baseline values) plus baseline use of medications [anti-diabetes agents, angiotensin-converting enzyme inhibitors (ACEI)/angiotensin II receptor blockers (ARB), beta-blockers, diuretics, digoxin, mineralocorticoid receptor antagonists, and statins); (ii) discharge values for the above covariates (when applicable) and discharge use of above medications. Lastly, because of previously described potential for an inverse relationship between readmission and mortality rates, additional adjusted sensitivity analyses were performed excluding patients who died within 30 days of discharge.16, 17
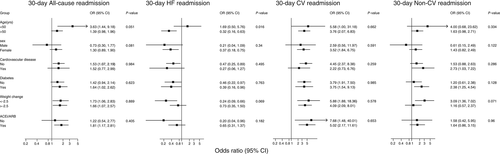
Rates of all-cause, HF, CV non-HF, and non-CV hospitalizations were assessed. To characterize shapes of associations, region-specific odds ratios (OR) were calculated by quintiles of LOS and were pooled on the log scale, by multivariable random-effects meta-analysis, and plotted against pooled mean lengths of stay within each quintile.18 Subgroup analyses were conducted using interaction tests to assess statistical evidence of heterogeneity in OR across select patient characteristics, including age, gender, history of diabetes mellitus, CV disease, and median change in weight between admission and discharge, and baseline ACEI/ARB use. For multivariable models, missing covariate data were imputed using multiple imputation by chained equations as introduced by van Buuren et al.19, 20 Parameter estimates and confidence intervals (CI) were obtained by combining five imputed datasets using the method described by Barnard and Rubin21 to account for possible error in missing value analysis. All statistical analyses were conducted using Stata version 13 (Stata Corp, College Station, TX, USA) with P < 0.05 considered statistically significant.
Results
Patient characteristics
Of the 4133 patients enrolled in EVEREST, 4020 patients were alive at discharge and included in this analysis (tolvaptan 2,022 patients; placebo 1,998 patients). Table 1 shows baseline characteristics of the study population stratified by 30-day readmission status. There were no significant differences in 30-day mortality (2.4% vs. 1.8%, P = 0.137) or any 30-day readmissions (all-cause 15.4% vs. 15.9%, P = 0.704; HF 5.6% vs. 5.5%, P = 0.8; CV non-HF 4.3% vs. 4.4, P = 0.814; and non-CV 5.5% vs. 6.2%, P = 0.367) between tolvaptan and placebo groups. Patients readmitted within 30 days were more likely to have comorbid conditions, lower systolic blood pressure and haemoglobin, and higher levels of natriuretic peptides, BUN, and serum creatinine.
| Variables | Overall (N = 4020) | Admitted within 30 days (N = 629) | Not admitted within 30 days (N = 3391) | P |
|---|---|---|---|---|
| Age (years) | 65.7 (11.8) | 66.5 (12.8) | 65.6 (11.6) | 0.083 |
| Female | 1020 (25.3%) | 149 (23.6%) | 871 (25.6%) | <0.001 |
| Race | <0.001 | |||
| White | 3432 (85.8%) | 500 (79.5%) | 2932 (86.5%) | |
| Black | 303 (7.7%) | 79 (12.6%) | 224 (6.6%) | |
| Other | 284 (6.6%) | 50 (7.9%) | 234 (6.9%) | |
| Region | <0.001 | |||
| Eastern Europe | 1589 (39.6%) | 125 (19.8%) | 1464 (43.1%) | |
| North America | 1217 (30.8%) | 287 (45.6%) | 930 (27.4%) | |
| South America | 672 (16.5%) | 104 (16.5%) | 568 (16.7%) | |
| Western Europe | 542 (13.2%) | 113 (17.9%) | 429 (12.7%) | |
| Smoking | 0.001 | |||
| Never | 1383 (34.1%) | 188 (29.9%) | 1195 (35.2%) | |
| Previous | 499 (12.1%) | 64 (10.1%) | 435 (12.8%) | |
| Current | 2134 (52.8%) | 377 (60.0%) | 1757 (51.8%) | |
| Unknown | 4(0.1%) | 0 | 4(1.2%) | |
| Comorbidities | ||||
| Atrial fibrillation | 1742 (43.3%) | 283 (45.0%) | 1459 (43.0%) | 0.361 |
| COPD | 405 (10.1%) | 83 (13.2%) | 322 (9.5%) | 0.005 |
| Coronary artery disease | 2831 (70.4%) | 445 (70.7%) | 2386 (70.4%) | 0.818 |
| Diabetes mellitus | 1554 (38.7%) | 291 (46.2%) | 1263 (37.2%) | <0.001 |
| Hypertension | 2859 (71.1%) | 441 (70.1%) | 2418 (71.3%) | 0.544 |
| Myocardial infarction | 2021 (50.2%) | 338 (53.7%) | 1683 (49.6%) | 0.055 |
| Baseline Medications | ||||
| ACE inhibitor/ARB | 3395(84.5%) | 485(77.1%) | 2910(85.8%) | <0.001 |
| Beta blocker | 2834(70.5%) | 440(69.9%) | 2394(70.6%) | 0.678 |
| MRA | 2219(55.2%) | 312(49.6%) | 1907(56.2%) | 0.002 |
| Diuretic | 3895(96.9%) | 610(96.9%) | 3285(96.8%) | 0.85 |
| Digoxin | 1936(48.2%) | 309(49.1%) | 1627(47.9%) | 0.635 |
| Anti-Diabetes agents | 1291(32.1%) | 243(38.6%) | 1048(30.9%) | <0.001 |
| Statin | 1405(34.9%) | 260(41.3%) | 1145(33.8%) | <0.001 |
| Discharge Medications | ||||
| ACE inhibitor/ARB | 3415(84.9%) | 475(75.5%) | 2940(86.7%) | <0.001 |
| Beta blocker | 3016(75.0%) | 449(71.4%) | 2567(75.7%) | 0.025 |
| MRA | 2456(61.1%) | 336(53.4%) | 2120(62.5%) | <0.001 |
| Diuretic | 3747(93.2%) | 592(94.1%) | 3155(93.0%) | 0.271 |
| Digoxin | 1913(47.6%) | 302(48.0%) | 1611(47.5%) | 0.794 |
| Anti-diabetes agents | 1257(31.2%) | 233(37.0%) | 1024(30.2%) | 0.001 |
| Statin | 1405(35.0%) | 260(41.3%) | 1145(33.8%) | <0.001 |
| Vital sign and laboratory data | ||||
| Systolic blood pressure, mmHg | 120.0 (105.0,132.0) | 110.0 (100.0,130.0) | 120.0 (107.0,134.0) | <0.001 |
| Diastolic blood pressure, mmHg | 70.0 (62.0,80.0) | 70.0 (60.0,78.0) | 71.0 (64.0,80.0) | <0.001 |
| Heart rate, per minute | 79.7(15.6) | 79.8(15.6) | 79.7(15.6) | 0.910 |
| Respiratory rate, per minute | 20.7 (3.6) | 20.7 (4.0) | 20.7 (3.5) | 0.678 |
| Weight changeb, kg | −3.2(3.4) | −2.9(3.5) | −3.3(3.4) | 0.013 |
| Blood urea nitrogen, mg/dL | 26.0 (19.0,35.0) | 31.0 (23.0,46.0) | 25.0 (19.0,33.0) | <0.001 |
| Creatinine, mg/dL | 1.2 (1.0,1.6) | 1.4 (1.1,1.9) | 1.2 (1.0,1.5) | <0.001 |
| eGFR, mL/min/1.73 m2 | 54.9 (40.5,70.3) | 46.9 (33.0,61.9) | 56.4 (42.4,71.5) | <0.001 |
| Ejection fraction, % | 28.0 (20.0,35.0) | 25.0 (20.0,32.0) | 29.0 (21.0,35.0) | <0.001 |
| BNP, pg/mlc | 679.8 (286.0,1458.7) | 1070.6 (548.0,1924.0) | 611.5 (261.0,1371.0) | <0.001 |
| Haemoglobin, g/dL | 13.5 (2.0) | 12.9 (2.0) | 13.6 (1.9) | <0.001 |
| Sodium, mEq/L | 139.7 (4.5) | 138.6 (4.9) | 139.9 (4.4) | <0.001 |
- ACE, angiotensin-converting enzyme; ARB, angiotensin II receptor blocker; BNP, B-type natriuretic peptide; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; MRA, mineralocorticoid receptor antagonists.
- a Data reflect baseline values unless otherwise noted and are expressed as mean +/− standard deviation, median (interquartile range), or n (%).
- b Change in weight calculated as difference in weight at discharge from weight at admission.
- c BNP data were not available for all patients in the trial.
Length of stay
Table 2 shows overall and region specific estimates of LOS during index hospitalization. Overall median LOS across all regions was 8 (4–11) days. Median LOS was highest in Eastern Europe at 11 (8–14) days and lowest in North America at 4 (3–7) days.
| Region | Patients, N | Length of stay, median (days) | Readmission rate, % | Correlation, r (95% CI)a | P |
|---|---|---|---|---|---|
| All-cause readmission | |||||
| Overall | 4020 | 8 (4, 11) | 15.7 | 0.09 (0.06–0.12) | <0.001 |
| Eastern Europe | 1589 | 11 (8,14) | 7.9 | 0.07 (0. –0.12) | 0.003 |
| North America | 1217 | 4 (3,7) | 23.5 | 0.10 (0.04–0.15) | 0.001 |
| South America | 672 | 5 (3,9) | 15.5 | 0.18 (0.11–0.25) | <0.001 |
| Western Europe | 542 | 8 (5,11) | 20.9 | −0.02 (−0.11–0.06) | 0.616 |
| Heart failure readmission | |||||
| Overall | 4020 | 8 (4, 11) | 5.6 | −0.01 (−0.05–0.02) | 0.367 |
| Eastern Europe | 1589 | 11 (8,14) | 1.8 | −0.00 (−0.05–0.05) | 0.922 |
| North America | 1217 | 4 (3,7) | 10.3 | −0.02 (−0.08–0.03) | 0.408 |
| South America | 672 | 5 (3,9) | 5.5 | 0.02 (−0.06–0.09) | 0.639 |
| Western Europe | 542 | 8 (5,11) | 6.1 | −0.05 (−0.14–0.03) | 0.225 |
| Cardiovascular non-heart failure readmission | |||||
| Overall | 4020 | 8 (4, 11) | 4.4 | 0.07 (0.04–0.10) | <0.001 |
| Eastern Europe | 1589 | 11 (8,14) | 3.7 | 0.08 (0.03–0.13) | 0.245 |
| North America | 1217 | 4 (3,7) | 4.8 | 0.07 (0.01–0.12) | 0.017 |
| South America | 672 | 5 (3,9) | 4.8 | 0.19 (0.12–0.26) | <0.001 |
| Western Europe | 542 | 8 (5,11) | 5.0 | 0.06 (−0.02–0.15) | 0.130 |
| Non-cardiovascular readmission | |||||
| Overall | 4020 | 8 (4, 11) | 5.8 | 0.07 (0.04–0.10) | <0.001 |
| Eastern Europe | 1589 | 11 (8,14) | 2.5 | 0.05 (−0.00–0.09) | 0.063 |
| North America | 1217 | 4 (3,7) | 8.6 | 0.12 (0.06–0.18) | <0.001 |
| South America | 672 | 5 (3,9) | 5.2 | 0.09 (0.01–0.16) | 0.022 |
| Western Europe | 542 | 8 (5,11) | 10.0 | −0.02 (−0.11–0.06) | 0.590 |
- a Partial correlations are adjusted for age and gender.
Length of stay and readmission risk
Data for unadjusted and adjusted risk of all-cause and cause specific readmission by LOS quintile are displayed in Table 3. Risk data adjusted for discharge characteristics and medication use, compared with baseline characteristics and medication use, are displayed in the Supplementary Material online, Table S1.
| Length of stay (days) | |||||
|---|---|---|---|---|---|
| Quintile 1 (≤3) | Quintile 2 (4–6) | Quintile 3 (7–9) | Quintile 4 (10–13) | Quintile 5 (≥14) | |
| All-cause readmission | |||||
| Unadjusted | 1.00 (Ref) | 1.19 (0.92–1.54) | 1.30 (0.97–1.74) | 1.37 (0.97–1.94) | 1.98 (1.32–2.98) |
| Adjusteda | 1.00 (Ref) | 1.06 (0.82–1.39) | 1.06 (0.78–1.44) | 1.10 (0.78–1.57) | 1.39 (0.92–2.11) |
| Adjusted including medicationsb | 1.00 (Ref) | 1.05 (0.80–1.38) | 1.06 (0.78–1.43) | 1.10 (0.77–1.57) | 1.41 (0.93–2.12) |
| Excluding those who died within 30-daysa | 1.00 (Ref) | 1.11 (0.84–1.46) | 1.14 (0.83–1.56) | 1.11 (0.77–1.60) | 1.53 (1.00–2.35) |
| Heart failure readmission | |||||
| Unadjusted | 1.00 (Ref) | 0.90 (0.63–1.30) | 0.89 (0.58–1.35) | 1.08 (0.67–1.74) | 0.71 (0.41–1.24) |
| Adjusteda | 1.00 (Ref) | 0.79 (0.54–1.16) | 0.68 (0.44–1.06) | 0.86 (0.53–1.42) | 0.43 (0.24–0.79) |
| Adjusted including medicationsb | 1.00 (Ref) | 0.77 (0.52–1.13) | 0.64 (0.41–1.00) | 0.82 (0.50–1.36) | 0.42 (0.23–0.77) |
| Excluding those who died within 30-daysa | 1.00 (Ref) | 0.84 (0.57–1.25) | 0.71 (0.45–1.13) | 0.78 (0.45–1.33) | 0.48 (0.26–0.90) |
| Cardiovascular non-heart failure readmission | |||||
| Unadjusted | 1.00 (Ref) | 1.34 (0.78–2.29) | 1.65 (0.93–2.91) | 1.47 (0.77–2.80) | 3.12 (1.63–5.96) |
| Adjusteda | 1.00 (Ref) | 1.33 (0.78–2.29) | 1.75 (0.99–3.10) | 1.54 (0.80–2.96) | 2.99 (1.49–6.02) |
| Adjusted including medicationsb | 1.00 (Ref) | 1.32 (0.77–2.28) | 1.69 (0.95–2.99) | 1.53 (0.80–2.93) | 3.07 (1.53–6.18) |
| Excluding those who died within 30 daysa | 1.00 (Ref) | 1.30 (0.75–2.27) | 1.79 (1.0–3.20) | 1.64 (0.85–3.15) | 3.26 (1.62–6.54) |
| Non-cardiovascular readmission | |||||
| Unadjusted | 1.00 (Ref) | 1.51(1.01–2.25) | 1.49 (0.96–2.31) | 1.54 (0.93–2.55) | 2.58 (1.61–4.12) |
| Adjusteda | 1.00 (Ref) | 1.36 (0.91–2.04) | 1.22 (0.78–1.91) | 1.19 (0.71–1.98) | 1.72 (1.05–2.81) |
| Adjusted including medicationsb | 1.00 (Ref) | 1.34 (0.89–2.02) | 1.26 (0.80–1.99) | 1.21 (0.72–2.03) | 1.74 (1.06–2.86) |
| Excluding those who died within 30-daysa | 1.00 (Ref) | 1.35 (0.89–2.05) | 1.30 (0.82–2.06) | 1.22 (0.72–2.07) | 1.85 (1.11–3.07) |
- Ref, referent quintile.
- a Odds ratios were adjusted for baseline variables including age, sex, region, race, history of diabetes, history of cardiovascular disease, body weight, haemoglobin levels, systolic blood pressure, heart rate, respiratory rate, serum creatinine, blood urea nitrogen, serum albumin, serum sodium, B-type natriuretic peptide levels and randomization group (tolvaptan vs. placebo).
- b Odds ratios adjusted for the covariates above plus baseline use of anti-diabetes agents, angiotensin-converting enzyme inhibitors/angiotensin II receptor blockers, beta-blockers, diuretics, digoxin, mineralocorticoid receptor antagonists, and statins.
All-cause readmissions
The 30-day all-cause readmission rate was 15.7% (95% CI 14.6%–16.8%) and weakly correlated with LOS (r = 0.09 P < 0.01). All-cause readmission rates varied across regions, with the lowest in Eastern Europe (7.9%, 95% CI 6.6–9.3%) and the highest in North America (23.5%, 95% CI 21.3–26.1%) (P < 0.001). The unadjusted OR for the top (≥14 days) vs. the bottom (≤3 days) LOS quintile was 1.98 (95% CI 1.32–2.98), which was attenuated to 1.39 (95% CI 0.92–2.11) after adjustment for baseline covariates, and 1.53 (95% CI 1.0–2.35) if patients who died within the first 30 days post-discharge were excluded. Adjustment for discharge characteristics, as opposed to baseline characteristics, did not materially change results.
HF readmissions
The 30-day HF readmission rate was 5.6% (95% CI 4.9%–6.3%) across all regions and was not correlated with the LOS (r = −0.01, P = 0.367). The HF readmission rate was lowest in Eastern Europe (1.8%, 95% CI 1.2–2.5%) and highest in North America (10.3%, 95% CI 8.7–12.1%, P < 0.001). Unadjusted OR for top vs. bottom quintile of LOS was 0.71 (95% CI 0.41–1.24), which changed to 0.43 (95% CI 0.24–0.79) after adjustment for baseline covariates. Adjustment for discharge characteristics and exclusion of patients who died within 30 days of discharge did not meaningfully change these results.
CV non-HF readmissions
The 30-day CV non-HF readmission rate was 4.4% (95% CI 3.8–5.1%) and modestly correlated with the LOS (r = 0.07, P < 0.01). The CV non-HF readmission rates were similar across regions with lowest being in Eastern Europe (3.7%, 95% CI 2.9–4.8%) and highest being in Western Europe (5.0%, 95% CI 3.4–7.2%) (P = 0.420). The unadjusted OR was 3.12 (95% CI 1.63–5.96) for CV non-HF readmissions comparing top vs. bottom quintile of LOS, which was not materially altered after adjustment for baseline covariates, adjustment for discharge covariates, or exclusion of patients who died within the first 30 days post-discharge.
Non-CV readmissions
The 30-day non-CV readmissions rate was 5.8% (95% CI 5.1%–6.6%) and modestly correlated with the LOS (r = 0.07, P<0.01). Readmission rates for non-CV events varied across regions with the lowest being in Eastern Europe (2.5%, 95% CI 1.9–3.4%) and the highest being in Western Europe (10.0%, 95% CI 7.7–12.8%) (P < 0.001). The unadjusted OR was 2.58 (95% CI 1.61–4.12) for non-CV readmissions comparing the top vs. bottom quintile, which was attenuated to 1.72 (95% CI 1.05–2.81, P < 0.001) after adjustment for baseline covariates. Similar adjusted results were seen when excluding patients who died within 30 days of discharge and when controlling for discharge characteristics.
Nature of association
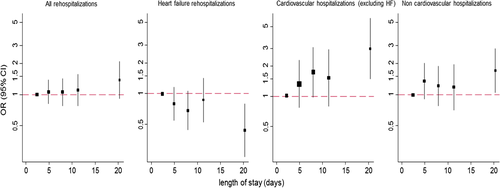
The shape of the association between 30-day readmissions and LOS is presented in Figure 1. All-cause and CV non-HF readmissions had a linear association while non-CV readmissions had a non-linear positive association with LOS. Readmissions for HF had an inverse association with LOS. Region-specific ORs for 30-day readmissions with LOS are presented in Figure 2 and odds ratios across select subgroups are shown in Figure 3.
Discussion
In this large multinational cohort of hospitalized HF patients with reduced EF, median LOS varied across geographic region, and was independently associated with 30-day risk of readmission. Longer LOS was positively associated with all-cause, CV non-HF and non-CV readmissions and inversely association with HF readmissions within 30 days of discharge.
To our knowledge, the only previous analysis of LOS from a hospitalized HF clinical trial population comes from the Acute Study of Clinical Effectiveness of Nesiritide in Decompensated Heart Failure Trial (ASCEND-HF).11, 22 Similar to the present results from EVEREST, in ASCEND-HF, increasing LOS was associated with lower risk of 30-day HF-specific readmission; contrary to our findings, longer LOS correlated with reduced risk of 30-day all-cause readmission following covariate adjustment in the USA and across countries.11 Reasons for these discordant findings may include differences in the study populations, with EVEREST patients more likely to be white males, to have a history of coronary artery disease and myocardial infarction, and less likely to have chronic obstructive pulmonary disease (COPD). Differing risk profiles may be reflected in absolute rates of all-cause 30-day readmissions between the two studies with rates of 11.4% and 15.7% in ASCEND-HF and EVEREST, respectively.11 By comparison, rates of 30-day HF-specific readmission were similar at 5.4% and 5.6%, respectively. Thus, when considered alongside the similar inverse relationship between LOS and HF readmission seen in these two studies, differing risk of non-HF-related readmission may explain conflicting results regarding all-cause readmission risk. However, the definition of the 30-day period used in each study also deserves attention. The present study started the 30-day follow-up at actual time of discharge, rather than time of randomization while the patient was still hospitalized (i.e. median time from hospitalization to randomization in ASCEND-HF placebo arm, 15.7 h).11 Thus, differences with regard to absolute rates of all-cause readmission and correlation with LOS may be explained by less ‘at risk’ time for readmission in the ASCEND-HF study (i.e. period where patient discharged from the hospital and within 30 days of randomization). By initiating follow-up at time of discharge, the present study used a more clinically relevant and generalizable measure of 30-day readmission that allowed LOS to be modelled as an independent exposure for predicting early rehospitalization.
It is notable that the statistically significant associations between LOS and readmission in the present study only became apparent with hospital stays ≥14 days. However, with the exception of HF readmission, LOS and all-cause and cause specific 30-day readmissions showed modest statistically significant correlations. This suggests some degree of continuous relationship and supports selection of our LOS group cutpoints as a potential reason for only one subgroup yielding statistically significant results. However, when designing this study, the authors felt that for the purposes of outcome analysis, organizing LOS by quintile would convey more clinically meaningful and easily interpretable results than continuous LOS. Nonetheless, in our study, it does appear that the independent influence of LOS on readmission becomes detectable only with particularly long hospital stays that approach 14 days in duration.
Consistent with previous reports, we observed substantial differences in LOS and rates of readmissions across regions, with Eastern Europe having significantly longer median LOS and lower all-cause and cause-specific 30-day readmissions.1, 11, 14, 23 However, following covariate adjustment, the relationship between LOS and readmission risk appeared largely homogeneous across regions, with the exception of Western Europe for the risk of all-cause and CV non-HF readmission. In Western Europe, longer LOS was associated with a trend towards reduced risk of all-cause readmission and had no apparent influence on CV non-HF readmission. These differences may reflect residual confounding from patient factors following multivariate modelling, unidentified regional differences in inpatient or post-discharge management practices and reimbursement, or access to care.
Characteristics of hospital and physician reimbursement systems present varying incentives that influence LOS. Pressures exist to discharge patients early to maximize revenue and open beds for new admissions.24, 25 Moreover, until recently, hospitals in the USA were not directly penalized for early readmission, but were, rather, reimbursed for the second hospitalization assuming it occurred >24 h post-discharge.26 The recent advent of financial penalties by the CMS for hospitals with higher standardized 30-day readmissions following HF hospitalization has refocused attention, research efforts, and quality improvement initiatives towards improving early post-discharge outcomes in the USA.27 However, the economic and public health burden of hospitalized HF and subsequent readmissions extends beyond North America with poor post-discharge outcomes across the world despite significant health-care expenditure.1, 28 Although rehospitalization risk and prevention have always been central to patient-centred care, the potential association between LOS and risk of readmission is becoming increasingly financially relevant.26
Unfortunately, there is a lack of evidence-based strategies definitively proven to reduce readmissions for patients hospitalized with HF.3, 29 Aside from optimization of guideline-directed medical therapy for chronic HF, contemporary approaches have centred on patient education, remote collection of subjective and objective data, and early follow-up with health-care providers.30-32 The best prospective support for these generic transitional care measures may come from a recent meta-analysis,33 but overall, the emphasis on these strategies is disproportionate to the underlying evidence and costs, and more proven approaches are urgently needed. The findings of the present study reiterate the need to recognize the reasons for readmission when developing potential interventions targeting overall readmission rate. Although, by definition, hospitalized HF patients present to the hospital in HF and may receive HF-centred care during the index admission, they get readmitted to the hospital for multiple reasons. While extending the length of the index hospital stay may allow added focus on HF optimization and theoretically prevent subsequent HF readmission, our data suggest these benefits come at the expense of increased risk for CV non-HF and non-CV admission. Potential underlying mechanisms for this finding may relate to suboptimal care for comorbidities by inpatient providers and added risk for nosocomial complications (e.g. infections, deconditioning) with added days in-hospital. Thus, our findings suggest added attention towards non-HF multi-disciplinary care during index admission may be an efficient means by which to improve the 30-day readmission rate. Such an approach would be consistent with data supporting associations between comorbidities such as diabetes, chronic kidney disease, and COPD with hospital admission among HF patients.34 Alternatively, reducing LOS while identifying and preserving aspects of care necessary to maintain lower HF-specific readmissions represents a plausible, albeit potentially more difficult approach.
The utility of the 30-day readmission rate as a quality metric has been debated.27 Inverse relationships may exist between a hospital's 30-day mortality and rehospitalization rates. Recent Medicare data suggest that LOS for HF admissions has decreased over the past decade, with a subsequent increase in 30-day readmission rates.35 Accordingly, alternative quality metrics have been proposed, such as total days in hospital during a specified time-frame.36 Although our data suggest a correlation between longer LOS and increased readmission risk, intuitively, each patient has a point in the hospital stay, albeit poorly defined, before which discharge would be unsafe, the hospitalization unhelpful, and the risk of readmission high. Future research must more precisely identify what interventions, and for which patients, justify the potential risks from increasing the length of the index HF hospitalization.
Limitations
This study analysed a highly selected clinical trial population with EF ≤40% that was well-treated with guideline-directed medical therapies and had prespecified early post-discharge follow-up. These results may not be applicable to patients with preserved EF and the study inclusion/exclusion criteria must be considered when generalizing these findings to other populations. Moreover, differences in country-specific reimbursement structures, post-discharge management, and readmission policies may represent confounding influences on LOS and readmissions that were unaccounted for. In addition, despite rigorous multivariable modelling, the retrospective nature of this study prevents determination of a definitive cause-and-effect relationship between LOS and readmissions. Lastly, determination of 30-day readmissions by the CMS excludes patients discharged to subacute nursing facilities or hospice care. This disposition information was not available within the EVEREST database.
Conclusions
In conclusion, in this large multinational cohort of hospitalized HF patients with reduced EF, longer LOS during index hospitalization was associated with increased risk of 30-day readmission for CV non-HF, non-CV, and all causes. By contrast, longer LOS correlated with lower risk for 30-day readmission for HF. Median LOS and its association with readmissions differed across geographic regions. These findings may inform development of interventions to prevent readmission, definitions of quality metrics, and the design of clinical trials in hospitalized HF patients.
Funding
The funding sources (Otsuka, Inc.) had no role in the study's design, conduct, or reporting. The authors take full responsibility for the design and conduct of the study, had authority over manuscript preparation, and controlled the decision to submit the manuscript for publication.
Conflict of interest
G.C.F. reports significant consulting for Novartis, and modest consulting for Amgen, Bayer, Gambro, Medtronic, and Janssen; Dr. Fonarow holds the Eliot Corday Chair of Cardiovascular Medicine at UCLA and is supported by the Ahmanson Foundation (Los Angeles, California). M.G. has been a consultant for Abbott Laboratories, Astellas, AstraZeneca, Bayer HealthCare AG, CorThera, Cytokinetics, DebioPharm S.A., Errekappa Terapeutici, GlaxoSmithKline, Ikaria, Johnson & Johnson, Medtronic, Merck, Novartis Pharma AG, Otsuka Pharmaceuticals, Palatin Technologies, Pericor Therapeutics, Protein Design Laboratories, Sanofi-Aventis, Sigma Tau, Solvay Pharmaceuticals, Takeda Pharmaceutical, and Trevena Therapeutics. All other authors have no conflicts of interest to declare.




