Measures of left atrial function predict incident heart failure in a low-risk general population: the Copenhagen City Heart Study
Abstract
Aims
This study investigated left atrial (LA) parameters as measured on transthoracic echocardiography as predictors of incident heart failure (HF) in a community cohort.
Methods and results
In a large general population study (n = 2221), participants underwent a health examination with echocardiography. The maximum and minimum LA volumes indexed to body surface area (LAVImax and LAVImin) were measured and the LA emptying fraction (LAEF) and LA expansion index (LAEI) were calculated. Among 1951 participants without atrial fibrillation or significant valve disease, the mean age was 59 ± 16 years and 58% were women. At baseline, 1% (n = 16) had a left ventricular ejection fraction of <50%, 44% had hypertension, and 10% had diabetes. During follow-up (median 15.8 years, interquartile range: 11.3–16.2 years), 187 (10%) participants were diagnosed with incident HF. Participants who were diagnosed with HF during follow-up had a larger LAVImax and LAVImin and a lower LAEF and LAEI compared to participants without HF. In unadjusted analysis, LAVImax, LAVImin, LAEF and LAEI were predictors of incident HF. After multivariable adjustment for clinical and echocardiographic parameters, only LAVImin remained an independent predictor of incident HF (hazard ratio per 1 standard deviation increase: 1.22 [95% confidence interval 1.01–1.47], p = 0.038).
Conclusion
In the general population, LAVImin is an independent predictor of incident HF. LAVImax, currently the only LA measure in a routine echocardiographic examination, was not an independent predictor of incident HF.
Graphical Abstract
Introduction
Globally, heart failure (HF) is a major cause of cardiovascular mortality and morbidity. The prevalence of HF is approximately 1%–2% in the western world and the 5-year mortality rate is as high as 35%.1, 2 Being able to detect subclinical signs of HF prior to development of signs of HF could indicate the need for early treatment that potentially could prevent HF.
In HF, the left ventricle cannot accommodate the cardiac output needed, or can only do so with a significant increase in the left ventricular (LV) filling pressure.3 The left atrium modulates the filling of the left ventricle.4 Changes in left atrial (LA) anatomical structure and function might therefore precede early changes in LV structure and function. These early changes in the left atrium and ventricle might predict future cardiac morbidity, some of which is potentially preventable. Indeed, several studies have demonstrated that LA size as measured on transthoracic echocardiography (TTE) is a strong predictor of atrial fibrillation, ischaemic stroke, HF and cardiovascular death, both in the general population and in selected clinical subpopulations.5-10
Currently, only the LA maximum volume indexed to body surface area (BSA) (LAVImax) is part of a routine echocardiographic examination.11 As mentioned, the relationship between LA anatomical changes and cardiovascular morbidity and mortality is well described, but whether LAVImax is the optimal LA parameter to describe the risk of developing cardiovascular disease, including HF, is not as well investigated.
We hypothesized that measures of LA function may reflect the relationship between the left atrium and ventricle better than the LAVImax, and thus be superior in predicting incident HF. Therefore, we aimed to investigate whether LAVImin, LA emptying fraction (LAEF), and LA expansion index (LAEI) are stronger predictors of incident HF than LAVImax in a low-risk general population. Additionally, we aimed to investigate whether LAVImin, LAEF and LAEI provided valuable prognostic information beyond an established clinical risk score for HF.
Methods
Study population
The study population consisted of the echocardiographic subpopulation of the 4th Copenhagen City Heart Study (CCHS), which is a longitudinal cohort study of cardiovascular risk factors and disease12, 13 (ClinicalTrials.gov Unique Identifier: NCT02993172). The 4th CCHS ran from 2001 to 2003. It was independent of the participants' risk factors and health status whether they were invited for an echocardiography as part of the 4th CCHS. All examinations were performed on the same day. All participants who had an echocardiographic examination, including colour tissue Doppler imaging (TDI), were included in this substudy (n = 2221).13-18 None of the participants had significant valvular stenosis or regurgitation. Participants with prevalent HF (n = 22), atrial fibrillation (n = 43), and inadequate quality of the echocardiographic examination (n = 205) were excluded. After exclusion, LA measures were available in a total of 1951 participants aged 20 to 93 years. The study was performed in accordance with the second Helsinki Declaration and approved by the regional ethics committee. All participants gave written informed consent.
Health examination
All participants underwent physical examinations and answered a self-administered questionnaire. Methods for blood samples and electrocardiogram recording, and the definitions of hypertension, diabetes mellitus, and ischaemic heart disease at baseline have been described elsewhere.17
Heart failure at baseline was defined by previous outpatient visit or hospital admissions for HF, according to the Danish National Patient Registry, using International Classification of Diseases (ICD)-10 codes (DI500-DI509 and DJ819).
Echocardiography
Three experienced sonographers performed echocardiography, using Vivid 5 ultrasound systems (GE Healthcare, Horten, Norway) with a 2.5-MHz transducer. All echocardiograms were conducted as a conventional two-dimensional echocardiography and colour TDI and were stored on magneto-optical disks and an external FireWire hard drive (LaCie, France). Echocardiograms were analysed offline with commercially available software (EchoPac, GE Medical, Horten, Norway).
Conventional echocardiography
Left vntricular ejection fraction (LVEF) was determined by one observer by an evaluation of the regional LV function as assessed by the standard 16-segment model, as suggested by the American Society of Echocardiography.11 LV systolic dysfunction was defined as an LVEF <50%.15, 16 LV mass index (LVMI) was calculated as the anatomic mass divided by BSA. LV hypertrophy (LVH) was defined as LVMI ≥96 g/m2 for women and ≥116 g/m2 for men.11 LV dilatation was defined as an LV internal diameter at end-diastole divided by height ≥3.3 cm/m.11 Global mitral annular plane systolic excursion (global MAPSE) was measured by TDI in the apical 4-, 2- and 3-chamber views. A sample volume was placed at the annular site and MAPSE was then measured by tracking the tissue from the start of the systolic ejection period to the aortic valve closure. Global MAPSE was averaged from all six annular sites. Aortic valve opening and closure were measured by placing a curved M-mode through the mitral leaflet in the colour TDI.
Mitral inflow velocity curves, including peak velocity of early (E) and atrial (A) diastolic filling and deceleration time of the E-wave (DT) were measured by placing a pulsed-wave Doppler sample at the top of the mitral leaflet. The E/A ratio was calculated.
In the 4-chamber view, colour TDI was used to measure the septal and lateral peak early diastolic longitudinal mitral annular velocity (e′). The average e′ was calculated from the septal and lateral e′ and used to calculate E/e′.
Left atrial measures
Left atrial volume was measured using the biplane area–length method in the 2- and 4-chamber apical views. If a 2-chamber apical view could not be visualized, the LA size was traced in a 3-chamber apical view.19 LA volume index was calculated as volume divided by BSA. The LAVImax was traced at the end of ventricular systole, when the chamber was at its largest. Minimum LA volume indexed to BSA (LAVImin) was traced at the end of the ventricular diastole, when the chamber was at its smallest. The LAEF was calculated using the formula: LAEF = (LAVImax- LAVImin)/LAVImax. The LAEI was calculated as LAEI = (LAVImax – LAVImin)/LAVImin. A normal LA volume was defined as LAVImax <34 ml/m2.
All echocardiographic analyses were performed by one investigator, who was blinded to all other information.
Follow-up and outcome
The examinations took place between 2001 and 2003, and participants were followed until outcome data extraction in April 2018 or time of event. Follow-up was 100%. The primary endpoint was defined as time to a hospital contact due to incident HF. Follow-up data on incident HF were obtained from the Danish National Patient Registry, using ICD-10 codes (DI500-DI509 and DJ819). Follow-up data on mortality were obtained from the Danish Civil Registration System.
Statistical analyses
Baseline characteristics for participants were stratified according to the primary endpoint (incident HF) and compared with Student's t-test for continuous Gaussian distributed variables, by a Wilcoxon rank-sum test for continuous non-Gaussian distributed variables, and by Pearson's chi-squared test for proportions and displayed in Table 1. Gaussian-distributed continuous variables are expressed as means ± standard deviation (SD). Non-Gaussian distributed continuous variables are expressed as medians (interquartile range, IQR). Categorical values are expressed as number (percentage).
| All participants (n = 1951) | Participants without HF (n = 1764) | Participants with HF at follow-up (n = 187) | p-value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 59 ± 16 | 58 ± 16 | 69 ± 11 | <0.001 |
| Women | 1125 (58) | 1036 (59) | 90 (48) | 0.005 |
| Clinical parameters | ||||
| Body mass index, kg/m2 | 25.5 ± 3.9 | 25.4 ± 3.9 | 27.0 ± 4.1 | <0.001 |
| Systolic blood pressure, mmHg | 136 ± 23 | 135 ± 23 | 145 ± 21 | <0.001 |
| Diastolic blood pressure, mmHg | 78 ± 12 | 78 ± 12 | 79 ± 13 | 0.27 |
| Heart rate, bpm | 67 ± 11 | 67 ± 11 | 69 ± 12 | 0.022 |
| Hypertension | 838 (44) | 719 (41) | 119 (64) | <0.001 |
| Diabetes mellitus | 189 (10) | 154 (9) | 35 (19) | <0.001 |
| COPD | 40 (2) | 30 (2) | 10 (5) | <0.001 |
| Ischaemic heart disease | 109 (6) | 78 (4) | 31 (17) | <0.001 |
| Ischaemic stroke | 30 (2) | 25 (1) | 5 (3) | 0.18 |
| Smoking status | ||||
| Previous smoker | 643 (34) | 570 (33) | 73 (40) | 0.11 |
| Current smoker | 651 (34) | 592 (34) | 59 (32) | |
| Never smoker | 616 (32) | 567 (33) | 49 (27) | |
| Laboratory results | ||||
| eGFR, ml/min/1.73 m2 | 77 ± 17 | 77 ± 17 | 74 ± 18 | 0.021 |
| Plasma proBNP, pmol/L | 17 (8–30) | 16 (7–29) | 25 (11–45) | <0.001 |
| Total plasma cholesterol, mmol/L | 5.6 ± 1.2 | 5.6 ± 1.2 | 5.5 ± 1.2 | 0.66 |
| Echocardiographic parameters | ||||
| LVEF <50% | 16 (1) | 7 (0.4) | 9 (5) | <0.001 |
| LVMI, g/m2 | 86 ± 22 | 85 ± 21 | 101 ± 27 | <0.001 |
| LV hypertrophy | 277 (17) | 225 (15) | 52 (36) | <0.001 |
| LV dilatation | 91 (5) | 68 (4) | 23 (16) | <0.001 |
| Global MAPSE, mm | 10.6 ± 2.2 | 10.7 ± 2.2 | 9.5 ± 2.2 | <0.001 |
| E wave, cm/s | 7.1 ± 1.6 | 7.1 ± 1.6 | 6.7 ± 1.8 | 0.004 |
| A wave, cm/s | 6.9 ± 1.8 | 6.9 ± 1.8 | 7.6 ± 2.0 | <0.001 |
| E/A ratio | 1.1 ± 0.4 | 1.1 ± 0.4 | 1.0 ± 0.4 | <0.001 |
| DT, ms | 167 ± 41 | 166 ± 40 | 181 ± 49 | <0.001 |
| e′, cm/s | 7 ± 3 | 7 ± 3 | 5 ± 2 | <0.001 |
| E/e′ | 10 (8–13) | 10 (8–12) | 13 (10–17) | <0.001 |
| LA parameters | ||||
| LAVImax, ml/m2 | 19 ± 6 | 19 ± 6 | 21 ± 8 | <0.001 |
| LAVImin, ml/m2 | 9 ± 4 | 9 ± 4 | 12 ± 6 | <0.001 |
| LAEF, % | 51 ± 13 | 51 ± 13 | 46 ± 14 | <0.001 |
| LAEI | 1.1 (0.7–1.5) | 1.1 (0.8–1.5) | 0.9 (0.6–1.3) | <0.001 |
- Values are mean ± standard deviation, n (%), or median (interquartile range).
- COPD, chronic obstructive pulmonary disease; DT, deceleration time; eGFR, estimated glomerular filtration rate; HF, heart failure; LAEF, left atrial emptying fraction; LAEI, left atrial expansion index; LAVImax, left atrial maximum volume indexed to body surface area; LAVImin, left atrial minimum volume indexed to body surface area; LV, left ventricular; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; MAPSE, mitral annular plane systolic excursion; proBNP, pro B-type natriuretic peptide.
Intraobserver and interobserver variability for LAVImax, LAVImin and LAEF was examined in 19 randomly selected participants from this cohort. We calculated coefficients of variation (CVs) and bias coefficients (mean difference ± SD).20
Tests were performed to assess whether clinical variables like age, sex and body mass index (BMI) modified the association between measures of LA structure and function and incident HF.
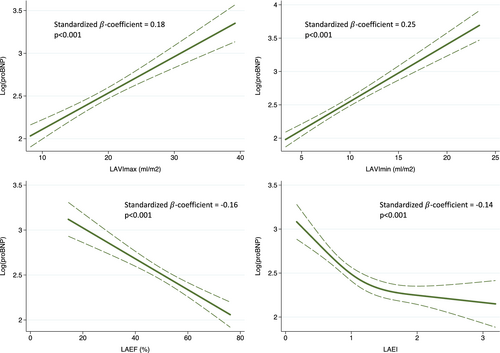
Linear regression analyses were conducted, and standardized β-coefficients were calculated to estimate the association between the natural logarithmic value of pro B-type natriuretic peptide [log(proBNP)] and LA measures (LAVImax, LAVImin, LAEF and LAEI). Restricted cubic spline curves were constructed using linear regression to illustrate the association between log(proBNP) and LA measures and displayed in Figure 1. Number of knots were chosen based on the lowest Akaike Information Criterion.
Hazard ratios (HR) were calculated by Cox proportional hazards regression analyses and displayed in Table 2 and online supplementary Table S1. Harrell's C-statistic was obtained from univariable Cox proportional hazards regression models in order to test the prognostic performance of LA volumes for predicting the endpoint. Missing variables were not imputed. LVEF was included in the multivariable Cox regression models as a dichotomous variable, with the cut-off for normality of LVEF ≥50%. In sensitivity analyses, Cox regression models with participants with LVEF ≥50% and LAVImax <34 ml/m2, were performed. ProBNP was transformed using the natural logarithmic value before being included in the multivariable Cox regression models. The assumptions of proportional hazards in the models were tested based on the Schoenfeld residuals.
| LAVImax | LAVImin | LAEF | LAEI | |||||
|---|---|---|---|---|---|---|---|---|
| HR per 1 SD increase (95% CI) | p-value | HR per 1 SD increase (95% CI) | p-value | HR per 5% decrease (95% CI) | p-value | HR per 10% decrease (95% CI) | p-value | |
| Unadjusted analysis (n = 1951) | 1.48 (1.30–1.68) | <0.001 | 1.70 (1.54–1.89) | <0.001 | 1.17 (1.11–1.23) | <0.001 | 1.09 (1.05–1.12) | <0.001 |
| C-statistic | 0.60 | 0.66 | 0.63 | 0.63 | ||||
| Model 1 (n = 1928) | 1.25 (1.10–1.42) | <0.001 | 1.37 (1.22–1.53) | <0.001 | 1.09 (1.04–1.15) | 0.001 | 1.05 (1.02–1.08) | 0.001 |
| Model 2 (n = 1821) | 1.25 (1.09–1.43) | 0.002 | 1.36 (1.20–1.55) | <0.001 | 1.08 (1.02–1.14) | 0.006 | 1.05 (1.01–1.08) | 0.005 |
| Model 3 (n = 1390) | 1.12 (0.92–1.35) | 0.27 | 1.22 (1.01–1.47) | 0.038 | 1.07 (1.00–1.15) | 0.063 | 1.04 (1.00–1.08) | 0.071 |
- CI, confidence interval; HR, hazard ratio; LAEF, left atrial emptying fraction; LAEI, left atrial expansion index; LAVImax, left atrial maximum volume; LAVImin, left atrial minimum volume; SD, standard deviation.
- Model 1: adjusted for age and sex.
- Model 2: adjusted for model 1, body mass index, estimated glomerular filtration rate, heart rate, systolic and diastolic blood pressure, cholesterol, log(pro B-type natriuretic peptide), baseline hypertension, diabetes, ischaemic heart disease, chronic obstructive pulmonary disease, ischaemic stroke and smoking status.
- Model 3: adjusted for model 2, left ventricular ejection fraction <50%, global mitral annular plane systolic excursion, left ventricular mass index, E/e′, deceleration time and E/A ratio.
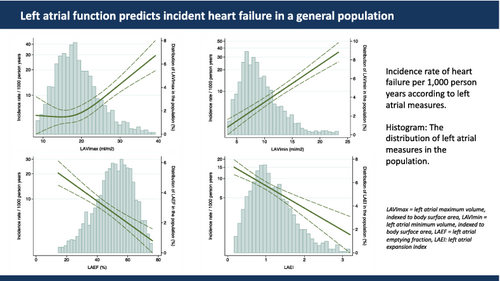
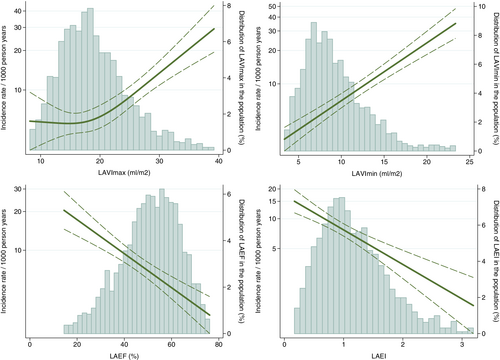
Restricted cubic spline curves were constructed using a Poisson model to estimate incidence rates (IRs) and number of knots were chosen based on the Akaike Information Criterion and displayed in Figure 2.
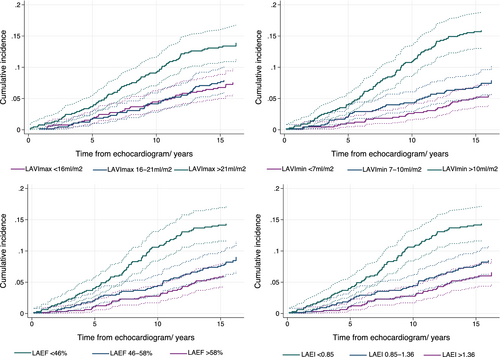
The cumulative incidence function stratified according to tertiles of the explanatory variable were computed, calculating the absolute risk of HF by time from baseline, with death as a competing risk, and displayed in Figure 3.
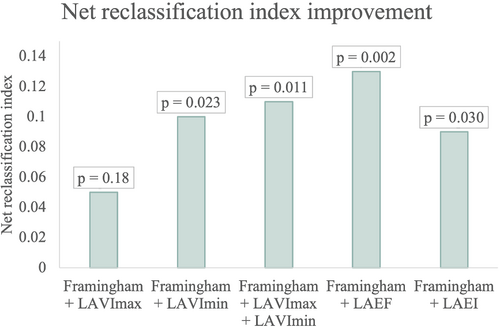
The Framingham ‘Probability of Congestive Heart Failure Within 4 Years for Men Aged 45 to 94 Years’ score and the ‘Probability of Congestive Heart Failure Within 4 Years for Women Aged 45 to 94 Years’ score were calculated for men and women, respectively,21 with a higher score indicating a higher probability for HF. The score includes age, systolic blood pressure, heart rate, LVH on electrocardiogram, and presence of coronary heart disease, valve disease and diabetes mellitus. For women, it also includes BMI.21 If participants were below the age of 45 years, they were considered to be the same age as a 45-year old which constituted 0 points. LVH on electrocardiogram was obtained from Minnesota codes 3.1 and 3.3. LAVImax, LAVImin, LAEF and LAEI were added to the Framingham HF probability score and the net reclassification index (NRI) was calculated by a statistical package in STATA22 to see whether the parameters added prognostic value to the score. The NRI is displayed in Figure 4.
A p-value ≤0.05 in 2-sided test was considered statistically significant.
All analyses were performed with STATA Statistics/Data analysis for Mac, SE 14.0 (StataCorp, College Station, TX, USA).
Results
Among 1951 participants, the mean age was 59 ± 16 years and 58% were women. One percent (n = 16) had a LVEF of <50% at baseline, 44% had hypertension, and 10% had diabetes mellitus.
Median follow-up time was 15.8 years (IQR: 11.3–16.2 years). During follow-up, 187 (10%) participants were diagnosed with incident HF.
Table 1 shows the baseline characteristics of the study population stratified according to whether they experienced an incident HF event during follow-up or not. Participants who developed HF were older, a higher proportion were men, and they had higher BMI. Likewise, participants who developed HF had higher systolic blood pressure, higher heart rate, higher proBNP, lower estimated glomerular filtration rate (eGFR) and a higher proportion had hypertension, diabetes mellitus, previous hospital visits for chronic obstructive pulmonary disease (COPD), and ischaemic heart disease. Regarding the echocardiographic parameters, patients suffering from a HF event during follow-up had lower LVEF, higher LVMI, lower global MAPSE, higher A wave, lower E wave, lower E/A ratio, lower e′, higher E/e′, longer DT, larger LAVImax and LAVImin, lower LAEF and LAEI (Table 1).
In intraobserver analysis, LAVImax and LAVImin both had a CV = 10.7%. LAEF had the lowest intraobserver variability (CV = 9.0%). In interobserver analysis, LAVImax had a lower variability (CV = 24.6%) than LAVImin (CV = 29.3%), with LAEF showing the lowest variability (CV = 19.9%). The bias coefficients were: LAVImax intraobserver: −1.85 ± 4.01 ml/m2; LAVImax interobserver: 1.41 ± 8.79 ml/m2; LAVImin intraobserver: 0.26 ± 1.89 ml/m2; LAVImin interobserver: 0.76 ± 5.12 ml/m2; LAEF intraobserver: −3.25 ± 4.75%; LAEF interobserver: 0.25 ± 10.16%.20
Age and sex did not modify the association between LA measures and HF. BMI significantly modified the association between LAVImax and HF (p = 0.007) as well as LAVImin and HF (p = 0.002) but not the association between LAEF and HF (p = 0.13) or LAEI and HF (p = 0.13). BMI was divided into a low BMI <25 kg/m2 and a high BMI ≥25 kg/m2 (p-value for interaction 0.003 and 0.006 for LAVImax and LAVImin, respectively). For LAVImax and LAVImin the risk for developing HF was significantly higher for a low BMI than a high BMI in univariable Cox regression analyses (LAVImax: HR 1.10, 95% confidence interval [CI] 1.06–1.14, p < 0.001 vs. HR 1.03, 95% CI 1.01–1.06, p = 0.011, for low and high BMI, respectively; LAVImin: HR 1.18, 95% CI 1.13–1.23, p < 0.001 vs. HR 1.10, 95% CI 1.06–1.14, p < 0.001, for low and high BMI, respectively).
Figure 1 illustrates the associations between log(proBNP) and LAVImax, LAVImin, LAEF and LAEI. The association between log(proBNP) and LA parameters was strongest for LAVImin but significant for all LA parameters (p < 0.001). Increasing proBNP was associated with increasing LAVImax and LAVImin and decreasing LAEF and LAEI.
Left atrial function as a predictor of incident heart failure in the general population
In unadjusted Cox regression analysis, LAVImax, LAVImin, LAEF and LAEI were all predictors of incident HF (Table 2). After multivariable adjustment for age and sex (Table 2, model 1) and age, sex, BMI, eGFR, heart rate, systolic and diastolic blood pressure, cholesterol, log(proBNP), hypertension, diabetes mellitus, smoking status, ischaemic heart disease, COPD, ischaemic stroke, (Table 2, model 2), all four measures remained independent predictors of incident HF.
After multivariable adjustment for the aforementioned clinical parameters as well as echocardiographic parameters (LVEF <50%, global MAPSE, LVMI, E/e′, DT, and E/A ratio), only LAVImin, remained an independent predictor of incident HF (Table 2, model 3).
In sensitivity analysis, we restricted the population to only include participants with a normal LVEF, defined as LVEF ≥50% and a normal LA volume, defined as LAVImax <34 ml/m2. Sixteen participants had a LVEF <50%, 52 participants had a LAVImax ≥34 ml/m2, one person had both low LVEF and a dilated left atrium, leaving 1884 participants for this analysis. Similar results were found as for the overall population, with the exception that LAVImax was not a significant predictor of HF in any of the adjusted models (online supplementary Table S1).
The continuous associations between LA measures and risk of HF are shown in Figure 2. For LAVImax, only an increase in LA size beyond 20 ml/m2 was associated with an increased risk of HF, whereas any increase in LAVImin posed an increased risk of HF. Decreasing LAEF and LAEI was also associated with a higher risk of HF. The highest IRs for incident HF for LA measures were found in the highest tertile of LAVImin (IR 13.1 per 1000 person-years), the lowest tertile of LAEF (IR 11.8 per 1000 person-years), the lowest tertile of LAEI (IR 11.8 per 1000 person-years) and the highest tertile of LAVImax (IR 10.6 per 1000 person-years) (p < 0.001 for all comparisons between highest and lowest tertile of each LA measure). The cumulative incidence of HF by these tertiles, while accounting for death as a competing event (n = 522), are shown in Figure 3.
When adding LAVImin, LAEF and LAEI to the Framingham ‘Probability of Congestive Heart Failure Within 4 Years for Men Aged 45 to 94 Years’ score and ‘Probability of Congestive Heart Failure Within 4 Years for Women Aged 45 to 94 Years’ score21 for men and women, respectively, the NRI was significantly improved by 0.10 for LAVImin (p = 0.023), 0.13 for LAEF (p = 0.002) and 0.092 for LAEI (p = 0.030). When LAVImax and LAVImin were added together to the Framingham score, the NRI was improved by 0.11 (p = 0.011). The NRI was not significantly improved by LAVImax (p = 0.18) (Figure 4).
Discussion
In this large prospective study of participants from the general population (n = 1951) undergoing TTE, including an assessment of LA size and function, we found that LAVImin, and measures of LA function, LAEF and LAEI, were independent predictors of incident HF after adjustment for clinical parameters. Only LAVImin continued to be a predictor of incident HF after adjustment for clinical and echocardiographic parameters. When restricting the analysis to only include individuals with a normal conventional echocardiography (normal LVEF and LAVImax), LAVImin, LAEF and LAEI were all independent predictors of incident HF. LAVImax, although currently the only LA parameter included in a standard echocardiographic examination,11 was not an independent predictor after adjustment for clinical and echocardiographic parameters. Additionally, LAVImin, LAEF and LAEI provided incremental prognostic information to an established risk score used to predict incident HF in the general population, as indicated by significant increases in the NRI.
Body mass index significantly modified the association between LAVImax, LAVImin and HF. The risk for developing HF was significantly higher for a low BMI than a high BMI for both LAVImax and LAVImin. Previous studies have shown that the indexed LA size was overcorrected by the BSA in obese patients, why an allometric correction could be considered instead.23
Our hypothesis was that LAVImin, LAEF and LAEI reflect the relationship between the left atrium and ventricle better than LAVImax. In diastole, when the mitral valve is open, the left atrium is directly exposed to the left ventricle. According to the Frank–Starling law, if LV end-diastolic pressure (LVEDP) is continuously increased, secondary changes in the left atrium will develop.24 In a general population, hypertension and other cardiac risk factors such as ischaemic heart disease, diabetes mellitus, atrial fibrillation, obesity, and dyslipidaemia, contribute to an increasingly stiffness in the left ventricle, which increases LVEDP and thus display changes in the left atrium, firstly seen as an increased LAVImin.25 While LAVImin is directly affected by LVEDP, LAVImax is affected by LV systolic function and mean LA pressure. Since mean LA pressure increases later than LVEDP, changes in LAVImax are seen later than changes in LAVImin.26
ProBNP is released from the heart in response to cardiac wall stress when the myocytes stretch.27 In our study, we found that proBNP was more closely associated with LAVImin than LAVImax, suggesting that LAVImin might reflect the endocrine function of the left atrium more closely than LAVImax.
These factors may explain the superior prognostic abilities of LAVImin in this study compared to LAVImax.
Recent studies have proposed how intrinsic factors in the left atrium, such as atrial remodelling, can contribute to the development of HF. Atrial remodelling include chamber enlargement, fibrosis, and arrhythmias (as a sign of electrical remodelling). Especially the development of atrial fibrosis results in LA stiffness.28, 29 LA stiffness was associated with low LAEF in a study examining patients with HF with right heart catheterization. Low LAEF due to LA stiffness was associated with pulmonary vascular disease and right-sided HF,30 that might present with symptoms such as dyspnoea and peripheral oedema. This could explain how LA dysfunction in itself could result in symptoms of HF. LAEI was not reported in the previous study, but as LA stiffness was associated with a decrease in LA diastolic function (during LV systole),30 and LAEI displays the reservoir function, which is part of LA diastole,4 one could speculate that LA stiffness and LAEI were associated with each other.
These factors may contribute to the explanation of the prognostic capabilities of LAEF and LAEI. As LAEF and LAEI can be derived mathematically from each other, it is questionable whether one is superior to the other, and as this study suggests, it seems more important to consider LAVImin than LAEF and LAEI.
To our knowledge, this is the largest study in a general population examining whether LA functional measures, as measured by TTE, are predictors of incident HF. Our results are in accordance with other echocardiographic studies. A community-based study (n = 740) found that LAEF was an independent predictor of a composite outcome, consisting of cardiovascular death, hospitalization for ischaemic heart disease, HF, or stroke. LAVImin was a predictor of the composite outcome in an adjusted model with clinical parameters, but not when echocardiographic parameters were added. LAVImax was not a predictor of the outcome in any of the adjusted models. The study had a shorter follow-up time than ours (median 4.9 years).31 In a population with stable ischaemic heart disease (n = 938) followed for a mean of 7 years, 147 participants were hospitalized due to HF. For a composite endpoint consisting of hospitalizations in a mean follow-up time of 7.3 years, it was shown that LAVImin was a better predictor of HF hospitalizations and cardiovascular outcomes than LAVImax. Also, a composite endpoint of HF hospitalizations, myocardial infarction, stroke, and heart disease death and an all-cause mortality was studied. LAVImin was a better predictor of all three endpoints than LAVImax.32 A study consisting of a population with underlying heart disease (n = 439) showed a significant better prediction for LAVImin than LAVImax of a composite outcome consisting of cardiac death, myocardial infarction, stroke, and admission due to HF, both for two-dimensional and three-dimensional echocardiography.33 A MESA substudy of LA sizes and function measured by magnetic resonance imaging, also revealed that LAVImin and LAEF were better predictors of incident HF than LAVImax.34
Previous studies have shown that LAVImax is a predictor of incident HF.6, 8-10 In this study, we did not find LAVImax to be a predictor of incident HF after adjusting for multiple clinical and echocardiographic parameters. Our study compared to previous studies6, 9, 10 is larger in size, has more events, has longer follow-up time, and accounted for more confounding factors, including clinical, biochemical and echocardiographic parameters. A large general population study (n = 851) with 255 cases of incident HF in people above 65 years, found that LAVImax was a predictor of incident HF, but it did not investigate LAVImin. Also, the study had a shorter follow-up time (3–4 years).8
In an aging population with increasing numbers of incident HF,2 it is important to be able to detect early signs of HF before structural and functional changes in the heart turn into fulminant disease. Being able to detect early signs of HF could implement new study designs for preventive treatment strategies and stratify the prognosis. TTE is an inexpensive, non-invasive, and non-ionizing procedure and can be used among other imaging modalities to measure LA size and function. Based on our findings, we suggest that LAVImin should be included alongside LAVImax in routine echocardiographic examinations. Newer echocardiographic fully automated software automatically quantifies LAVImin and LAVImax and is expected to limit time spent on measuring size and function and improve the reproducibility of the measurements.35 Another LA parameter of interest is LA strain that represents a more novel technique that can directly assess LA reservoir function and has also shown a potential for predicting cardiovascular outcomes in the general population.36 Future studies should aim at comparing volumetric measures of LA structure and function with LA strain to determine whether LA strain adds clinical information beyond volumetric measures of LA structure and function for the prediction of HF, similar to what has recently been reported for atrial fibrillation.37
Strengths and limitations
The large number of participants from a community cohort, and the long follow-up with the resulting high number of incident HF events makes the results found in the present study strong. Due to the prospective design, we were able to collect data on important confounders.
Limitations include that LVEF and LA size were not measured by the Simpson biplane method. As for LA size, the area–length method overestimates LA volume compared to the Simpson biplane method38; however, this method was used to obtain both LAVImax and LAVImin and would therefore not affect the associations found with incident HF. As the echocardiograms and analyses were done before global longitudinal strain (GLS) was widely used, the images were not optimized for strain analysis by speckle tracking. We were not able to estimate GLS for 39% (n = 752) of the participants and because of that, we did not have enough HF events to include GLS in the Cox regression model with echocardiographic data. Instead, we included global MAPSE, which like GLS is a measure of longitudinal LV function.
The information about HF was obtained from Danish nationwide registries and registered as ICD-10 codes. The diagnosis has previously been validated with a positive predictive value of 100% (95% CI 92.9–100), nevertheless using ICD-10 codes is obviously not as precise as adjudicated events.39 The HF diagnosis is not specified as either HF with reduced ejection fraction or HF with preserved ejection fraction.
In sensitivity analysis, the Cox regression model 2 and 3 is on the brink of being overfitted which should be taken into account when interpreted. This can be accepted though, as the focus is not on building prediction models but controlling for confounders.40
Conclusion
The LAVImin is an independent predictor of incident HF in a general population. LAVImax, currently the only LA measurement included in a standard clinical echocardiographic report, was not an independent predictor after adjustment for clinical and echocardiographic parameters. LAVImin, LAEF and LAEI provided incremental prognostic information to an established risk score for prediction of incident HF in the general population.
Acknowledgments
The authors would like to sincerely thank our late Professor Jan Skov Jensen, MD, PhD, DMSc for his immense contribution to the Copenhagen City Heart Study.
Funding
This work was supported by Herlev & Gentofte Hospital's internal funds (grant received by DMA). The sponsors had no role in the study design, data collection, data analysis, data interpretation, or writing of the manuscript.
Conflict of interest: D.M.A. has received consulting fees from Ambu. E.P. has received research grants from NIH. Her employer has received support from Novartis for consulting outside the submitted work. She has consulted for scPharmaceuticals outside the submitted work. T.B.S. has received research grants from Sanofi Pasteur and GE healthcare, consulting fees from Amgen, Sanofi Pasteur and lecture fees from Novartis and Sanofi Pasteur. All other authors have nothing to disclose.