Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure
Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association
This article is co-published in Journal of Cardiac Failure and European Journal of Heart Failure.
Abstract
In this document, we propose a universal definition of heart failure (HF) as a clinical syndrome with symptoms and/or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion. We also propose revised stages of HF as: At risk for HF (Stage A), Pre-HF (Stage B), Symptomatic HF (Stage C) and Advanced HF (Stage D). Finally, we propose a new and revised classification of HF according to left ventricular ejection fraction (LVEF). This includes HF with reduced ejection fraction (HFrEF): symptomatic HF with LVEF ≤40%; HF with mildly reduced ejection fraction (HFmrEF): symptomatic HF with LVEF 41–49%; HF with preserved ejection fraction (HFpEF): symptomatic HF with LVEF ≥50%; and HF with improved ejection fraction (HFimpEF): symptomatic HF with a baseline LVEF ≤40%, a ≥10 point increase from baseline LVEF, and a second measurement of LVEF > 40%.
Abbreviations and acronyms
-
- ACC
-
- American College of Cardiology
-
- AHA
-
- American Heart Association
-
- ARVC
-
- arrhythmogenic right ventricular cardiomyopathy
-
- BNP
-
- B-type natriuretic peptide
-
- CKD
-
- chronic kidney disease
-
- EF
-
- ejection fraction
-
- eGFR
-
- estimated glomerular filtration rate
-
- EHR
-
- electronic health record
-
- ESC
-
- European Society of Cardiology
-
- GDMT
-
- guideline-directed management and therapy
-
- HFA
-
- Heart Failure Association
-
- HF
-
- heart failure
-
- HFimpEF
-
- heart failure with improved ejection fraction
-
- HFpEF
-
- heart failure with preserved ejection fraction
-
- HFmrEF
-
- heart failure with mildly reduced ejection fraction
-
- HFrEF
-
- heart failure with reduced ejection fraction
-
- HFrecEF
-
- heart failure with recovered ejection fraction
-
- HFSA
-
- Heart Failure Society of America
-
- JHFS
-
- Japanese Heart Failure Society
-
- LVEF
-
- left ventricular ejection fraction
-
- MI
-
- myocardial infarction
-
- NT-proBNP
-
- N-terminal pro-B-type natriuretic peptide
-
- NYHA
-
- New York Heart Association
-
- SGLT2
-
- sodium–glucose co-transporter 2
-
- WHO
-
- World Health Organization
Summary of key points
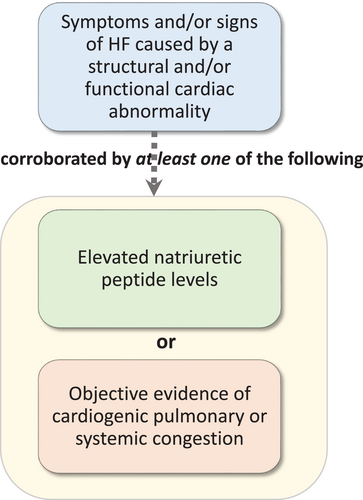
- In this document, we propose a universal definition of heart failure (HF) as the following: HF is a clinical syndrome with symptoms and/or signs caused by a structural and/or functional cardiac abnormality and corroborated by elevated natriuretic peptide levels and/or objective evidence of pulmonary or systemic congestion.
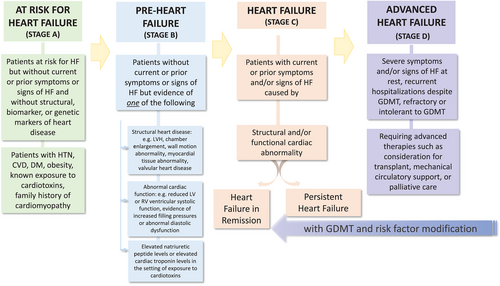
- We propose revised stages of HF as: At risk for HF (Stage A), for patients at risk for HF but without current or prior symptoms or signs of HF and without structural or biomarker evidence of heart disease. Pre-heart failure (Stage B), for patients without current or prior symptoms or signs of HF but evidence of structural heart disease or abnormal cardiac function, or elevated natriuretic peptide levels. HF (Stage C), for patients with current or prior symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality. Advanced HF (Stage D), for patients with severe symptoms and/or signs of HF at rest, recurrent hospitalizations despite guideline-directed medical therapy (GDMT), refractory or intolerant to GDMT, requiring advanced therapies such as consideration for transplant, mechanical circulatory support, or palliative care.
- Finally, we propose a new and revised classification of HF according to left ventricular ejection fraction (LVEF). The classification includes HF with reduced ejection fraction (HFrEF): HF with LVEF ≤40%; HF with mildly reduced ejection fraction (HFmrEF): HF with LVEF 41–49%; HF with preserved ejection fraction (HFpEF): HF with LVEF ≥50%; and HF with improved ejection fraction (HFimpEF): HF with a baseline LVEF ≤40%, a ≥10 point increase from baseline LVEF, and a second measurement of LVEF >40%.
Preamble
Currently available definitions of heart failure (HF) are ambiguous and lack standardization.1-8 Some definitions focus on the diagnostic features of the clinical syndrome,3-5 whereas other definitions approach the definition as a characterization of the haemodynamic and physiological aspects.2, 8 There is significant variation in different platforms,1-5 and a growing need for standardization of the definition of HF.6, 9
A universal definition of HF is of critical importance to clinicians, investigators, administrators, health care services, institutions, legislators and payers alike. The increasing prevalence and burden of HF,10, 11 an increased recognition of growing health care disparities12 and deficiencies in the optimal treatment of HF with guideline-directed management and therapy (GDMT) strategies,13, 14 all underline the necessity for a universally recognizable definition of HF. Evolving evidence for new effective preventive and treatment strategies in HF will require clarity in the different stages and/or ejection fraction (EF) subgroups of HF,15, 16 along with an increased emphasis on performance measures with a need for accuracy in patient diagnoses and treatment indications17-19; a need for improved communication and understanding of the definition of HF with patients and for shared decision-making and transitions of care between different levels of care and health care professionals3; and an increased recognition and emphasis of standard diagnoses and endpoints in the settings of research, clinical trials and registries.20, 21
The objectives of this document are to provide a universal definition of HF that is clinically relevant, simple but conceptually comprehensive, with the ability to sub-classify and to encompass stages within, with universal applicability globally, and with prognostic and therapeutic validity and acceptable sensitivity and specificity. We envision the proposed universal definition and classifications to be used in a standardized fashion across scientific societies and guidelines, employed by clinicians and used in research studies.
1 Methodology
1.1 Writing committee composition
The Heart Failure Society of America (HFSA), the Heart Failure Association (HFA) of the European Society of Cardiology (ESC) and the Japanese Heart Failure Society (JHFS) selected the members of the writing committee. The writing committee consisted of 38 individuals with domain expertise in HF, cardiomyopathy and cardiovascular disease.
1.2 Consensus development
On 20 August 2020, in response to the necessity for consensus for definition of HF, the HFSA, HFA/ESC and JHFS convened a virtual consensus conference to develop a universal definition of HF with participation from 14 different countries and 6 continents. The work of the writing committee was accomplished via a series of teleconference and web conference meetings, along with extensive email correspondence. The review work was distributed among subgroups of the writing committee based on interest and expertise. The proceedings of the workgroups were then assembled, resulting in the proposed universal definition. All members reviewed and approved the final vocabulary.
1.3 Peer review and approval
The 2020 Universal Definition of HF was reviewed by official reviewers nominated by the HFSA, HFA/ESC and JHFS. The writing committee anticipates that the proposed definition and classification will require review and updating in the same manner as other published universal definitions.22 The writing committee, therefore, plans to review the universal definition on a periodic basis, starting with the anniversary of publication of the definition, to ascertain whether modifications should be considered.
2 Current definitions of heart failure
Heart failure is a clinical syndrome with different aetiologies and pathophysiology rather than a specific disease. This makes defining HF more complex than diseases that have a pathologic gold standard for diagnosis such as cancer. Not surprisingly, definitions of HF vary widely in the medical literature, in contemporary guidelines, and in medical practice. Differing definitions have been developed for different purposes, ranging from ‘textbook’ definitions of HF, which are typically focused on pathophysiology, to case definitions such as the Framingham criteria23 that are primarily used in research. The traditional textbook definition of HF, which is usually defined as a ‘condition in which the heart cannot pump enough blood to meet the body's needs’1 or ‘abnormality of cardiac structure or function leading to failure of the heart to deliver oxygen at a rate commensurate with the requirements of the metabolizing tissues’,2 is a complex and impractical definition that often cannot be verified in practice and apply to only a certain subgroup of patients with HF. As such, in a study of patients with advanced HF awaiting left ventricular assist device implantation, cardiac output was shown to be insufficient to meet the metabolic needs of the body only in 25% of these patients with advanced HF at rest, demonstrating the inadequacy of such definitions in the majority of the HF population.24 In clinical care, other diagnostic criteria such as measurement of plasma natriuretic peptides play an important role in clarifying the diagnosis of HF.3-5 A summary of contemporary definitions of HF from the American College of Cardiology/American Heart Association (ACC/AHA), HFA/ESC, and JHFS guidelines is provided in Table 1. Although the definitions of HF used in current practice guidelines from the ACC/AHA,3 HFA/ESC,4 and JHFS5 differ in some details, they share the following common elements: they identify HF as a clinical syndrome, i.e. a recognizable cluster of signs and symptoms; they require the presence of at least some of the cardinal symptoms of HF including dyspnoea, fluid retention/oedema, fatigue, activity intolerance and exercise limitation; and they require some form of structural or functional heart disease. Some also specify a reduced cardiac output and/or elevated intra-cardiac pressures at rest or during stress.4 Overall, the existing definitions of HF comprise three elements: evidence of structural heart disease, a history of symptoms that are commonly reported in HF and objective signs commonly seen in HF.
| ACCF/AHA (2013)3 | HF is a complex clinical syndrome that results from any structural or functional impairment of ventricular filling or ejection of blood. The cardinal manifestations of HF are dyspnoea and fatigue, which may limit exercise tolerance, and fluid retention, which may lead to pulmonary and/or splanchnic congestion and/or peripheral oedema. Some patients have exercise intolerance but little evidence of fluid retention, whereas others complain primarily of oedema, dyspnoea, or fatigue. |
| ESC (2016)4 | HF is a clinical syndrome characterized by typical symptoms (e.g. breathlessness, ankle swelling and fatigue) that may be accompanied by signs (e.g. elevated jugular venous pressure, pulmonary crackles and peripheral oedema) caused by a structural and/or functional cardiac abnormality, resulting in reduced cardiac output and/or elevated intracardiac pressures at rest or during stress. |
| JCS/JHFS (2017)5 | HF is a clinical syndrome consisting of dyspnoea, malaise, swelling and/or decreased exercise capacity due to the loss of compensation for cardiac pumping function due to structural and/or functional abnormalities of the heart. |
- ACCF/AHA, American College of Cardiology Foundation/American Heart Association; ESC, European Society of Cardiology; HF, heart failure; JCS, Japanese Circulation Society; JHFS, Japanese Heart Failure Society.
2.1 Definitions of heart failure used in current clinical trials and registries
The definitions and inclusion criteria used in clinical trials and registries in HF differ from those in clinical practice, guidelines and textbooks. Most trials in HF with reduced EF (HFrEF) (Table 2),25-29 and in HF with preserved EF (HFpEF) (Table 3)30-33 reflect inclusion criteria that usually include a left ventricular EF (LVEF) threshold, an established HF diagnosis with specific New York Heart Association (NYHA) functional class categories, certain levels of natriuretic peptides and may sometimes include a requirement of past HF hospitalizations, depending on the severity of HF targeted for the trial. HFpEF studies also may include corroborative evidence by imaging reflecting structural and/or functional changes. Nonetheless, a number of gaps remain in standardizing the criteria for clinical trials. These include sensitivity and specificity of diagnostic criteria for HF, establishing standardized natriuretic peptide criteria; the complexity of additional requirements to ascertain the diagnosis of HF; challenges with HFpEF including multiple comorbidities that are often excluded in clinical trials, how to handle patients with EF recovery or changes in clinical trajectory, competing diagnoses that may mimic findings of HF and the generalizability of the trial criteria to the ultimately intended treatment population. It is also important to distinguish between clinical trial inclusion criteria that aim to select target populations, from clinical trial endpoint definitions that facilitate measurement of outcomes secondary to the disease process. For example, natriuretic peptides, which are commonly used in entry criteria in HF trials, are not commonly required for clinical endpoint definitions.21
| Trial | Age, NYHA class | LVEF | Natriuretic peptides | HF hospitalization or other |
|---|---|---|---|---|
| PARADIGM-HF25 | Age ≥18 years NYHA II–IV | LVEF <35% |
If previous hospitalization, BNP ≥100 pg/mL or NT-proBNP ≥400 pg/mL If no previous hospitalization, BNP ≥150 pg/mL or NT-proBNP ≥600 pg/mL |
Within previous 12 months |
| VICTORIA26 | Age ≥18 years NYHA II–IV | LVEF <45% |
Within past 30 days: NSR, BNP >300 pg/mL, NT-proBNP >1000 pg/mL AF, BNP >500 pg/mL, NT-proBNP >1600 pg/mL |
Within 6 months or outpatient IV diuretics for HF within 3 months |
| DAPA-HF27 |
Age ≥18 years NYHA II–IV |
LVEF ≤40% |
If HF hospitalization within 12 months: NT-proBNP ≥400 pg/mL If no hospitalization, NT-proBNP ≥600 pg/mL AF, NT-proBNP ≥900 pg/mL |
Diagnosis of HF for at least 2 months |
| EMPEROR-Reduced28 |
Age ≥18 years NYHA II–IV |
LVEF ≤40% |
LVEF ≤30%, NT-proBNP ≥600 pg/mL (NSR) or ≥1200 pg/mL in AF LVEF 31–35%, NT-proBNP ≥1000 pg/mL (NSR) or ≥2000 pg/mL in AF LVEF 36–40%, NT-proBNP ≥2500 pg/mL (NSR) or ≥5000 pg/mL in AF LVEF <40% but HF hospitalization within 12 months, NT-proBNP ≥600 pg/mL (NSR) or ≥1200 pg/mL in AF |
NYHA class II–IV for at least 3 months |
| GALACTIC-HF29 |
Age ≥18 and <85 years NYHA II–IV |
LVEF ≤35% |
NT-proBNP ≥400 pg/mL (NSR) or ≥1200 pg/mL in AF; or BNP ≥125 pg/mL (NSR) ≥375 pg/mL |
Currently hospitalized for HF (inpatients) or had either made an urgent visit to the emergency department or been hospitalized for HF within 12 months (outpatients) |
- AF, atrial fibrillation; BNP, B-type natriuretic peptide; HF, heart failure; IV, intravenous; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; NSR, normal sinus rhythm.
| Trial | Age, NYHA class | LVEF | Natriuretic peptides | HF hospitalization |
|---|---|---|---|---|
| TOPCAT30 | Age ≥50 years NYHA II–IV | LVEF ≥45% |
BNP ≥100 pg/mL or NT-proBNP ≥360 pg/mL |
Within previous 12 months, with management of HF as a major component |
| PARAGON-HF31 | Age ≥50 years NYHA II–IV | LVEF ≥45% and LAE LVH |
If NSR, NT-proBNP >200 pg/mL If AF: >600 pg/mL or If no previous hospitalization and If NSR: NT-proBNP >300 pg/mL If AF: NT-proBNP >900 pg/mL |
Within previous 9 months |
| EMPEROR-Preserved32 |
Age ≥18 years NYHA II–IV (at least 3 months) |
LVEF >40% (no prior history of LVEF ≤40%) | NT-proBNP >300 pg/mL in NSR or >900 pg/mL in AF | Within 12 months OR evidence of structural changes (LAE or increased LVM) on echo |
| DELIVER33 |
Age ≥40 years NYHA II–IV |
(LVEF >40% and evidence of structural heart disease (i.e. LAE or LVH) | Elevated natriuretic peptides | Medical history HF ≥6 weeks before enrolment with at least intermittent need for diuretic treatment |
- AF, atrial fibrillation; BNP, B-type natriuretic peptide; HF, heart failure; LAE, left atrial enlargement; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; LVM, left ventricular mass; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; NSR, normal sinus rhythm.
2.2 Gaps in current definitions of heart failure
2.2.1 Combined definition with haemodynamic characterization of heart failure
The current definitions that include a haemodynamic characterization such as the HFA/ESC definition which defines HF as ‘a clinical syndrome characterized by typical signs and symptoms, caused by a structural and/or functional cardiac abnormality, resulting in a reduced cardiac output and/or elevated intra-cardiac pressures at rest or during stress’,4 have the following limitations: while accurate, this type of definition is hard to apply in public health or epidemiological settings, because of the subjectivity of the symptoms counterbalanced by the unfeasibility (invasive) or unreliability of measurements of cardiac output or filling pressures. For a definition to be also useful for the non-specialist, it should be assessable easily and with relatively low inter-observer variability. The Framingham criteria, which were developed for just such a purpose,23 are now considered insufficiently specific for adoption as a definition of HF in the contemporary setting.
2.2.2 Cardiomyopathy and heart failure
A key distinction that has led to persistent confusion in many discussions of the definition of HF is that between the concepts of ‘heart failure’ and ‘cardiomyopathy.’ As defined elsewhere in this document, HF is a clinical syndrome, that is, a recognizable pattern of signs and symptoms. ‘Cardiomyopathy’ is a term, itself with widely differing definitions, that describes features of structural and functional heart muscle dysfunction. These different definitions may lead to potential confusion. In clinical practice, the term ‘cardiomyopathy’ is often used as a more general term encompassing types of cardiac dysfunction, which may be further qualified with the underlying cause (e.g. ischaemic cardiomyopathy, non-ischaemic cardiomyopathy, etc.). Alternatively, cardiomyopathy may be understood to be a specific form of myocardial disease that excludes forms of HF with a clearly established cause (such as ischaemic heart disease). Even guideline statements from various scientific bodies have varied in their use of this term.34, 35 Furthermore, the maladaptive haemodynamic and compensatory mechanisms in HF may result in development of or worsening of cardiomyopathy.36 Classification systems have been proposed that attempt to incorporate both the classification of HF and cardiomyopathy into a unified system, most notably the proposed MOGES criteria (Morpho-functional phenotype-M, organ(s) involvement-O, genetic inheritance pattern-G, etiological annotation-E including genetic defect or underlying disease/substrate, and the functional status-S), but these have not been widely adopted due to their complexity.37 HF encompasses a broader spectrum of cardiac disorders, not only cardiomyopathies that could be an underlying cause of the HF syndrome. In this statement, we do not provide specific classification strategies for cardiomyopathies, which we believe to be outside the scope of this document.34
2.2.3 Biomarkers in the definition of heart failure
Natriuretic peptides such as B-type natriuretic peptide (BNP) and N-terminal pro-B-type natriuretic peptide (NT-proBNP) are elevated in most forms of HF and are an integral component of making a diagnosis of HF in many clinical settings, especially when the diagnosis is uncertain.3-5, 38 The use of these biomarkers has the highest class of recommendation to support a diagnosis or exclusion of HF4, 38 in contemporary practice guidelines, but are notably absent from most definitions of HF. This is in contrast with the universal definition of myocardial infarction (MI), where elevations of a circulating biomarker (troponin) are both central to the clinical diagnosis and fundamental to the universal definition itself.22 Although a biomarker-based approach has incremental diagnostic value, especially in the context of clinical uncertainty, in some communities with limited resources, natriuretic peptide measurements currently may not be readily available, but their availability is rapidly increasing, and natriuretic peptide measurements are becoming part of standard care. Furthermore, certain clinical conditions other than HF, such as chronic kidney disease (CKD), atrial fibrillation, pericardial disease, pulmonary embolism, and even aging can also result in an increase in natriuretic peptide levels, and obesity is associated with lower natriuretic peptide levels, underlining the importance of an individualized interpretation of biomarker levels, particularly in special populations and in the setting of competing diagnoses and comorbidities. It is important to recognize that though measuring natriuretic peptide levels may improve diagnostic accuracy and guide risk stratification in patients with HF, in certain patients with HF, such as patients with HFpEF or obesity, natriuretic peptide levels can be lower than those with HFrEF (though usually higher than those without HF); this may complicate their use for diagnosis and prognosis. Differences according to race/ethnicity, sex and age will need to be taken into consideration in their interpretation and different thresholds are commonly used for patients with atrial fibrillation, a very common comorbidity in HF that can lead to increased natriuretic peptide levels. A potential influence of comorbidities is also relevant for troponin interpretation in patients with suspected acute MI; however, despite similar limitations, the introduction of a quantitative biomarker element to a disease definition has improved the accurate classification of disease states and proven to be of value in MI and other diseases.22, 39 In general, both BNP and NT-proBNP values track similarly, and either can be used in patient care settings as long as their respective absolute values and cut-points are not used interchangeably. Notably, BNP, but not NT-proBNP, is a substrate for neprilysin. Angiotensin receptor–neprilysin inhibitor may result in an increase in BNP levels, but not NT-proBNP levels.38 Furthermore, patient-level changes need to be interpreted according to baseline levels; natriuretic peptides are higher during periods of decompensation compared with compensated periods, reflecting dynamic temporal changes.
2.2.4 Clinical and research aspects of defining heart failure
Clinical research requires standardized definitions for identifying cases of HF and the collection of endpoints of interest, including especially HF-related hospitalizations.21 Given the increased use of electronic health records (EHR) as research tools, there is growing interest in the use of computer algorithms to identify cases of HF from EHR data for research purposes. Although classical signs and symptoms are often included in EHR data, they may not be codified as discrete data fields, leading to increased interest in the use of machine learning techniques to identify cases.40 Definitions of HF are important not only for clinical practice or research entry criteria, but also for the generalizability of research findings to the HF population, uniformity in endpoints of clinical trials; reliability and appropriateness of data captured in clinical, administrative and billing registries and performance measures.
2.2.5 Patient and clinician perspective
A syndrome that is based solely on symptoms can be confusing for clinicians and patients, both because they are often not specific to a single disease (e.g. fatigue and dyspnoea) and because they are highly subjective, for example with the same objective limitation being considered disabling by one person and perceived as being normal for age by another. Once diagnosed, and with effective therapy, patients may become asymptomatic (NYHA class I); however, structural, cellular and molecular abnormalities may continue to worsen silently.41 Although Stage C HF uses the wording ‘current or previous symptoms’ in the definition, patients may believe that lack of signs and symptoms equates to ‘being out of HF’, and be less likely to adhere to care.42 Health care professionals may be less likely to optimize GDMT when symptoms are mild or absent.43 Removing the word ‘congestive’ in the term HF was an important reminder to providers that there is a range of signs and symptoms once diagnosed. Further, patients may not understand or recognize when HF worsens, until symptoms are severe enough to prompt emergency care.44 In the era of shared decision-making and patient understanding of chronic conditions, it will be important to acknowledge and incorporate different stages that are understandable by patients after diagnosis.
2.2.6 Competing diagnoses
There are many conditions that may mimic HF, either in isolation (mimicry) or when co-existing with HF (co-causative). The combination of acute dyspnoea, hypervolaemia and cardiorenal syndrome is often labelled as HF in an emergency care setting, although the problem could be confounded by, or even be predominantly due to, anaemia and iron deficiency. Recognizing proportionate contributions of a clinical picture, to dissect out the element that is specifically HF-related, will be an important part of establishing a HF diagnosis, and it may not be an easy differentiation to make in all situations. It is HF only if the cardiac component is considered ‘important’. However, it is also important to recognize that HF can co-exist with other diagnoses. For example, HF syndromes with lesser degrees of systolic impairment such as HFpEF frequently present with a wide range of cardiac and non-cardiac abnormalities.45 Newer, sometimes inconsistent terminology regarding mildly reduced EF has further complicated subcategorization of HF. It is important to promote greater clarity and specificity in the diagnosis of HF.
3 Current classifications of heart failure
An important part of defining HF is that of creating a ‘usable’ classification scheme. There are a variety of classification frameworks in current use that attempt to define distinct subsets of HF (Table 4).3, 4, 37, 46, 47 Some of these, such as NYHA class and EF categories, have been subsequently used as entry criteria for clinical trials, resulting in their incorporation into product labelling and guideline recommendations about which patients should receive a given therapy.3-5 Others, such as classifying patients by HF aetiology, may have important implications for prognosis or differential response to therapy.48
| NYHA class3 | I, II, III, IV based on symptom severity |
| Ejection fraction4 | HFrEF, HFmrEF, HFpEF based on left ventricular ejection fraction |
| Aetiology34 | Specific aetiology of HF, e.g. ischaemic/non-ischaemic, valvular, hypertensive, infiltrative cardiomyopathy such as cardiac amyloidosis, peripartum cardiomyopathy, viral myocarditis, chemotherapy-induced cardiomyopathy |
| Disease progression (ACCF/AHA)3, 46 | Stages A, B, C, D according to presence of HF symptoms and signs and cardiac structural changes |
| MOGES37a | Morpho-functional phenotype (M), organ(s) involvement (O), genetic inheritance pattern (G), etiological annotation (E) including genetic defect or underlying disease/substrate, and the functional status (S) |
| INTERMACS profiles of advanced HF47 | Profiles 1 through 7 according to symptoms, functional capacity, haemodynamic stability for patients who are considered for advanced HF therapies |
- ACCF/AHA, American College of Cardiology Foundation/American Heart Association; HF, heart failure; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support; NYHA, New York Heart Association.
- aMOGE(S) nosology system.
3.1 Current subclassification of heart failure according to ejection fraction and its limitations
Because clinical trial inclusion criteria, and hence evidence of benefit, have often been restricted to patients with a reduced EF, HF has traditionally been subcategorized according to EF when defining recommended treatments in clinical practice guidelines.3-5All guidelines use the terminology of HFrEF and HFpEF (Table 5) but differ in the terminology used in patients with EFs between 40% and 49%. The 2013 ACC/AHA guidelines have used the terminology of HFpEF-borderline for patients with EF between 41–49%, and HFpEF-improved for those whose EF improved from a lower level to an EF of >40% under the subgrouping of patients with HFpEF.3 The HFA/ESC and JHFS guidelines have defined a third category of HF with mid-range EF (HFmrEF) or mildly reduced EF for those with an EF of 41% to 49%.4, 5 The concept of HFmrEF is not necessarily accepted by all guidelines.49
| Society | HF classification according to LVEF | LVEF | Additional requirements |
|---|---|---|---|
| ACCF/AHA3 |
|
≤40% | Symptoms and signs |
|
≥50% | Symptoms and signs | |
| a) HFpEF, borderline | 41–49% | Symptoms and signs | |
| b) HFpEF, improved | >40% | Symptoms and signs | |
| ESC4 |
|
<40% | Symptoms and signs |
|
40–49% | Symptoms and signs, elevated levels of natriuretic peptides and at least one additional criterion of relevant structural heart disease (LVH or LAE) or diastolic dysfunction | |
|
≥50% | Symptoms and signs, elevated levels of natriuretic peptides and at least one additional criterion of relevant structural heart disease (LVH or LAE) or diastolic dysfunction | |
| JCS/JHFS5 |
|
<40% | |
|
40% to <50% | ||
|
≥50% | ||
|
≥40% |
- ACCF/AHA, American College of Cardiology Foundation/American Heart Association; ESC, European Society of Cardiology; HF, heart failure; JCS, Japanese Cardiology Society; JHFS, Japanese Heart Failure Society; LAE, left atrial enlargement; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy.
In an effort, through a public-private partnership with the US Food and Drug Administration (FDA) and with an intent to standardize terminology and LVEF cut-points used in US clinical trials, the Heart Failure Collaboratory and Academic Research Consortium proposed the following definitions and EF ranges as their most recent recommendations: (i) HFrEF: HF with LVEF ≤40%; (ii) HFpEF: HF with LVEF ≥50%; (iii) HFmrEF: HF with LVEF >40% and LVEF <50%.50
The dichotomization of LVEF of above or below, for example, 40% has been helpful to apply therapies that have been shown to work in patients with reduced EF. Further classification into HFmrEF has potential utility as well as challenges due to its ambiguity, uncertainty and dynamic trajectory.15, 51 Post-hoc analyses of certain HF trials have suggested that standard therapy for HFrEF may be effective and extended to patients with HFmrEF,52-55 but meta-analyses report diverse findings with neurohormonal antagonism in patients with HFmrEF, specifying benefit in certain subgroups and underlining heterogeneity of this category15, 55-57 The characteristics of HFmrEF overlap with HFrEF and HFpEF, straddling either category, sometimes one more than the other depending on the clinical circumstance or patients studied.15 In population-based studies, usually without exclusions of specific aetiologies, HFmrEF comprises 10–20% of the HF population,54, 58 resembles the HFrEF group, but with similar57 or better survival than HFrEF patients.15, 58 Although some patient characteristics of HFmrEF are just between those of HFrEF and HFpEF, the prognosis of patients is not necessarily related to EF,59 and the relation between mortality and BNP is not affected by EF.59, 60 In many patients, HFmrEF reflects a transitional trajectory for a dynamic temporal change; either to improvement or recovery from HFrEF,57, 61 or to deterioration to HFrEF.15, 57, 61, 62 Although HFrEF and HFpEF have different clinical spectrums and proposed pathophysiological mechanisms, there is no clear defining syndrome recognized or postulated for HFmrEF. It is likely that patients in this range may have aetiologies that are similar to those in lower or higher LVEF groups, and may be in transition from higher to lower LVEF or vice versa. Persistent HFmrEF can be seen in some patients, including heterogeneous aetiologies such as those with ischaemic, infiltrative, restrictive or hypertrophic cardiomyopathies.57, 61, 62 Therefore, lower than a normal EF does not necessarily represent one phenotype and does not always entail the maladaptive deleterious mechanisms seen in patients with HFrEF. Furthermore, patients with restrictive, infiltrative and hypertrophic cardiomyopathies, who may have HFmrEF, have traditionally been excluded from some clinical trials, emphasizing the necessity to focus on aetiology rather than LVEF. The prevalence of HFmrEF, without overlap of other categories, has posed a major challenge for recruitment in trials, resulting in termination due to enrolment futility63 and in some clinical trials and epidemiological studies, patients with LVEF 40–49% have been categorized as HFpEF.
Another criticism is the accuracy of the measurement of EF in clinical practice. Echocardiography is widely used to assess EF in patients with cardiovascular diseases, but the inter-observer and intra-observer variability are not small enough to allow precise quantification of differences in one integer place values such as 39% vs. 41%. Although other cardiovascular imaging modalities can be used to assess EF, there is substantial variation between modalities as well.64 Furthermore, EF is not a reliable measure of contractile performance, is load-dependent, and can vary according to haemodynamic status and loading conditions. Other imaging modalities such as global longitudinal strain are evolving to better characterize the ventricle, structural abnormalities, contractile performance, reverse remodelling, response to therapy, and will likely expand the structural phenotyping beyond EF.
Finally, the trajectory of EF over time in addition to a single absolute value of EF, and severity of left ventricular dysfunction even among HFrEF may need to be taken into account to further classify patients with HF. Despite all these reservations, classification by EF has proven to be clinically and epidemiologically useful.
3.2 Current classification according to stages of heart failure and its limitations
The ACC/AHA stages are categorized as Stage A, patients at high risk for HF but without structural heart disease or symptoms of HF; Stage B, structural heart disease but without signs or symptoms of HF; Stage C, structural heart disease with prior or current symptoms of HF; Stage D, refractory HF requiring specialized interventions..3, 4, 46 The original ACC/AHA definition of stages of HF46 has been ubiquitously adapted throughout other HF guidelines globally.3-5 Although these stages of HF are well recognized amongst health care professionals, they are not standard nomenclature for general practitioners, patients, payers, or among the literature or education platforms provided by patient advocacy groups. Patients living with HF are less likely to identify with stages of HF in comparison to the familiarity with EF and subjective symptom burden. Contemporary clinical trials have not enrolled or randomized based on stages of HF and most treatment strategies are not guided by the stages in HF.
The ACC/AHA stages are based on symptoms and the presence or absence of structural heart disease and are applicable to both HFrEF and HFpEF. Certainly, there are prognostic nuances that are missed in such a broad staging classification, and opinions also vary as to whether those individuals solely identified with risk factors should be labelled as having a disease state, especially given that they have risk factors for many different diseases (not just HF risk factors). In comparison, classification schemas such as the Society for Cardiovascular Angiography and Interventions (SCAI) cardiogenic shock stages65 classified their stages based on detailed parameters of laboratory values, and haemodynamics as well as physical examination findings and exemplifies a more detailed approach to staging. Furthermore, the definitional progression along the ACC/AHA stages A through D is a unidirectional path with little appreciation of a possibility to revert to a lower stage with appropriate GDMT.
If the HF process were to be defined as a continuum from Stage A through D, the highest number of patients would be in Stage A or Stage B.66-69 This is due to the fact that the prevalence of hypertension, diabetes, coronary artery disease, obesity/metabolic syndrome – the risk factors with significant relative risk and population attributable risk for development of HF – are present in approximately one-third of the US population.10 By population-based registries, more than 40 to 50% of the adult population has been categorized to be in Stages A or B.66-68 The high prevalence of HF risk in the general population raises the question of whether Stage A patients should really be defined to have HF. From the public and health care perspective, being called HF, regardless of such an early status as Stage A, raises important concerns, since HF is usually perceived as an advanced chronic disease with symptoms and very adverse outcomes, and may have implications for health and life insurance. Of course, it is critical to focus on prevention, with recognition, prevention and treatment of these risk factors, but it is also important to differentiate those who have HF from those at risk for HF. Similarly, clinicians in general or HF practice have not adopted the terminology of Stage A HF beyond academic circles, partly due to lack of actionable specific treatment recommendations according to stages, and most of their assessment and management focuses on management of left ventricular dysfunction (Stage B) or symptomatic HF (Stages C/D). When clinicians address risk factors such as hypertension, diabetes, obesity or coronary artery disease, they do not refer to those as Stage A HF or pre-HF but rather independent diagnoses. Furthermore, despite recognized increased adverse outcome risk and possibility of progress to symptomatic HF in some patients,66, 69, 70 the data on the likelihood of progression from Stages A/B to C/D are limited.67, 69, 70 Thus most clinicians do not commonly use the HF terminology for Stage A patients, and do not commonly educate patients regarding risk of progression from Stages A/B to C.
Another important development that needs to be taken into consideration of stages in HF is the advances in prevention of future risk of HF by specific therapies. Although in the past, prevention and holistic treatment of risk factors by standard treatment strategies were felt to prevent HF,3 there is growing evidence that certain treatment strategies are better for the prevention of HF, and not all treatment strategies of hypertension and diabetes prevent HF equally or at all. For example, in the treatment of hypertension, diuretic-based antihypertensive therapies, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers have been shown to prevent HF in a wide range of target populations, whereas calcium channel blockers have not.71 There is growing evidence that treatment with sodium–glucose co-transporter 2 (SGLT2) inhibitors prevent HF hospitalizations among patients with type 2 diabetes72-74 or in patients with HFrEF regardless of diabetes27, 28 whereas other glucose treatment strategies do not. It is also interesting to note that patients with a higher future HF risk identified by risk scores that include biomarkers such as albuminuria, seem to derive greater benefit from SGLT2 inhibitor therapy among patients with type 2 diabetes.75 The biomarker profile may identify patients with cardiometabolic, cardiovascular and cardiac structural changes in patients predestined to develop HF or in other words pre-HF. Supporting this concept was the STOP-HF trial which provided evidence that screening with natriuretic peptides among individuals with cardiovascular disease or with cardiovascular risk factors such as diabetes and hypertension, can be helpful to prevent development of HF or left ventricular systolic or diastolic dysfunction.76 Accordingly, the 2017 ACC/AHA/HFSA focused update of the guidelines for the management of HF incorporated recommendations for natriuretic peptide-based screening in the prevention of HF as a Class IIa recommendation.38 Similarly, high-sensitivity cardiac troponin levels are associated with future development of incident HF in the general population77, 78 and in those with evidence of cardiotoxicity or cardiac injury in high-risk populations79 allowing for treatment strategies to prevent development of HF. Thus, biomarker elaboration can further identify risk and presence of ultrastructural abnormalities in HF among asymptomatic patients and could be a marker for Stage B HF without development of macroscopic structural changes detectable by imaging or electrocardiography.
4 Gaps in definitions according to trajectory of changes in heart failure
The HF syndrome is dynamic, with changing clinical trajectories based on signs, symptoms, and disease progression, driven by underlying pathophysiologic processes. Changes in HF may be captured in several ways, including alterations in cardiac structure and function and by clinical status.
4.1 Trajectory changes in ejection fraction
Guideline-directed management and therapy can result in improvement in LVEF and reverse remodelling in patients with HFrEF.80 The phenomenon of improvement and recovery of LVEF has led to a growing interest in the long-term outcomes and management of these patients and how they differ from ‘non-responders’, or individuals whose LVEF does not improve with treatment. Currently, there is no consensus definition for patients with HFrEF whose LVEF improves, which has led to a variety of terms describing this phenotype, including patients with ‘improved’ LVEF, HFpEF (borderline), HFpEF, and HF with recovered EF (HFrecEF). The magnitude of change that defines ‘recovery’ of LVEF is not standardized, but it is recognized that distinguishing HFrecEF from HFmrEF requires serial measurements of the LVEF to appropriately capture change over time as this group might represent HFrecEF or deteriorated HFpEF. Moreover, because the measurement of the LVEF is subject to significant intra- or inter-reader variability, small changes in the LVEF need to be interpreted cautiously. Thus, a recent scientific panel put forth a working definition of HFrecEF that includes: a baseline LVEF of ≤40%, a ≥10% increase from baseline LVEF, and a second measurement of LVEF of >40%.80 In this formulation, recovered EF signifies improvement of LVEF to over 40% but not necessarily totally normalized. There have been other attempts to characterize improvement in EF as an increase in LVEF by more than 10%.80 It is also important to recognize that the trajectory might not be linear and unidirectional, and a patient may have improvement followed by a decline in EF or vice versa depending on the underlying aetiology, duration of disease, adherence to GDMT, comorbidities or re-exposure to cardiotoxins.
4.2 Trajectory changes in clinical status
Another method that captures the HF trajectory relies on an assessment of the patient's clinical status, which can inform the risk for hospitalization for HF or for mortality. A de novo diagnosis of HF, also referred to as ‘new-onset HF’, carries an increased risk for adverse clinical outcomes since the patient is not likely to be treated with optimal GDMT at the time of diagnosis.
Most patients with HF have episodes of clinical worsening of HF, which has been previously defined as worsening signs or symptoms in concert with a hospitalization.81 Data from more contemporary studies resulted in expansion of worsening HF to also include patients who require escalation of outpatient therapies, such as diuretics, even without a hospitalization.82 This is because the need for intensifying diuretic therapy, regardless of location (inpatient or outpatient), portends a worse prognosis than a patient who does not require intensification of therapy. Worsening HF implies a period of stability preceding a deterioration of signs and symptoms. However, the phrase ‘stable’ HF may be a misnomer, as patients with HF always carry a residual risk for hospitalization or sudden cardiac death, even when minimally symptomatic or asymptomatic receiving optimal treatment. For such patients remission may be a more suitable term.83 When a patient with worsening HF does not improve with therapy escalation and continues to decline, she or he can be referred to as refractory to treatment. These patients are often assessed for advanced therapies such as mechanical circulatory support or cardiac transplant, or if they do not qualify for advanced therapies, clinicians can consider referral for palliative care.
Patients may have improvement in HF symptoms, functional capacity, quality of life and exercise performance with GDMT. Some patients with reversible or treatable causes of HF such as cardiomyopathy due to hypertensive heart disease, alcoholic cardiomyopathy, peripartum cardiomyopathy, or tachycardia-induced cardiomyopathy may even recover from HF with treatment and manifest resolution of HF symptoms, as well as normalization of the EF and cardiac structure. These patients require close follow-up and continuation of treatment to ascertain that HF symptoms or left ventricular dysfunction do not re-occur in the future.84
5 Learning from other disease definitions
Disease definitions are not all the same. Some are categorical, where the disease is present or it is not. In some there may be a single pathognomonic feature that defines the disease state, such as many cancers and infectious diseases. In others, where numerical thresholds are used, a disease may be defined against a quantitative threshold of abnormality in an anatomical and/or functional feature. Examples of these include hypertension, osteoporosis, sarcopenia and CKD. In some (e.g. CKD, hypertension) the presence of this numerical abnormality alone is sufficient to define the disease, whereas in others (e.g. HF, sarcopenia) the loss of function must be symptomatic or functionally evident for the disease to be defined. In the current universal definition of MI, elevation of cardiac troponin is central to the clinical diagnosis and fundamental to the universal definition.22
There are many other corollaries and lessons to learn from other areas of cardiology and medicine in regard to disease definition and classifications. Current ACC/AHA classification of valvular heart disease is very similar to the current ACC/AHA HF categorization into Stages A–D.3, 85 Such categorization is an epidemiology-based system where the disease stage is defined based on stages of susceptibility from ‘at risk’ to subclinical disease to clinical disease, and finally, recovery, disability, or death. Atrial fibrillation is also based on an epidemiology-based system where patients are categorized as paroxysmal (≤48 h), persistent (>7 days or cardioverted), long-standing (>1 year), and permanent.86 However, in atrial fibrillation, clinicians also use the CHA2DS2-VASc risk score to determine potential stroke risk and thereby guide management.86 A similar parallel in HF is the MAGGIC model for prediction of mortality and other attempts at scoring to help risk stratify patients who may have worsening HF, rehospitalization, or a greater chance of dying.87
In regard to non-cardiovascular strategies for disease definition, there are quite a few examples. CKD is classified based on albuminuria and estimated glomerular filtration rate (eGFR).39 Albuminuria states are similar to numeric categorization of disease, like LVEF in HF, whereas eGFR ranging from normal to end-stage renal disease provides prognostic information and guides management decisions, such as drug dosing and the need for dialysis. Liver disease is categorized based on pathology using imaging and tissue sampling to define levels of steatosis, hepatitis, fibrosis, and cirrhosis.88 Much like CKD, liver disease also supplements disease categorization with risk scores like the MELD score.89 Lung disease is assessed using pulmonary function tests which help clinicians stratify patients based on air-flow limitation and the GOLD system.90 Chronic obstructive pulmonary disease also stratifies patients based on symptoms and risk of exacerbations similar to congestion–perfusion91 categorization in HF. Pulmonary hypertension classification [World Health Organization (WHO) groups 1–5]92 is similar to the aetiology-based groupings for cardiomyopathies34, 35 with genetic, acquired, and mixed categories and is a potential model for future HFpEF93 disease stratification. Finally, the field of cancer groups disease using a combination of epidemiology-based staging (i.e. at risk for cancer, pre-cancer, carcinoma in situ, localized, early/late locally advanced, and metastasized) coupled with disease-specific markers that determine treatment course and targeted therapies. Cancer, which is a chronic disease similar to HF, reflects one of the most comprehensive combined approaches of classification using epidemiology, biomarker thresholds and trajectory.
Future attempts at defining HF will need to draw on principles of categorization used in other disease states. Each organ system has a unique pathophysiology that helps to determine its disease categorization, and ultimately, all organ systems are interconnected. Indeed, HF represents an end-stage phenotype for most (if not all) cardiovascular diseases. In the terminal stages of disease, the universal element is disseminated disease and multiorgan failure. However, unlike other organ systems, the heart is unique in that haemodynamics play a central role in the disease state. Many disease states are moving towards a combination of epidemiology-based, numeric, and targeted marker-based therapies. Disease definitions are critical to patients' and clinicians' understanding of their pathology, inform clinical decision-making, categorization for financial billing, and creation of future health policies.
6 Proposed universal definition of heart failure
In this section, we provide a consensus opinion on a new proposed universal definition of HF.
6.1 Symptoms
Heart failure, like many non-categorical diseases, is widely held to be a clinical syndrome, devoid of any single pathognomonic histological or biochemical signal, and being the possible end result of many quite distinct and varied clinical disease states. Common symptoms and signs of HF are listed in Table 6.
| Symptoms of heart failure | |
| Typical | Breathlessness |
| Orthopnoeaa | |
| Paroxysmal nocturnal dyspnoeaa | |
| Reduced exercise tolerancea | |
| Fatigue, tirednessb | |
| Ankle swellinga | |
| Inability to exercisea | |
| Swelling of parts of the body other than ankles | |
| Bendopnoea | |
| Less typical | Nocturnal cough |
| Wheezing | |
| Bloated feelingc | |
| Postprandial satietyc | |
| Loss of appetite | |
| Decline in cognitive function, confusion (especially in the elderly)b | |
| Depression | |
| Dizziness, syncopeb | |
| Signs of heart failure | |
| More specific | Elevated jugular venous pressurea |
| Third heart sounda | |
| Summation gallop with third and fourth heart sounds | |
| Cardiomegaly, laterally displaced apical impulse | |
| Hepatojugular reflux | |
| Cheyne–Stokes respiration in advanced heart failureb | |
| Less specific | Peripheral oedema (ankle, sacral, scrotal) |
| Pulmonary ralesa | |
| Unintentional weight gain (>2 kg/week) | |
| Weight loss (in advanced heart failure) with muscle wasting and cachexia | |
| Cardiac murmur | |
| Reduced air entry and dullness to percussion at lung bases suggestive of pleural effusion | |
| Tachycardia, irregular pulse | |
| Tachypnoea | |
| Hepatomegaly/ascites | |
| Cold extremitiesb | |
| Oliguria | |
| Narrow pulse pressure | |
- a Commonly used in clinical trials, registries, risk scoring and have been tested for sensitivity and specificity.
- b Common in low perfusion, low cardiac output states.
- c Can be typical in the setting of right heart failure or biventricular failure.
The current ACCF/AHA classification of HF3 includes two pre-symptomatic stages, A and B. Although we restrict the definition of the syndrome of HF to being a symptomatic clinical condition, our proposed revised stages still straddle the pre-symptomatic stages. To not lose the advantage that the A/B/C/D staging system offered, to incorporate the asymptomatic phases under the HF umbrella, and to enhance understandability of these asymptomatic phases, we propose a new categorization of Stages A and B into ‘at risk’ and ‘pre-HF’ in Section 9 below.
6.2 Objective marker
In learning from other disease states that incorporated a core and frequently measured variables in their definition, such as acute MI, eGFR in CKD, glycated haemoglobin in diabetes, bone mineral density in osteoporosis or forced expiratory volume in the first second in COPD, making the diagnosis more accessible to non-specialists and more reliable and consistent between observers, hospitals and health care systems, we propose the incorporation of an objective measurement in addition to the symptoms in the HF definition.
In HF, possible candidates for such a measurement might theoretically be haemodynamic measures such as elevated pulmonary capillary wedge pressure and right atrial pressure by right heart catheterization, biomarkers associated with congestion such as natriuretic peptides, measures of neurohormonal overactivity or measures of exercise limitation such as maximal oxygen consumption. None of these are commonly or reliably associated with the disease states of HF; for example, the LVEF can vary from low through normal to high and still be part of an HF syndrome; no single haemodynamic measure is adequate to serve as a practical, non-invasive and reliable measurement; measurement of exercise limitation with cardiopulmonary exercise testing with expired gas exchange is not practical or universally available; and to date, neurohormone levels have not universally been considered reliable measures of the disease state. The closest have been the natriuretic peptides, which are recommended in modern guidelines as both diagnostic tests of reasonable clinical usefulness with prognostic utility and as good tests to rule out HF as a cause of breathlessness in certain settings.4, 38 Contemporary guidelines already state that natriuretic peptides can be used as an initial diagnostic test, and that patients with normal plasma natriuretic peptide concentrations are unlikely to have HF.4, 38 A detailed diagnostic algorithm will require specific operational thresholds based on individual natriuretic peptides and assay systems, as well as detailing other clinical features which can affect natriuretic peptide levels, but for common clinical purposes simple thresholds can be established that have sufficient operational accuracy to be incorporated usefully into a universal definition of HF.
6.3 Proposed new heart failure definition
-
UNIVERSAL DEFINITION OF HF
HF is a clinical syndrome with current or prior
- Symptoms and/or signs (Table 6) caused by a structural and/or functional cardiac abnormality (as determined by EF <50%, abnormal cardiac chamber enlargement, E/E′ >15, moderate/severe ventricular hypertrophy or moderate/severe valvular obstructive or regurgitant lesion)
-
and corroborated by at least one of the following:
- Elevated natriuretic peptide levels (for values refer to Table 7)
- Objective evidence of cardiogenic pulmonary or systemic congestion by diagnostic modalities such as imaging (e.g. by chest X-ray or elevated filling pressures by echocardiography) or haemodynamic measurement (e.g. right heart catheterization, pulmonary artery catheter) at rest or with provocation (e.g. exercise).
| Ambulatory | Hospitalized/decompensated | |
|---|---|---|
| BNP, pg/ml | ≥35 | ≥ 100 |
| NT-proBNP, pg/ml | ≥ 125 | ≥ 300 |
| Causes of elevated natriuretic peptide levels other than primary diagnosis of heart failure | ||
| Cardiovascular causes | Acute coronary syndrome, myocardial infarction | |
| Pulmonary embolism | ||
| Myocarditis | ||
| Hypertrophic cardiomyopathy | ||
| Valvular heart disease | ||
| Congenital heart disease | ||
| Atrial or ventricular arrhythmias | ||
| Heart contusion, cardiac infiltration or malignancy | ||
| Cardioversion, ICD shock | ||
| Pericardial disease | ||
| Invasive or surgical procedures involving the heart | ||
| Pulmonary hypertension, right ventricular failure | ||
| Infiltrative cardiomyopathies | ||
| Non-cardiovascular causes | Advanced age | |
| Kidney disease | ||
| Critical illnesses including sepsis syndrome, cytokine syndrome | ||
| Ischaemic or haemorrhagic stroke | ||
| Pulmonary disease (pneumonia, chronic obstructive pulmonary disease) | ||
| Liver disease | ||
| Severe anaemia | ||
| Severe metabolic and hormone abnormalities (e.g. thyrotoxicosis, diabetic ketoacidosis, severe burns) | ||
| Causes of lower natriuretic peptide levels | ||
| Obesity, or increased BMI | ||
| Pericardial diseasea | ||
- BMI, body mass index; BNP, B-type natriuretic peptide; ICD, implantable cardioverter-defibrillator; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
- a In certain patients with pericardial disease and effusion, natriuretic peptides may be lower and rise after pericardiocentesis.
Such a definition is comprehensive and practical enough to form the base which allows further subclassifications and which can encompass formal disease stages, both with universal applicability, prognostic and therapeutic validity, and an acceptable sensitivity and specificity. Please note that the definition of HF requires not only symptoms or signs (Table 6) but also presence of either elevated natriuretic peptides or objective evidence of pulmonary or systemic congestion by diagnostic modalities. For example, it would be important for peripheral oedema or ascites (Table 6) to be corroborated by presence of elevated right-sided cardiac filling pressures or rales by presence of elevated left-sided cardiac filling pressures; or elevated natriuretic peptides. It is also important to note that elevated jugular venous pressure estimated by an experienced clinician could be accepted as an objective evidence.
Please also note that in certain patients, congestion and haemodynamic abnormalities may become manifest with provocation such as exercise, especially in patients with HFpEF. This can support the diagnosis of HF. It is also critical to note that in patients with low perfusion and hypovolaemic state, there may not be any evidence of congestion or elevated filling pressures, but rather decreased cardiac output accompanied with low or normal ventricular filling pressures94 (e.g. in the setting of over-diuresis in patients with HF). Once the hypovolaemic state is corrected, patients with HF usually have elevated filling pressures.
In the definition above, we did not specify left or right HF. Though left heart HF, and in advanced stages, biventricular HF are common, right HF can also be recognized as part of the above definition when patients present with symptoms or signs (Table 6) caused by a cardiac abnormality and have elevated natriuretic peptide levels or objective evidence of cardiogenic pulmonary or systemic congestion. Right HF primarily due to cardiac abnormalities such as arrhythmogenic right ventricular cardiomyopathy (ARVC) would be part of this definition.
We recognize that asymptomatic stages with patients at risk (former Stage A HF), or patients with structural heart disease or cardiomyopathies (former Stage B HF) would not be covered under the above definition as having HF, which emphasizes symptoms and signs of HF, but we conceptualize the HF syndrome as a continuum of disease with certain stages, such as pre-HF. This is similar to the approach with other disease states such as cancer, which defines those at risk and pre-cancer. The stages preceding the symptomatic phases as those at risk and pre-HF will be discussed in the following section.
We also realize certain patients with competing diagnoses such as CKD with marked volume overload, can present with symptoms and signs of HF, have elevated natriuretic peptides, and may even have evidence of congestion by imaging or elevated filling pressures. Although some of these patients may have concomitant HF, these patients have a primary abnormality that may require a specific treatment beyond that for HF. In the following section, we will address such other syndromes.
7 Other syndromes related to heart failure
As noted above, the definition of HF comprises a combination of symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality, and evidence of elevated filling pressures by natriuretic peptides or by imaging/haemodynamic assessment. Although many clinicians will initially envision patients with left HF as embodying this definition, it is important to note that there are other syndromes that may fulfil this definition of HF, addressed below. These aetiologies require specific treatment and management strategies targeting the underlying or proximate cause, as well as treating the HF itself.
7.1 Right heart failure
The most common cause of right HF is left HF. However, right HF is characterized not only by signs and symptoms of right-sided HF but also by right atrial enlargement or right ventricular dysfunction. The presence of right HF in the setting of left HF is typically due to post-capillary, WHO group 2 pulmonary hypertension and may require modified treatment approaches and portends a poor prognosis; therefore, recognition of biventricular HF is important.92 Given the importance of these distinctions, the classification of types of ventricular failure in HF commonly includes three categories: left ventricular failure, right ventricular failure and combined left and right ventricular failure usually termed as biventricular failure. We believe isolated right HF due to primary pulmonary hypertension aetiologies (WHO groups 1, 3, 4), though may have symptoms or signs which may mimic HF and may have elevated natriuretic peptide levels, would not be categorized under HF, as the signs and/or symptoms are not caused primarily by a structural and/or functional cardiac abnormality. On the other hand, right HF due to primary right ventricular conditions such as ARVC would be categorized under HF.
7.2 Acute myocardial infarction/acute coronary syndrome
Acute MI may be complicated by HF. Given its acuity, specific pathophysiology and specific treatment strategies, we believe acute MI would be the overarching definition for the episode in proximity to acute MI. It is also possible that these patients may recover with timely treatment strategies and not progress to chronic HF, but also many may progress to chronic HF. In clinical trials, patients with acute MI or acute coronary syndrome within 6 weeks are usually excluded from clinical trials in HF. These patients may present with asymptomatic left ventricular dysfunction or pre-HF or symptoms and signs of HF due to a cardiac abnormality and may have elevated natriuretic peptides or evidence of congestion by imaging or haemodynamics. During the acute phase, these patients are diagnosed as having an MI complicated by HF rather than with HF alone. This does not mean acute MI should be replaced by HF alone, but it does mean the setting and specific aetiology of HF can be an important feature that determines specific therapeutic approaches. This setting has also been subject to specific clinical trial evaluation.95-97 In addition to specific therapies for acute MI, these patients have indications for specific treatment for asymptomatic left ventricular dysfunction (pre-HF or Stage B HF) or symptomatic HF complicating acute MI during the acute phase, or as primary diagnoses in the chronic phase post-MI.
7.3 Cardiogenic shock
Another important form of HF is cardiogenic shock, which is the clinical state of organ hypoperfusion due to severe cardiac dysfunction. In cardiogenic shock, the symptoms and signs reflecting HF include hypotension unresponsive to volume repletion, altered mental status, cool extremities, and laboratory evidence of end-organ dysfunction such as elevated lactate levels due to hypoperfusion.65 Cardiogenic shock is an extreme form of HF which requires some form of definitive therapy such as intravenous inotropes, vasopressors, or mechanical circulatory support. Cardiogenic shock is a type of HF, but due to its specific haemodynamic and clinical characterization requiring specific therapies such as vasoactive agents, circulatory support and/or revascularization depending on the aetiology, we believe keeping the descriptor ‘cardiogenic shock’ will help to identify a patient cohort with specific and urgent treatment needs. Cardiogenic shock may occur as an acute de novo presentation (e.g. large acute MI, fulminant myocarditis) or with progressive deterioration in a patient with chronic HF. Subacute cardiogenic shock may be in continuum of the wet and cold advanced HF patient with low cardiac output state. Such patients may meet the criteria for cardiogenic shock especially when they have evidence of end-organ dysfunction. A system describing stages of cardiogenic shock has been proposed by SCAI and other societies and characterizes the patients as Stage A ‘at risk’ for cardiogenic shock, stage B ‘beginning’ shock, stage C ‘classic’ cardiogenic shock, stage D ‘deteriorating’, and E ‘extremis’.65 Such classification is important to characterize the severity and stage of shock, but it is also important to acknowledge the presence of HF as the preceding cause of shock in such patients, and identify advanced HF complicated with cardiogenic shock as the diagnosis.
7.4 Hypertensive emergency and hypertensive heart disease
Hypertensive emergencies encompass a spectrum of clinical presentations of uncontrolled blood pressure associated with end-organ damage that can include acute left ventricular dysfunction, pulmonary oedema, MI/ischemia and/or aortic dissection. All of these complications may result in or be complicated with an acute presentation of HF. Hypertension increases HF risk by two to threefold98 and accounts for almost half of the HF cases in the US population as a population attributable risk.99 Thus, both acutely hypertensive emergency and chronically, hypertensive heart disease can be complicated with HF. Treatment of hypertension is of utmost importance in the prevention and treatment of HF, underlined as a Class I recommendation with strong level of evidence in guidelines.4, 38
7.5 Valvular heart disease
Aortic stenosis and mitral regurgitation can result in HF. Valvular heart disease is acknowledged as a specific disease, as it results in specific haemodynamic and ventricular alterations and requires specific treatment strategies targeting valvular abnormality. Most HF clinical trials exclude significant valvular heart disease for these reasons.
7.6 Congenital heart disease
Some types of congenital heart disease can result in HF. Incomplete or palliative correction of a congenital lesion leading to a chronic state of haemodynamic stress may result in subsequent HF, especially in complex congenital heart diseases such as tetralogy of Fallot, single ventricle defects and transposition of the great arteries. Additional myocardial, coronary or conduction system injury can occur due to complications of corrective surgery and can lead to progressive contractile dysfunction in some patients. The treatment should target the underlying anomaly and specific haemodynamic conditions.
7.7 High-output failure
High-output HF presents with similar symptoms and signs of systemic or pulmonary congestion, frequently associated with rapid heart rate and signs of peripheral vasodilatation. Cardiac dysfunction may be represented by pathologically elevated cardiac output, echocardiographic signs of right ventricular dilatation or dysfunction, and elevated natriuretic peptide concentrations. High-output HF is a response to extracardiac causes including liver disease, arteriovenous shunt, lung disease, thiamine deficiency, anaemia, thyroid disease or myeloproliferative disorders. Treatment is generally directed to the underlying causes. Given the unique nature of high-output failure, it is appropriate that it has a separate classification.
7.8 Other overlapping and competing diagnoses with heart failure
Patients can experience clinical deterioration as specific events that may not necessarily meet the universal definition of a diagnosis of HF. Such occurrences consist of events of a primary disease process that may be associated with signs and symptoms of HF as a result of the primary cause that is not HF at that encounter. These can include cardiovascular causes such as acute MI or acute coronary syndrome, hypertensive emergency as mentioned above, and also other cardiovascular primary diagnoses such as atrial fibrillation with rapid ventricular response, prolonged ventricular arrhythmias, pulmonary embolus, pericardial diseases, and acute valvular dysfunction. In these cardiovascular diagnoses, complication with HF is associated with worse prognosis and outcomes and underlines the urgency of addressing the underlying problem as well as the HF.
Other non-cardiovascular entities such as renal failure, liver failure, morbid obesity with peripheral oedema and chronic respiratory failure hypoventilation syndrome may present with symptoms and signs that mimic HF. Due to volume overload and neurohormonal compensatory mechanisms involved in some of these disease states, symptoms, signs and even haemodynamic characterization and biomarker profile can overlap with HF, and these patients may indeed also have concomitant HF. In these cases, the proximate cause of the signs and symptoms of volume overload is a distinct entity to which treatment is often primarily directed, in addition to HF. These events are often of significant interest to clinical events committees of clinical trials, where they may be considered as an event ‘with HF’ rather than a primary HF event. Another important concept that supports the principality of these competing diagnoses are that the symptoms and signs of HF may disappear once the underlying primary cause is treated, for example symptoms and signs that mimic HF may resolve with haemodialysis in a patient with end-stage CKD who may have missed a dialysis appointment. Thus, it is important not to catalogue every presentation with shortness of breath and oedema that requires treatment with fluid management strategies or diuretics as HF. It is, however, also important to not miss the complication with HF which requires timely management of HF as well as the proximate cause. Many of these factors can contribute to worsening outcomes in a complementary fashion in patients with HF. For example, patients with HF associated with CKD or diabetes are at much higher risk than those without. Rather than ‘competing’, these diagnoses can become complementary comorbidity risk factors to HF for worse outcomes.
8 Proposed revised stages of the heart failure continuum
- At risk for HF (Stage A): patients at risk for HF but without current or prior symptoms or signs of HF and without structural cardiac changes or elevated biomarkers of heart disease. Patients with hypertension, atherosclerotic cardiovascular disease, diabetes, obesity, known exposure to cardiotoxins, positive family history of cardiomyopathy or genetic cardiomyopathy would be in this category. Not all of these patients will develop HF, but risk factor intervention may be warranted.
-
Pre-HF (Stage B): patients without current or prior symptoms or signs of HF but evidence of one of the following:
- Structural heart disease: e.g. left ventricular hypertrophy, cardiac chamber enlargement, ventricular wall motion abnormality, myocardial tissue abnormality (e.g. evidence of myocardial oedema, scar/fibrosis abnormality by cardiac magnetic resonance T2 or late gadolinium enhancement imaging), valvular heart disease.
- Abnormal cardiac function: e.g. reduced left or right ventricular systolic function, evidence of increased filling pressures (by invasive or non-invasive measures), abnormal diastolic dysfunction.
- Elevated natriuretic peptide levels (for levels, refer to Table 7) or elevated cardiac troponin levels (over 99th percentile in a normal reference population) especially in the setting of exposure to cardiotoxins.
- HF (Stage C): patients with current or prior symptoms and/or signs of HF caused by a structural and/or functional cardiac abnormality.
-
Advanced HF (Stage D): severe symptoms and/or signs of HF at rest, recurrent hospitalizations despite GDMT, refractory or intolerant to GDMT, requiring advanced therapies such as consideration for transplantation, mechanical circulatory support, or palliative care.
- Abnormal cardiac function: e.g. reduced left or right ventricular systolic function, can be characterized by reduced EF, abnormal ventricular strain, or other non-invasive or invasive modalities.
Although certain genetic markers may be associated with structural cardiac changes and future HF, we did not specifically include genetic markers in the definition of pre-HF or Stage B HF as the penetrance, expressivity, phenotypic characterization and prognosis with genetic markers vary significantly. Because the evidence for precision for risk evolves with biomarkers, genetics, omics and/or risk calculators, alternative approaches can be developed in the future to identify risk categories beyond traditional risk factors, and pre-HF beyond cardiac structure and biomarkers alone, and support expansion of indications for preventive treatment strategies for patients at risk or with pre-HF.
Please note that the cut-offs provided for natriuretic peptide levels in Table 7 represent thresholds lower than inclusion criteria used in some clinical trials for symptomatic HF,27, 28 but similar to those used in former guidelines.4 Thresholds proposed in the table have higher sensitivity and may have lower specificity especially in older patients, or patients with atrial fibrillation or CKD. Usually, higher cut-off values are recommended for the diagnosis of HF in these patients.100 For example, for ages 50–75 years, NT-proBNP threshold value of 900 pg/mL; for ages >75 years, NT-proBNP value of 1800 pg/mL provide reliable sensitivity and specificity for the diagnosis of HF, compared with an NT-proBNP value of 450 pg/mL for ages <50 among patients requiring hospitalization.100 Similarly, in patients with atrial fibrillation, an increase by 20–30% have been suggested in natriuretic peptide level thresholds for trial enrolment in HF,100 since atrial fibrillation is known to result in increased concentrations of natriuretic peptides even in the absence of HF. Furthermore, it is important to note that natriuretic peptide cut-offs selected for population screening for pre-HF (Stage B HF) may be lower than 99% reference limits76 and will need to be defined according to the population at risk.
8.1 NYHA classification
The NYHA funtional classification is important to characterize symptoms and functional capacity of patients with symptomatic (Stage C) HF or advanced HF (Stage D). The NYHA classification system categorizes HF on a scale of I to IV; Class I: no limitation of physical activity, Class II: slight limitation of physical activity, Class III: marked limitation of physical activity, and Class IV: symptoms occur even at rest; discomfort with any physical activity. We believe it is important to specify NYHA class at baseline after the initial diagnosis, and after treatment through the continuum of care of a patient with HF. A patient with symptomatic HF (Stage C) may become asymptomatic with treatment. Since that patient will still be categorized as HF Stage C, NYHA class I can further specify his/her absence of current symptoms. Worsening NYHA class is associated with worse prognosis and any symptomatic patient with HF (NYHA class II–IV HF) should have further optimization of GDMT.
8.2 Recognition of clinical trajectory in heart failure
It is well-recognized that the natural history of HF encompasses changes in the clinical risk of hospitalization and death over time, with risk increasing from ‘pre-HF’ to ‘new onset/de novo HF’, and further increasing with each episode of ‘worsening HF’ where there is deterioration of HF signs and symptoms despite ongoing therapy, requiring hospitalization or outpatient escalation of therapy.101 It is crucial to identify both the stage of the patient's natural history, as well as recognize the patient's clinical trajectory (improving vs. stalled or persistent vs. worsening)102 for optimal treatment, risk mitigation strategies, and patient-centred discussions. Gaining perspective of not only where the patient stands at the point in time, but in which direction the patient is headed, is a critical element of determining whether to continue along the current therapeutic course or to change direction. Thus, a patient with ‘worsening chronic HF’ following initial stabilization of ‘new onset/de novo HF’ would alert a physician of the immediate high risk for recurrent hospitalization or death, particularly in the period of close proximity to the worsening HF event, and trigger escalation of disease-modifying therapies rather than a focus on decongestion with diuretics alone. Of note, we caution against a terminology of ‘stable HF’, as patients are expected to improve with GDMT. These patients should have further optimization of therapies despite perceived stability or improvement, as there is evidence for significant improvement in outcomes with additional therapies in these patients. Lack of improvement is a marker of worse prognosis and should be termed as ‘persistent’ rather than ‘stable’, and prompt clinicians to further optimize therapy. For those patients who have resolution of symptoms and signs of HF along with resolution of previously present structural and functional heart disease after a phase of symptomatic HF, we recommend ‘HF in remission’ or NYHA class I HF status rather than ‘recovered HF’, which should be reserved for patients who have persistent resolution of HF symptoms and signs, normalization of cardiac structure, function and biomarker profile following resolution and treatment of a fully reversible cause, especially in view of the TRED-HF trial results which demonstrated that many patient deemed to have ‘recovered’ from dilated cardiomyopathy will relapse following treatment withdrawal suggesting remission rather than recovery84 (Figure 2). Full and persistent recovery is rare, and even in the setting of reversible causes, patients may have recurrence of symptoms and or develop left ventricular dysfunction in the future.
8.3 Acute versus decompensated heart failure
In this document, we do not use the terms ‘acute new-onset HF’ or ‘acute decompensated HF’, which are the terminologies commonly used to describe patients requiring hospitalization or urgent care. The indications for hospitalization and/or urgent care utilization vary, and most patients who require hospitalization for HF may have chronic progressively worsening HF, rather than an acute singular event. We realize these patients may present with rapid onset or progressively escalating symptoms and/or signs of HF which are associated with adverse outcomes, requiring urgent evaluation and treatment. We have elected to characterize these patients as having ‘decompensated HF’ which may represent acutely decompensated patients due to an inciting event (e.g. atrial fibrillation with rapid ventricular response) or chronically and progressively worsening patients with marked deterioration of HF signs and symptoms despite ongoing therapy requiring urgent intervention, hospitalization, or rapid escalation of therapies including advanced therapies.
We recognize that there are a variety of acute presentations of HF (e.g. myocarditis, peripartum, cardiotoxicity, stress cardiomyopathy, etc.) and other entities associated with acute presentations of HF such as hypertensive emergency and acute MI, that will require specialized treatment strategies targeting the underlying aetiology. These have been addressed by other investigators103-105 and are beyond the scope of this document.
9 Proposed new classifications of heart failure according to ejection fraction
The strongest argument to use LVEF to categorize HF is that LVEF defines a group known to respond to life-prolonging therapy from randomized controlled trials.3, 4, 25, 30, 31, 38, 52, 53, 83, 95, 97, 106 While LVEF also provides prognostic information, this reason alone does not justify using LVEF to define HF. Accordingly, LVEF categories were created that define groups where treatment differs.
- HF with reduced EF (HFrEF): HF with LVEF ≤40%
- HF with mildly reduced EF (HFmrEF): HF with LVEF 41–49%
- HF with preserved EF (HFpEF): HF with LVEF ≥50%.
-
HF with improved EF (HFimpEF): HF with a baseline LVEF ≤40%, a ≥10 point increase from baseline LVEF, and a second measurement of LVEF >40%.
We acknowledge the growing body of evidence that standard therapy for HFrEF may be effective and extended to select patients with HFmrEF.52-55 It is however important to recognize the heterogeneity of this category, underlined by diverse findings from meta-analyses with neurohormonal antagonism, specifying benefit in certain subgroups.15, 55-57
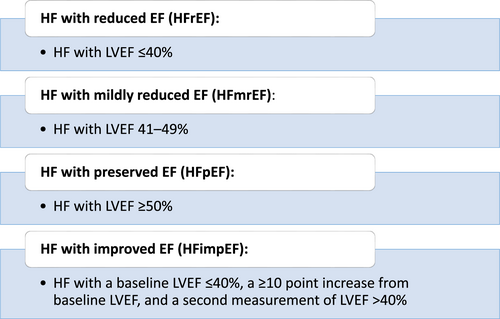
Evidently, LVEF is not a singular measurement by which left ventricular function is assessed in isolation. Chamber volumes and other cardiac structural and functional parameters are important and other diagnostic modalities can be complementary. Though the above classification is provided for targeting GDMT according to LVEF indications, other cardiac features are also important for phenotypic characterization, aetiology or prognosis. Development of left ventricular dilatation in a patient with HFpEF or HFmrEF may imply impending HFrEF. It is important to recognize that cardiac structural and functional information in addition to LVEF is important to guide management of the patient.
Since GDMT can result in improvement in LVEF and reverse remodelling in patients with HFrEF, the trajectory of improvement and recovery of EF has been of interest to determine the types (e.g. device, medical, advanced) and duration of treatment.80 In cases where longitudinal surveillance of LVEF is available, clinicians should also consider the trajectory of LVEF, in addition to the LVEF at the point in time, recognizing that a significant decrease in LVEF over time is a poor prognostic factor calling for consideration of intensification of therapy and advanced management strategies according to patient goals. Importantly, EF can decline after withdrawal of pharmacological treatment in many patients who had improved EF to normal range with GDMT.84 This implies that there is not full recovery in cardiac structure and function in most patients despite improvement in EF. Therefore, we recommend use of the improved terminology rather than recovered EF. We believe ‘improved EF’ deserves a separate classification and should not be classified as HFmrEF or HFpEF even after improvement in LVEF to 41–49% or ≥50% respectively, as discontinuing HFrEF therapy in this group leads to poor outcome.84 GDMT should be continued in patients with HFimpEF regardless of whether it has improved to normal range (LVEF ≥50%), especially in view of the TRED-HF trial results.84 We also recognize that patients with baseline LVEF of 41-49% who have improved LVEF to ≥50% may be categorized as HFimpEF.
10 Approaches to specific aetiologies of heart failure
In addition to the recognition of the syndrome of HF and its classifications, it is critical that every effort should be made to diagnose and define the specific aetiology/aetiologies of HF. Understanding the underlying aetiological processes of HF can provide important information in selecting the most appropriate therapy beyond standard approaches guided by EF phenotypic characterization, especially when specific targeted treatment strategies are indicated,34 provided the diagnostic and/or specific treatment strategies are cost-effective, with favourable benefit risk ratios and are in line with patient goals. For example, a patient with cardiac amyloidosis requires different treatment strategies than standard HF therapies. The diagnosis of such a patient solely as HFpEF or HFrEF without further work-up to confirm the diagnosis of cardiac amyloidosis may deprive the patient potentially life-saving therapies for amyloidosis.
In clinical practice, the aetiology of HF has often been placed into two categories: ischaemic and non-ischaemic cardiomyopathy. However, further diagnostic work-up for aetiology should be carried out beyond the first step of defining ischaemic or non-ischaemic aetiology, especially for dilated, infiltrative, hypertrophic and idiopathic cardiomyopathies.34 Many attempts have been made for morpho-functional classifications of cardiomyopathies in the past.34, 35, 37, 107 In this statement, we do not provide recommendations for classifications of specific cardiomyopathies, as we feel those remain outside the scope of this document.
11 Perspective for the non-cardiologist
The majority of the HF care is provided by non-cardiologists, including general practitioners, internal medicine or family medicine clinicians, hospitalists, emergency room providers, and other specialists. We believe the universal definition will be useful to these clinicians for the timely diagnosis and management of patients with HF. Important points for the non-cardiologists are as follows. it is critical to optimally identify and treat patients at risk for HF to prevent or delay the development of HF; recognize that pre-HF patients, such as asymptomatic patients with elevated natriuretic peptide levels likely will require referral to a cardiologist for further diagnostic and treatment strategies to prevent progression of HF,76, 108 that the diagnosis and timely treatment of HF should not be missed or delayed in patients with symptoms and signs of HF, and elevated natriuretic peptide levels or patients with evidence of systemic or pulmonary congestion/elevated filling pressures, and patients with advanced HF would be considered for referral to HF specialists according to their goals.
12 Appendix
Author relationships with industry and other entities
| Writing Committee Member | Employment/affiliation | Consultant | Speakers Bureau | Ownership/Partnership/Principal | Personal research | Institutional, organizational, or other financial benefit | Expert witness |
|---|---|---|---|---|---|---|---|
| Magdy Abdelhamid | Professor of Cardiovascular Medicine Faculty of Medicine, Kasr Al Ainy, Cairo University, Egypt | None | None | None | None | None | None |
| Stamatis Adampoulos |
Director, Heart Failure and Transplant Units, Onassis Cardiac Surgery Centre, Athens, Greece |
None | None | None | None | None | None |
| Nancy Albert | Associate Chief Nursing Officer, Research and Innovation- Nursing Institute and Clinical Nurse Specialist, Kaufman Center for Heart Failure- Heart, Vascular and Thoracic Institute, Cleveland Clinic, Cleveland, OH, USA | Amgen, AstraZeneca, Boston Scientific, Merck, Novartis | None | None | None | None | None |
| Stefan Anker |
Department of Cardiology (CVK), Charité - Universitätsmedizin Berlin; Berlin Institute of Health Center for Regenerative Therapies (BCRT); German Centre for Cardiovascular Research (DZHK), Berlin, Germany |
Vifor, Bayer, Boehringer Ingelheim, Novartis, Servier, Abbott, Actimed, Cardiac Dimensions, Impulse Dynamics | None | None | Vifor Int and Abbott | None | None |
| John Atherton | Director of Cardiology, Royal Brisbane and Women's Hospital, Faculty of Medicine, University of Queensland, Brisbane, QLD, Australia | Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly, Impedimed, Novartis, Otsuka, Vifor Pharma. | Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, Novartis | None | None | None | None |
| Michael Böhm | Klinik für Innere Medizin III, Universitätsklinikum des Saarlandes, Saarland University, Homburg/Saar, Germany | None | Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Medtronic, Novartis, Servier, Vifor | None | None |
Deutsche Forschungsgemein- schaft (DFG, SFB-TTR 219, S-01) |
None |
| Biykem Bozkurt | Baylor College of Medicine, Winters Center for Heart Failure Research, Cardiovascular Research Institute and Michael E. DeBakey VA Medical Center Houston, TX, USA | Abbott, Amgen, Baxter, Bristol-Myers Squibb, Liva Nova, RELYPSA/Vifor Pharma, Respicardia Registry Steering Committee, Sanofi-Aventis, scPharmaceuticals, Inc | None | None | None | None | None |
| Javed Butler | University of Mississippi Medical Center |
Abbott, Adrenomed, Amgen, Applied Therapeutics, Array, AstraZeneca, Bayer, Boehringer Ingelheim, CVRx, G3 Pharma, Impulse Dynamics, Innolife, Janssen, LivaNova, Luitpold, Medtronic, Merck, Novartis, NovoNordisk, Relypsa, Sequana Medical, V-Wave Limited, and Vifor |
Novartis, BI-Lilly, Astra Zeneca, Janssen |
None | None | None | None |
| Andrew Coats | University of Warwick, UK and Monash University, Australia | AstraZeneca, Bayer, Boehringer Ingelheim, Menarini, Novartis, Nutricia, Servier, Vifor, Abbott, Actimed, Arena, Cardiac Dimensions, Corvia, CVRx, Enopace, ESN Cleer, Faraday, Gore, Impulse Dynamics, Respicardia | None | None | None | None | None |
| Mark Drazner | Clinical Chief of Cardiology, University of Texas Southwestern Medical Center, Department of Internal Medicine/Division of Cardiology | None | None | None | None | None | None |
| G. Michael Felker | Duke University School of Medicine, Durham, NC, USA | Novartis, Amgen, BMS, Cytokinetics, Medtronic, Cardionomic, Innolife, Boehringer Ingelheim, American Regent, Abbott, AstraZeneca, Eidos Therapeutics, Reprieve, and Sequana, and has served on clinical endpoint committees/data safety monitoring boards for Amgen, Merck, Medtronic, EBR Systems, V-Wave, LivaNova, Siemens, and Rocket Pharma | None | None | None | None | None |
| Gerasimos Filippatos | National and Kapodistrian University of Athens; Department of Cardiology, Athens University Hospital Attikon, Athens, Greece | Medtronic, Vifor, Servier, Novartis, Boehringer Ingelheim, Bayer, Amgen. | None | None | None | European Union | None |
| Mona Fiuzat | Duke University | None | None | None | None | None | None |
| Gregg Fonarow | Division of Cardiology, University of California, Los Angeles, CA, USA | Abbott, Amgen, AstraZeneca, Bayer, CHF Solutions, Edwards, Janssen, Medtronic, Merck, Novartis | None | None | NIH | None | None |
| Juan Esteban Gomez-Mesa | Head of the Cardiology Service of the Fundación Valle del Lili. Cali, Colombia; Facultad de Medicina of the Universidad Icesi. Cali, Colombia; Coordinator of the Interamerican Council of Heart Failure & Pulmonary Hypertension of the Interamerican Society of Cardiology. | None | None | None | None | None | None |
| Paul Heidenreich | VA Palo Alto Health Care System, Stanford University School of Medicine | None | None | None | None | None | None |
| Teruhiko Imamura | Second Department of Internal Medicine, University of Toyama | None | None | None | None | None | None |
| Ewa Jankowska |
Department of Heart Diseases, Wroclaw Medical University, Poland and Centre for Heart Diseases, University Hospital, Wroclaw, Poland |
Bayer, Abbott, Vifor Pharma, Pfizer, Servier, AstraZeneca, Boehringer Ingelheim, Berlin Chemie, Novartis, Cardiac Dimensions | Bayer, Vifor Pharma, Pfizer, Servier, AstraZeneca, Boehringer Ingelheim, Fresenius, Berlin Chemie, Gedeon Richter, Novartis | None | None |
Unrestricted research grant for Wroclaw Medical University Co-PI role in the AFFIRM-AHF trial sponsored by Vifor Pharma |
None |
| James Januzzi | Massachusetts General Hospital | Roche Diagnostics, Siemens, Quidel, Abbott, Novartis, Merck, Jana Care, Imbria | None | None | Novartis, Roche | Endpoint committees: AbbVie, Amgen, Bayer, CVRx, Intercept | None |
| Prateeti Khazanie | The University of Colorado School of Medicine, Division of Cardiology, Department of Medicine, Aurora, Colorado, US | None | None | None | None | None | None |
| Koichiro Kinugawa | University of Toyama | None | None | None | None | None | None |
| Carolyn S.P. Lam | National Heart Centre, Singapore & Duke-National University of Singapore | Abbott Diagnostics, Amgen, Applied Therapeutics, AstraZeneca, Bayer, Biofourmis, Boehringer Ingelheim, Boston Scientific, Corvia Medical, Cytokinetics, Darma Inc., Eko.ai Pte Ltd, JanaCare, Janssen Research & Development LLC, Medtronic, Menarini Group, Merck, MyoKardia, Novartis, Novo Nordisk, Radcliffe Group Ltd., Roche Diagnostics, Sanofi, Stealth BioTherapeutics, The Corpus, Vifor Pharma and WebMD Global LLC | None | co-founder & non-executive director of EKo.ai Pte Ltd | Boston Scientific, Bayer, Roche Diagnostics, AstraZeneca, Medtronic, and Vifor Pharma | None | None |
| Yuya Matsue | Department of Cardiovascular Biology and Medicine, Juntendo University Graduate School of Medicine and Cardiovascular Respiratory Sleep Medicine Juntendo University, Graduate School of Medicine | Otsuka Pharmaceutical Co., Ltd. and Novartis Japan | Otsuka Pharmaceutical Co. and Pfizer, Inc. | Philips Respironics, ResMed, and Fukuda Denshi | |||
| Marco Metra | University of Brescia, Brescia, Italy | Actelion, Amgen, AstraZeneca, Bayer, Livanova, Servier, Vifor pharma, WindTree Therapeutics, as member of Committees for clinical trials or advisory boards | Abbott Vascular, Amgen, Edwards Therapeutics, Servier | none | none | None | none |
| Tomohito Ohtani |
Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine |
None |
Bristol-Myers Squibb Company, Daiichi-Sankyo, Nippon Boehringer Ingelheim, Otsuka Pharmaceutical |
None | None | None | None |
| Massimo Francesco Piepoli | Cardiac Unit, Guglielmo da Saliceto Hospital, Piacenza and University of Parma, Italy | AstraZeneca, Novartis, Servier, Vifor, TRX Italy | None | None | None | None | None |
| Piotr Ponikowski | Department of Heart Diseases, Wroclaw Medical University, Poland; Centre for Heart Diseases, University Hospital, Wroclaw, Poland |
Vifor Pharma, Amgen, Servier, Novartis, Bayer, Pfizer, Cibiem, Coridea, Impulse Dynamics, Renal Guard Solutions, Boehringer Ingelheim, AstraZeneca, Corvia, BMS, V-wave Limited |
Vifor Pharma, Amgen, Servier, Novartis, Pfizer, Impulse Dynamics, Renal Guard Solutions, Boehringer Ingelheim, AstraZeneca, Berlin Chemie, Radcliffe Group; Ono Pharmeceutical | None | None |
Abbott Vascular – co-PI of the RESHAPE-2 trial Vifor Pharma – PI of the AFFIRM-AHF trial Vifor Pharma - research grant for the institution Medical Research Agency – Poland: grant for IIT |
None |
| Giuseppe Rosano | IRCCS San Raffaele, Rome, Italy | None | None | None | None | None | None |
| Yasushi Sakata | Department of Cardiovascular Medicine, Osaka University Graduate School of Medicine | None | Otsuka Pharmaceutical, Daiichi-Sankyo, Novartis Pharma K.K., Nippon Boehringer Ingelheim | None | None |
Astellas Pharma Inc. Otsuka Pharmaceutical, Daiichi Sankyo, Medtronic Japan, Nippon Boehringer Ingelheim, Bristol-Myers Squibb K.K. |
None |
| Petar Seferovic |
Vice-president, European Society of Cardiology President, Heart Failure Association of the ESC (2018–2020) Academician, Serbian Academy of Sciences and Arts Professor of Cardiology, University of Belgrade Faculty of Medicine and Heart Failure Center, Belgrade University Medical Center President, Heart Failure Society of Serbia |
Boehringer Ingelheim, Novartis, Vifor Pharma | Boehringer Ingelheim, Novartis, Medtronic, Abbott, Servier, AstraZeneca, Respicardia | None | None | None | None |
| Randy C. Starling |
Employee Cleveland Clinic Heart, Vascular and Thoracic Institute Cleveland, OH, USA Member FDA Circulatory System Devices Panel |
Novartis PARAGLIDE steering committee. No honoraria. Cardiac Dimensions Steering Committee Carillon Trial |
None | None | None | None | None |
| John R. Teerlink | Section of Cardiology, San Francisco Veterans Affairs Medical Center and School of Medicine, University of California San Francisco | Abbott, Amgen, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Cytokinetics, Daxor, EBR Systems, Lilly, LivaNova, Medtronic, Merck, Novartis, Relypsa, Servier, Windtree Therapeutics, ZS Pharma | None | None | None | None | None |
| Hiroyuki Tsutsui |
Department of Cardiovascular Medicine, Faculty of Medical Sciences, Kyushu University |
Nippon Boehringer Ingelheim, Bayer Yakuhin, Novartis Pharma K.K., Ono Pharmaceutical | MSD K.K, Astellas Pharma, Pfizer Japan, Bristol-Myers Squibb, Otsuka Pharmaceutical, Daiichi-Sankyo, Mitsubishi Tanabe Pharma, Nippon Boehringer Ingelheim, Takeda Pharmaceutical, Bayer Yakuhin, Novartis Pharma K.K., Kowa Pharmaceutical, Teijin Pharma Ltd. | None | Actelion Pharmaceuticals Japan, Japan Tobacco Inc., Mitsubishi Tanabe Pharma Corp., Nippon Boehringer Ingelheim, Daiichi-Sankyo, IQVIA Services Japan, Omron Healthcare |
Astellas Pharma Inc. Novartis Pharma K.K., Daiichi-Sankyo, Takeda Pharmaceutical, Mitsubishi Tanabe Pharma Corporation, Teijin Pharma, MSD K.K. |
None |
| Orly Vardeny | Minneapolis Veterans Administration Health Care Center and University of Minnesota | Sanofi-Pasteur, Novartis | None | None | NIH, FDA, AstraZeneca, Bayer | American Heart Association | None |
| Kazuhiro Yamamoto |
Department of Cardiovascular Medicine, and Endocrinology and Metabolism Faculty of Medicine Tottori University |
None | Otsuka Pharmaceutical, Daiichi-Sankyo, Novartis | None | None | Abbott, Otsuka Pharmaceutical Co. Ltd., Medtronic Japan Co., Ltd., Daiichi-Sankyo, Biotronik Japan Inc., Japan Lifeline, Mitsubishi Tanabe Pharma, Fukuda Denshi, Takeda Pharmaceutical, Novartis, Ono Pharmaceutical, Boston Scientific | None |
| Clyde Yancy |
Northwestern University, Feinberg School of Medicine |
Spousal Employment, Abbott, Inc. | None | None | None | Deputy Editor, JAMA Network | None |
| Jian Zhang | Fuwai Hospital, Chinese Academy of Medical Science and Peking Union Medical College | No | No | No | No | No | No |
| Shelley Zieroth | Cardiac Sciences Program, University of Manitoba | Abbott, Akcea, AstraZeneca, Amgen, Alnylam, Boehringer Ingelheim, Eli-Lilly, Merck, Novartis, Otsuka, Pfizer, Servier, Vifor | Amgen, AstraZeneca, Boehringer Ingelheim, Eli-Lilly, Novartis, Servier, Vifor | None | Novartis | None | None |




