Sex, fat and the heart: it's all in the waist
The opinions expressed in this article are not necessarily those of the Editors of the European Journal of Heart Failure or of the European Society of Cardiology. doi: 10.1002/ejhf.2563
This article refers to ‘Sex and central obesity in heart failure with preserved ejection fraction’ by H. Sorimachi et al., published in this issue on pages 1359–1370.
The link between sex and heart failure (HF) is well known: women predominate in HF with preserved ejection fraction (HFpEF), whereas men predominate in HF with reduced ejection fraction.1 Similarly, the link between obesity and HF is well recognized: obesity is a stronger risk factor for HFpEF than HF with reduced ejection fraction,2 and the ‘obese HFpEF phenotype’ has been well characterized.3-5 Only recently, we have begun to understand the link between all three factors: sex, obesity and HFpEF. In a combined analysis of diverse community-based cohorts, the association of obesity with incident HFpEF was stronger in women than men.2 In large HFpEF outcomes trials, women with HFpEF were more obese than men with HFpEF.6
Beyond excess body weight per se, the pathophysiologic importance of body composition, fat distribution and visceral obesity in HFpEF is increasingly recognized, particularly in women.2, 7 With fat (vs. lean mass) gain, whether an individual expands the body's subcutaneous store versus the visceral adipose depot significantly impacts the metabolic consequences of obesity. Specifically, a substantial gain in visceral adipose tissue, with preferential fat storage in the abdominal subcutaneous area (vs. lower body), is associated with increased cardiometabolic risk. Accurately assessing regional fat distribution in routine clinical practice can be challenging. Gold standard imaging of visceral fat (using computed tomography [CT], magnetic resonance imaging and dual-energy X-ray absorptiometry) is costly, involves radiation and has limited availability. While body mass index (BMI) is a convenient and simple metric of obesity, the weak correlation with body fat or cardiovascular risk limits its clinical utility. Abdominal measures of adiposity (e.g. waist circumference, waist-to-height ratio, waist-to-hip ratio) quantify abdominal girth relative to the rest of the body (i.e. central obesity) and provide independent, additive information to BMI for predicting morbidity and mortality in HFpEF.2, 8 Despite being an established surrogate measure of visceral fat, waist circumference is often neglected in routine clinics and HFpEF trials.
In this issue of the Journal, Sorimachi and colleagues utilized waist circumference to investigate the prevalence and association of central obesity with metabolic, cardiac and exercise haemodynamic abnormalities in HFpEF, while exploring potential sex differences.9 Patients with HFpEF (n = 229, 54% women) who underwent invasive supine haemodynamics testing for unexplained dyspnoea, and with available anthropometric indices within 6 months of catheterization, were identified in this retrospective single centre study. All patients also had comprehensive echocardiography and blood sampling within 1 month of catheterization, as well as upright exercise testing (median 10 days from supine exercise). Overall, central obesity (defined by waist circumference ≥88 cm for women and ≥102 cm for men) was more prevalent (77% vs. 64%) than general obesity (defined by BMI ≥30 kg/m2) among patients with HFpEF, with no sex differences. Compared to those without central obesity, patients with central obesity had more comorbidities, worse metabolic profile, more left ventricular (LV) structural and functional abnormalities (larger LV diastolic size, higher LV mass, more impaired LV relaxation) and worse exercise haemodynamics (higher right-sided pressures, higher wedge pressure) with more impaired aerobic capacity (reduced peak oxygen consumption). Findings were similar in patients with central adiposity without general obesity (BMI <30 kg/m2, n = 38) compared to their non-obese counterparts without central adiposity. Sex-stratified analyses showed that higher (vs. lower) waist circumference was associated with a higher prevalence of metabolic abnormalities and haemodynamic perturbations in women but not in men (Figure 1), although there were no significant sex interactions for these exploratory analyses. The only significant sex interaction was observed for workload-corrected wedge pressure scaled to body weight, which increased to a greater extent with waist circumference among women compared to men with HFpEF (23.3 vs. 12.1 mmHg/W/kg for every 10 cm increase in waist circumference, pinteraction = 0.02). In both sexes, higher waist circumference was similarly associated with reduced exercise capacity (peak oxygen consumption normalized to body weight; pinteraction = 0.7). Taken together, among individuals with HFpEF, central obesity was associated with worse metabolic profiles, more cardiac structural/functional abnormalities and poorer exercise haemodynamics, with some suggestion of greater exercise deficit in women than men.
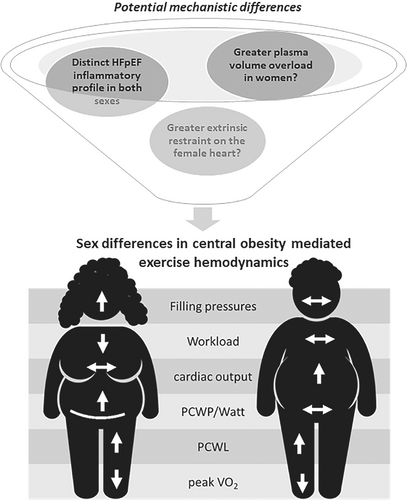
This current work extends prior knowledge of central obesity in HFpEF, including work from the same authors (Table 1).7-11 In TOPCAT (Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist), a direct relationship was found between increased waist circumference and risk of hospitalization in both women and men.10 Sorimachi et al. showed that, compared to age-, sex- and BMI-matched controls, women with HFpEF, but not men, had excess visceral adipose tissue as measured by abdominal CT; and increased visceral adiposity was associated with impaired exercise haemodynamics in women but not men with HFpEF.7 The same group now corroborates the prior CT findings with a simple, pragmatic measure of central adiposity – waist circumference.9 While important, the current study is limited by selection bias, lack of adjustment for clinical covariates or multiple comparisons, no non-HFpEF controls and limited power to detect sex differences. Indeed, it is not uncommon for such sex comparisons to be underpowered in HFpEF cohorts, and the sex-specific results, although only hypothesis-generating, are valuable. Sex comparisons in cardiology are fraught with controversies regarding how best to account for body size differences between women and men. For instance, opinions are divided regarding whether to normalize haemodynamic parameters and aerobic capacity to body weight, height, body surface area, or other anthropometric measures – considerations that are even more complex when obesity is involved.12
| Study | Population | Obesity index | Comparator group | Follow-up | Key findings (pooled analyses) | Sex-specific sub-analyses |
|---|---|---|---|---|---|---|
| Haykowsky et al., 201811 | Obese HFpEF (BMI ≥30 kg/m2) healthy controls | Total body adiposity (DEXA) and regional adiposity (CMR) | Obese HFpEF (n = 100) vs. age-, sex-, race-, body surface area-adjusted healthy controls (n = 61) | Not applicable |
Compared to controls, obese HFpEF: - lower peak VO2 (15.7 vs. 23.0 ml/kg/min) - lower 6MWD (427 vs. 538 m) - lower SPPB score (10.3 vs. 10.9) - lower leg power (117 vs. 152 W) peak VO2, 6MWD, leg power showed: (i) negative associations with - abdominal subcutaneous fat - thigh subcutaneous fat - thigh intermuscular fat/skeletal muscle ratio (ii) positive associations with: - epicardial fat. Intra-abdominal fat was the strongest independent predictor of peak VO2 and 6MWD (partial R2 = 0.38 and 0.2, respectively) |
Not available |
| Tsujimoto et al., 20178 | Patients with HFpEF (n = 3310) | WC of ≥102 cm in men and ≥88 cm in women | HFpEF with high WC (n = 2413) vs. HFpEF without high WC (n = 897) | 3.4 ± 1.7 years |
HFpEF subjects with abdominal obesity (vs. without): - higher risk of all-cause mortality (aHR: 1.52; 95% CI 1.16–1.99) |
Not available |
| Fu et al., 202210 | Patients with HFpEF (n = 3320) | WC of ≥102 cm in men and ≥88 cm in women |
Men (n = 1620) vs. women (n = 1700) with HFpEF Obese vs. non-obese (sex-stratified) |
3.4 ± 1.7 years |
Women vs. men with HFpEF: - women were older and had higher NYHA functional classes - lower quality of life in women - worse diastolic function and higher EF in women; higher LV mass index, higher posterior wall thickness, LV enlargement in men |
Women (obese vs. non-obese): - abdominal obesity associated with higher risk of hospitalization (aHR 1.15, 95% CI 1.18–3.28). Men (obese vs. non-obese): - abdominal obesity associated with higher risk of all-cause mortality (aHR 1.32, 95% CI 1.02–1.71) and higher risk of hospitalization (aHR 1.39, 95% CI 1.01–1.93) |
| Sorimachi et al., 20217 | HFpEF (n = 105) vs. controls (n = 105) |
VAT (abdominal CT) >126 cm2 in women, >203 cm2 in men |
Pooled HFpEF vs. controls Sex-stratified high vs. normal VAT |
Not applicable | Each 100 cm2 increase in VAT area associated with 4.0 mmHg higher PCWP (4.0, 95%CI 2.1–6.0 mmHg, p < 0.0001) in women with HFpEF, but not in men with HFpEF (pinteraction = 0.009) |
Women: - higher VAT area in HFpEF vs. controls (186 vs. 139 cm2) - higher PCWP during exercise in those with increased VAT vs. normal VAT (28 ± 10 vs. 21 ± 10 mmHg) Men: - no difference in VAT area in HFpEF vs. controls (294 vs. 252 cm2) - no difference in PCWP with excercise in those with increased VAT (vs. normal VAT) |
| Sorimachi et al., 20229 | HFpEF with central obesity (n = 177) vs. without central obesity (n = 52) | WC ≥88 cm for women and ≥102 cm for men | Sex-stratified (n = 124 women, n = 105 men) | Not applicable |
Prevalence of central obesity (defined by WC) more than general obesity (defined by BMI ≥30 kg/m2) (77% vs. 62%), no sex differences HFpEF (high vs. low WC): - higher % body fat (32 ± 5 vs. 26 ± 6%) - higher prevalence of comorbidities (diabetes, dyslipidaemia, COPD) - greater LV diastolic dimensions, higher LV mass index, lower e' - with exercise, higher filling pressures, PCWP, PCWP/W and PCWL - lower body weight scaled-peak VO2 in supine and upright exercise - increased WC was associated with elevated PCWP in both sexes |
Women (high vs. low WC): - increased filling pressures - no change in CO - reduced peak VO2 Men (high vs. low WC): - no change in filling pressures - increased CO - reduced peak VO2 Pooled regression analyses (per 10 cm increase in WC by sex): - higher PCWL/W only in women (pinteraction = 0.08) - higher PCWL in women vs. men (pinteraction = 0.02) |
- 6MWD, 6-min walk distance; aHR, adjusted hazard ratio; BMI, body mass index; CI. confidence interval; CMR, cardiac magnetic resonance; CO, cardiac output; COPD, chronic obstructive pulmonary disease; DEXA, dual-energy X-ray absorptiometry; EF, ejection fraction; HFpEF, heart failure with preserved ejection fraction; LV, left ventricular; NYHA, New York Heart Association; PCWL, workload-corrected pulmonary capillary wedge pressure normalized to body weight; PCWP, pulmonary capillary wedge pressure; PCWP/W, pulmonary capillary wedge pressure normalized to workload; SPPB, Short Physical Performance Battery; VAT, visceral adipose tissue; VO2, oxygen consumption; WC, waist circumference.
One of the most important clinical take-home messages from the current and prior studies is that a simple measure of waist circumference can identify a high-risk phenotypic subset of HFpEF with greater metabolic derangement, worse exercise haemodynamics, and poorer prognosis. Given the simplicity and widespread accessibility of waist circumference measurement, routine clinical application, including in the context of clinical studies, appears warranted. The question remains if waist circumference may even serve as a target for treatment or predictor of response to therapies. Based on the premise that the severity of exercise intolerance correlates with increased body adiposity and skeletal muscle adipose infiltration, the SECRET (Exercise Intolerance in Elderly Patients With Diastolic Heart Failure) trial randomized obese HFpEF individuals (n = 100, 80% women) to caloric restriction and/or aerobic exercise and showed that both lifestyle interventions increased peak oxygen consumption and percentage lean body mass within 20 weeks.13 The marked improvement in functional capacity in the PRESERVED-HF trial of dapagliflozin in HFpEF,14 but not the EMPERIAL-Preserved trial of empagliflozin in HFpEF,15 has been postulated to relate to differences in the degree of obesity in the respective study populations, although central versus general obesity was not specifically studied. Emerging evidence from metabolic/bariatric surgery in HFpEF and incretin-based therapies (glucagon-like peptide-1 receptor agonists [GLP-1RA] or dual GLP-1/glucose-dependent insulinotropic polypeptide [GIP] or GLP-1/glucagon receptor co-agonists)16 in reducing regional adiposity are showing promise. Liraglutide plus lifestyle intervention in community-dwelling obese adults (BMI ≥27 kg/m2 with metabolic syndrome or BMI ≥30 kg/m2) showed a significant reduction in visceral adipose tissue within 40 weeks.16 We await results from three ongoing randomized clinical trials that are examining the role of pharmacologic weight-loss therapies in HFpEF, two using the GLP-1RA semaglutide and one the dual GIP/GLP-1 receptor agonist tirzepatide (NCT04788511, NCT04916470, NCT04847557).
The intriguing findings in subgroup analysis relating to non-obese central adiposity carry implications for other HFpEF populations in which frank obesity may not be as highly prevalent as central adiposity and cardiometabolic disease. Such ‘lean-fat HFpEF’ (with low BMI and high waist-to-height ratio) has been described in Asian HFpEF cohorts and shown to predominantly affect women with a high prevalence of diabetes and poor outcomes.17 While abdominal visceral adiposity was not directly measured, epicardial adipose tissue mass was assessed by magnetic resonance imaging and found to relate to greater LV dysfunction and fibrosis in Asian HFpEF (but not HF with reduced ejection fraction).18 Among asymptomatic Asian adults, higher BMI (even in the non-obese range) and larger waist circumference were associated with a greater degree of LV remodelling and subclinical LV contractile dysfunction in Asian women than men, at much lower anthropometric cut-offs than World Health Organization criteria (BMI >23.4 kg/m2, waist circumference >83 cm).19
We are still far from understanding the mechanistic translation of how central obesity impacts cardio-mechanical adipose interaction differently in men and women with HFpEF. Sex differences in proteomic correlates of microvascular dysfunction in HFpEF exist.20 Transcriptomic analyses of endomyocardial biopsies identified a distinct cluster within HFpEF, consisting exclusively of obese women with elevated pro-inflammatory signalling and enriched immune pathways.21 While oestrogen receptors are known to be expressed in the adipose tissue, little is known about the cardio-mechanical adaptions in post-menopausal women with central obesity and HFpEF. Other postulations include a potential sex-selective role for adipokine-mediated sodium retention and plasma volume expansion, or greater adipocyte-derived inflammatory mediators in women than men with HFpEF. An increase in intra-abdominal pressure from central obesity could potentially increase the extrinsic restraint load to a greater extent in women than men.22 While further work is clearly required to unravel the complex relationships among sex, fat and HFpEF in the search for potential sex-specific central obesity-targeting interventions, Sorimachi et al. have reminded us of the value of waist circumference as a simple measurement that may be applied now.
Conflict of interest: C.C. has received research grants from National Medical Research Council Singapore and Lee Foundation Singapore, speaker/writing fees from Roche Diagnostics, Boehringer Ingelheim, Sanofi Aventis, and consulting fees from Us2.ai. C.S.P.L. is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore; has received research support from AstraZeneca, Bayer, Boston Scientific and Roche Diagnostics. has served as consultant or on the advisory board/ steering committee/executive committee for Actelion, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Boehringer Ingelheim, Boston Scientific, Cytokinetics, Darma Inc., Us2.ai, Janssen Research & Development LLC, Medscape, Merck, Novartis, Novo Nordisk, Radcliffe Group Ltd., Roche Diagnostics, Sanofi and WebMD Global LLC; and serves as co-founder & non-executive director of Us2.ai.




