Cardiac resynchronization therapy in mild heart failure should be reserved for true dyssynchronopathy
Abstract
This editorial refers to ‘Cardiac resynchronization therapy in heart failure patients with less severe left ventricular dysfunction’†, by Hay O.Y. et al., published in the February issue, European Journal of Heart Failure (2015); 17: 135–143.
Heart failure (HF) is a syndrome that can be caused by a wide variety of insults, but once it develops it is considered as a single disorder and treated identically. This contention is particularly true for patients with dilated cardiomyopathy (DCM), which may be caused by an underlying genetic, inflammatory, infiltrative, toxic, or electrical disorder, but who are classified and treated as having one disease. Cardiac resynchronization therapy has become a well-established therapy for patients with systolic HF and evidence of electromechanical dyssynchrony. Recent evidence has shown that the chance of responding to CRT is greatest in patients with DCM and a true LBBB. Guidelines therefore favour CRT implantation in these patients rather than in patients with a broad QRS due to a non-specific intraventricular conduction delay (IVCD) or right bundle branch block.1 A true LBBB is caused by failure of the conduction system, whereas IVCD is thought to reflect intramyocardial fibrosis or infiltration. The superior efficacy of CRT in patients with a true LBBB begs the question of whether this ECG pattern can identify a subgroup of DCM patients with primary conduction system disease, highly amendable to CRT.
Randomized multicentre studies testing the efficacy of CRT have transitioned from patients with severe HF to patients with less severe symptoms.2 Also in the latter group, CRT is effective in reducing hard endpoints such as HF hospitalization and mortality. Criteria for QRS width have also decreased over time. In fact, the recent EchoCRT study even (unsuccessfully) tested the efficacy of CRT in patients with isolated mechanical dyssynchrony, but with a narrow QRS complex.3 Remarkably, however, CRT trials have so far been restricted to patients with severe LV dysfunction (LVEF <30–35%). In this issue of the journal, Hai et al. discuss the evidence supporting the use of CRT in patients with higher LVEF and also propose methods for finding the ‘sweet spot’ for CRT implantation.4 CRT efficacy might be diminished if we are too late (lack of contractile reserve) but being too early might expose the patient to unnecessary risk of intervention-related complications if the expected benefit is small. While this review addresses an interesting topic that requires attention, there are a number of steps that need to be taken before we can begin to define the ‘sweet spot’.
Criteria for efficacy
The efficacy of CRT depends on a variety of factors. In addition to selection criteria, optimal delivery of CRT requires optimal lead positioning,5 effective programming,6 careful re-titration of medical therapy, and cardiac rehabilitation.7 It is essential to verify that all these steps have been taken before we assess the individual response. Improvement of functional class is a poor measure of response, as only reverse remodelling of the left ventricle is associated with a reduction of hard endpoints. Current prospective studies will hopefully reveal pre-implantation prognostic parameters that allow better prediction of expected magnitude of reverse remodelling. The team effort of implanter and HF cardiologist could then be assessed on the background of expected response. Currently, an objective response rate is judged in black and white by using a uniform cut-off, e.g. 15% reduction in LV end-systolic volume (LVESV). However, a 16% reduction in LVESV could be considered a poor outcome in a woman with true LBBB and non-ischaemic cardiomyopathy, whereas a 14% reduction of LVESV could be outstanding in a male patient with IVCD and previous myocardial infarction.
Selection criteria
In addition to a need for a change in perception of CRT response, we are also ready for a paradigm change in selecting patients for CRT. Rather than focusing on LVEF or functional class, we probably need to focus on those factors that have been established as predictors of CRT response. In the PROSPECT study, which aimed to include only patients with LVEF <35%, but unintentionally also included those with higher LVEFs, patients with higher LVEF showed similar response rates to those with more severely depressed LVEF.8
What are the best predictors of CRT success? Severe dyssynchrony that is mostly present in true LBBB remains the best and most easily assessable parameter in addition to QRS duration. Dyssynchrony can be aggravated at higher heart rates, but we tend to measure LVEF at rest. The discrepancy between poor functional class and a relatively preserved systolic function could be explained by this phenomenon. Studies have already demonstrated the usefulness of the presence of a contractile reserve in guiding CRT.9 We hypothesize that in patients with a relatively preserved systolic function, the opposite phenomenon could be true. The poor outcome and lack of effective medical treatment of heart failure with preserved ejection fraction (HFpEF) make these patients an attractive target population for alternative therapies even if it might only benefit the subgroup with true LBBB.10 Atrial dyssynchrony is another attractive target for device therapy in patients with HFpEF.11 Correction of chronotropic incompetence has not yet been proven to be efficacious in HFpEF, but is an additional target for treatment that is simple to assess.
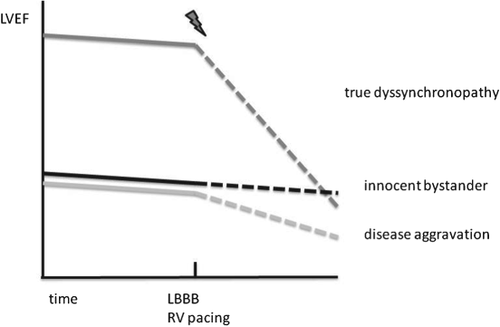
An important criterion for success of CRT is the sequence of events (see Figure 1). If HF is present and QRS is broadening without further decline in cardiac function, LBBB (often atypical in this case) can be an innocent bystander and patients will not improve with CRT. A different scenario is the decline in LV function after the development of LBBB or chronic right ventricular (RV) pacing. Some patients might even have dyssynchrony as the only pathomechanism of heart failure.12 These patients with ‘true dyssynchronopathy’ are often super-responders to CRT, with normalization of LV function even though arrhythmia risk often remains high.13
Feasibility
Can we define the ‘sweet spot’ for CRT? A pragmatic approach is to restrict CRT in mild HF with objectively assessed good functional capacity to patients with true LBBB, rather broad QRS complexes, and severely depressed systolic function. Patients with more severe HF symptoms should undergo objective assessment of functional capacity with cardiopulmonary exercise testing. Further studies have to prove whether adding measurement of systolic function during exercise or stress can predict response to CRT. If there is indeed objectively reduced functional capacity, a differentiated approach could be taken: CRT might be effective in patients with true LBBB and less severely depressed systolic function as well as in those with IVCD and low LVEF. Patients with IVCD or RBBB and HFpEF have a poor chance of response to CRT and it might even be detrimental, as has been shown for patients with a narrow QRS.3
Finally, if future studies confirm the efficacy of CRT in patients with HFpEF and true LBBB, we will need to consider LBBB-associated heart disease as a separate cardiomyopathy and design tools to identify patients with primary electromechanical dyssynchronopathy. If this disease entity exists, these patients may only need a CRT to recover. We might even consider discontinuing their HF medication, but this provocative hypothesis certainly needs further testing before being implemented.
Conflicts of interest: A.H.M. has received lecture fees from Medtronic, Biotronik, Boston Scientific, St. Jude. and Sorin. B.D.W. has no conflicts to declare.




