Epidemiology of heart failure
Abstract
The heart failure syndrome has first been described as an emerging epidemic about 25 years ago. Today, because of a growing and ageing population, the total number of heart failure patients still continues to rise. However, the case mix of heart failure seems to be evolving. Incidence has stabilized and may even be decreasing in some populations, but alarming opposite trends have been observed in the relatively young, possibly related to an increase in obesity. In addition, a clear transition towards heart failure with a preserved ejection fraction has occurred. Although this transition is partially artificial, due to improved recognition of heart failure as a disorder affecting the entire left ventricular ejection fraction spectrum, links can be made with the growing burden of obesity-related diseases and with the ageing of the population. Similarly, evidence suggests that the number of patients with heart failure may be on the rise in low-income countries struggling under the double burden of communicable diseases and conditions associated with a Western-type lifestyle. These findings, together with the observation that the mortality rate of heart failure is declining less rapidly than previously, indicate we have not reached the end of the epidemic yet. In this review, the evolving epidemiology of heart failure is put into perspective, to discern major trends and project future directions.
Introduction
The purpose of this paper is to provide a contemporary overview of the epidemiology of heart failure for the practicing physician, covering prevalence and incidence, aetiology and prognosis.
The heterogeneity of the heart failure syndrome is reflected in the existing variety of definitions and categorizations leading, in turn, to large variance in reported estimates of occurrence, hospitalizations and mortality rates of heart failure. This paper is not intended to be a comprehensive presentation of all epidemiological reports, but rather as a clinical summary of the evolving heart failure epidemic and as a guide in the understanding of differences across studies.
Occurrence of heart failure
Measuring an epidemic
Heart failure is a heterogeneous syndrome, and both case ascertainment and categorization of patients in epidemiological research are challenging. Left ventricular ejection fraction (LVEF) is generally viewed as a clinically useful phenotypic marker indicative of underlying pathophysiological mechanisms1, 2 and sensitivity to therapy.3, 4 Currently, heart failure patients are most often categorized as having heart failure with reduced (HFrEF; LVEF <40%), mid-range (HFmrEF; LVEF 40–49%), or preserved ejection fraction (HFpEF; LVEF ≥50%).5 Cut-off values are arbitrary and differ across guidelines, and LVEF categorization itself has been criticized for leading to oversimplification of a complex syndrome.4
Several research and clinical criteria have been proposed to diagnose heart failure, each with its own advantages and disadvantages (Table 1).6 Prevalence and incidence are ideally estimated in a (repeated) random sample of the general population, using validated criteria that remain unchanged over time and include objective methods (such as natriuretic peptide measurement and echocardiography) to evaluate cardiac dysfunction. Because a generally accepted ‘gold standard’ is lacking and substantial observer variability occurs even at the expert level of test interpretation,7-9 the use of an expert panel to determine presence or absence of heart failure based on pre-defined echocardiography criteria and biomarkers seems an appropriate method of case validation.10-13
| Definition | Advantages | Disadvantages |
|---|---|---|
| Framingham criteria | ||
| Major and minor signs and symptoms | Widely used and well-validated | Poor sensitivity, especially for early heart failure |
| Chest X-ray | High specificity | |
| 2016 ESC criteria | ||
| Signs and symptoms | Incorporate signs and symptoms with objective measures of cardiac dysfunction | |
| Natriuretic peptides | Natriuretic peptides are easy to measure and widely available | Many patients with proven HFpEF have normal natriuretic peptide levels |
| Echocardiography or other cardiac imaging | EF and diastolic dysfunction can be readily measured with echocardiography | Measurement variability of echocardiographic parameters may be high |
| Gothenburg criteria | ||
| Symptoms and rales | Easily applicable in primary care | Poor sensitivity |
| Atrial fibrillation on ECG | ||
| Boston criteria | ||
| Signs and symptoms | Predicts adverse outcomes | Heavily relies on dyspnoea, which is often absent in the elderly |
| Chest X-ray |
- ECG, electrocardiogram; EF, ejection fraction; HFpEF, heart failure with preserved ejection fraction; ESC, European Society of Cardiology.
- Adapted from Pfeffer et al.6
More often, studies use administrative registry data and billing codes, the accuracy of which may be questioned.14 Concerns include misspecification of diagnoses and ‘upcoding’ (whether or not for reimbursement incentives), which can lead to significant misrepresentation of trends in prevalence and incidence over time. International Classification of Disease (ICD) codes are used worldwide in both primary and hospital care to facilitate comparability in epidemiologic studies. While ICD-10 codes were fairly good at predicting presence of heart failure (sensitivity 68.6%, specificity 99.3%, positive and negative predictive value 90.2% and 97.2%, respectively) when compared to chart reviewing,15 relevant ICD-9 codes were lacking in an estimated one-third of patients hospitalized for acute heart failure exacerbations.16 Self-report data, such as used by the large National Health and Nutritional Examination Survey (NHANES),17 may be more inclusive than registries, but relies on patients knowing their diagnoses.
Hospital records cannot capture all cases of heart failure as patients with chronic heart failure increasingly receive care in an outpatient setting, and may not be referred.18 Furthermore, because heart failure patients are frequently hospitalized for non-cardiovascular causes, accurate identification of hospitalization for acute decompensated heart failure (as opposed to hospitalization for comorbidity) is difficult. As shown in the ARIC study, performance of different classifications for the identification of acute decompensated heart failure was variable.8
Prevalence
An estimated 64.3 million people are living with heart failure worldwide.19 In developed countries, the prevalence of known heart failure is generally estimated at 1% to 2% of the general adult population (Table 2).20-37 Notwithstanding variances in diagnostic criteria, most studies estimated that over half of all heart failure patients in the general population have a preserved LVEF and that this proportion is increasing.34, 38
| First author | Years | Study population | Diagnostic criteria | Prevalence | Incidence |
|---|---|---|---|---|---|
| Studies using non-standardized criteria | |||||
| Loehr20 | 1987–2002 | USA, ARIC study, age-adjusted | First heart failure hospitalization or death certificate |
White men: 6.0/1000 p-y White women: 3.4/1000 p-y Black men: 9.1/1000 p-y Black women: 8.1/1000 p-y |
|
| Jhund21 | 1986 | Scotland, age-adjusted | First heart failure hospitalization |
Men: 1.2/1000 persons Women: 1.3/1000 persons |
|
| 2003 |
Men: 1.1/1000 persons Women: 1.0/1000 persons |
||||
| Curtis22 | 1994 | USA, Medicare beneficiaries, age ≥ 65 years, age-adjusted | Inpatient and outpatient billing codes | 9% | 32/1000 p-y |
| 2003 | 12% | 29/1000 p-y | |||
| Bibbins-Domingo23 | 1985–2006 | USA, CARDIA study, age 18–30 years at enrolment | Hospitalization for heart failure |
Black women: 1.1% Black men: 0.9% (cumulative incidence in 20 years) |
|
| Yeung24 | 1997 | Canada, age ≥ 20 years, age- and sex-standardized | Inpatient and outpatient billing codes | 4.5/1000 persons | |
| 2008 | 3.1/1000 persons | ||||
| Zarrinkoub25 | 2006–2010 | Sweden, all ages, age- and sex-adjusted | Hospital, outpatient and primary care registry | 2.2% | 3.8/1000 p-y |
| Christiansen26 | 1995 | Denmark, age > 18 years, age- and sex-adjusted | First time in-hospital diagnosis of heart failure |
>74 years: 16.4/1000 p-y 65–74 years: 6.3/1000 p-y 55–64 years: 2.0/1000 p-y 45–54 years: 0.50/1000 p-y 35–44 years: 0.13/1000 p-y 18–34 years: 0.04/1000 p-y |
|
| 2012 |
>74 years: 11.5/1000 p-y 65–74 years: 3.5/1000 p-y 55–64 years: 1.7/1000 p-y 45–54 years: 0.64/1000 p-y 35–44 years: 0.20/1000 p-y 18–34 years: 0.07/1000 p-y |
||||
| Conrad27 | 2002 | UK, all ages, age- and sex- standardized | Hospital and primary care health records | 1.5% | 3.6/1000 p-y |
| 2014 | 1.6% | 3.3/1000 p-y | |||
| Störk28 | 2009–2013 | Germany, all ages, age- and sex-standardized | Healthcare claims data | 4.0% | 6.6/1000 persons |
| Benjamin17 | 2011–2014 | USA, NHANES, age ≥ 20 years, age-adjusted | Self-report |
Men: 2.4% Women: 2.6% |
|
| Smeets29 | 2000 | Belgium, aged ≥45 years, age-standardized | Primary care health registry |
Men: 1.5% Women: 1.4% |
Men: 3.1/1000 persons Women: 2.2/1000 persons |
| 2015 |
Men: 1.2% Women: 1.3% |
Men: 2.8/1000 persons Women: 2.3/1000 persons |
|||
| Studies using standardized criteria | |||||
| Bahrami30 | Enrolled 2000–2002, median follow-up 4 years | USA, MESA cohort, not adjusted | MESA criteria |
African-American: 4.6/1000 p-y Hispanic: 3.5/1000 p-y White: 2.4/1000 p-y Chinese-American: 1.0/1000 p-y |
|
| Ho31 | 1981–2008 | USA, FHS cohort | Framingham criteria | 5/1000 p-y | |
| Meyer32 | 1997–2010 | Netherlands, PREVEND cohort | ESC criteria |
Men: 3.7/1000 p-y Women: 2.4/1000 p-y |
|
| Tsao33 | 1990–1999 | Combined FHS and CHS cohort, age ≥ 60 years, age-standardized | Framingham criteria and CHS criteria | 19.7/1000 persons | |
| 2000–2009 | 18.9/1000 persons | ||||
| Gerber34 | 2000 | USA, Olmsted County cohort | Framingham criteria | 3.2/1000 p-y | |
| 2010 | 2.2/1000 p-y | ||||
| Meta-analysis of studies using echocardiographic case validation | |||||
| van Riet35a | 1989–2010 | 28 articles, age ≥ 60 years | Echocardiographic validation using various scores |
≥60 years: 11.8% (median) All ages: 4.2% (calculated) |
|
- ARIC, Atherosclerosis Risk in Communities; CARDIA, Coronary Artery Risk Development in Young Adults; CHS, Cardiovascular Health Study; ESC, European Society of Cardiology; FHS, Framingham Heart Study; MESA, Multi-Ethnic Study of Atherosclerosis; NHANES, National Health and Nutritional Examination Survey; PREVEND, Prevention of Renal and Vascular End-stage Disease; p-y, person-years.
- a Studies that were included in the meta-analysis by van Riet and colleagues are not represented separately.
The NHANES investigators estimated heart failure prevalence in the USA to be 2.5% based on self-reported data.17 The estimated prevalence of heart failure in Germany based on health care claims data of over 3 million inhabitants was 4% for both men and women.39 Based on primary care data of ∼50 practices in Belgium, prevalence of heart failure was 1.2% in men and 1.3% in women in 2015.29 In a population-based study by Conrad and colleagues, using routine primary care data from the UK Clinical Practice Research Datalink of 4 million individuals, prevalence was 1.6%.27 However, since none of these large studies included previously unrecognized cases (nor removed misclassified cases), they cannot account for all heart failure patients in the population at large.
A meta-analysis based on echocardiographic screening studies in the general population—thus also counting previously unrecognized cases—showed that the prevalence of ‘all type’ heart failure in developed countries is around 11.8% in those aged 65 years and over. This would account for a calculated prevalence in the general population of 4.2%; thus around twice as high as some of the reported prevalence based on registries containing only established cases.35 Given that by convention a sample of people from the general population must be included in the denominator, the prevalence rate of 4.2% is a more realistic estimate.
The difference between 4.2% and 2% illustrates that even a prognostically severe syndrome as heart failure may remain undetected in over half of the cases. Especially HFpEF is easily missed: up to 76% of the unrecognized cases of heart failure are patients with a preserved ejection fraction.35 Reasons for not recognizing heart failure are multiple, and include misclassification as chronic obstructive pulmonary disease, deconditioning, ageing, or obesity due to a similarity in symptoms, and unavailability of echocardiography in primary care.40
The absolute numbers of patients living with heart failure have been increasing, as a result of aging of the population, global population growth and improved survival after diagnosis.41, 42 Part of this increase is ‘artificial’, caused by the recognition of HFpEF as an important heart failure subtype.
Incidence
The incidence of heart failure in European countries and the USA ranges widely from 1 to 9 cases per 1000 person-years and strongly depends, again, on the population studied and the diagnostic criteria used (Table 2). In developed countries, incidence rates have stabilized between 1970 and 1990 and are now thought to be decreasing.
In the population-based study by Conrad and colleagues, a decline of 7% of all-type heart failure was noted between 2002 and 2014 from 3.6 to 3.3/1000 person-years [adjusted incidence rate ratio (IRR) 0.93, 95% confidence interval (CI) 0.91–0.94].27 The decline was largely driven by a decline in patients aged 60–84 years. Worrisomely, incidence remained stable or increased in younger patients (<55 years) and in the very old (>85 years), groups that have thus far received fairly little attention. Particularly the very old have been excluded from most randomized controlled trials on which treatment guidelines are based, and the risk–benefit balance of standard therapies might well differ in the very old and frail.43 Data on the occurrence of all-type heart failure in Denmark from 1995 to 2012, based on a national sample of hospitalized patients, showed similar trends.26
An even larger decline in heart failure incidence was observed in the USA. Investigators of the Olmsted County cohort have used diagnostic criteria (ICD-9 codes and rigorous case validation sampling using the Framingham score) that remained uniform over time and can therefore report relatively reliable trends in incidence within the cohort. Importantly, echocardiographic data allowed examination of the respective contributions of HFpEF (LVEF ≥ 50%) and HFrEF (LVEF < 50%) to the population burden of heart failure. Between 2000 and 2010, age- and sex-adjusted incidence declined substantially, from 3.2 to 2.2 cases per 1000 person-years.34 The decline was greater in women (43%) than in men (29%), and greater in HFrEF (45%) than in HFpEF (28%). The proportion of incident HFpEF cases in this cohort increased, simultaneously to an increase in the proportion of heart failure patients with hypertension, atrial fibrillation and diabetes. Recent reliable European estimates of the incidence of heart failure stratified by type come from the Prevention of Renal and Vascular End-stage Disease (PREVEND) study. In this Dutch community-based cohort following 8592 subjects between 1998 and 2010, the European Society of Cardiology criteria were applied by an expert panel to diagnose heart failure. Incidence rate was 3.7/1000 person-years in men and 2.4/1000 person-years in women; 34% of the cases were classified as HFpEF (LVEF ≥ 50%).32, 44
In view of the spectacular decline in incidence of myocardial infarction (MI) of about one third starting in the early '70s of the last century, the decline in standardized heart failure incidence seems modest by comparison.45 The smaller decline in heart failure incidence is likely due to decreased severity of MI, along with better therapeutic management and survival after post-MI ventricular dysfunction. Despite the decline in standardized incidence of heart failure, the total number of new cases of heart failure has increased by 12%, mainly as a result of a growing and aging population and the post-war baby boom, which is now reaching the age at high risk for heart failure.27
Lifetime risk
In the Cardiovascular Lifetime Risk Pooling project, data from several American cohorts (Cardiovascular Health Study, ARIC and the Chicago Heart Association Cohort) was pooled, including nearly 40 000 individuals. At age 45 years, lifetime risks for heart failure through age 95 years were 30% to 42% in white men, 20% to 29% in black men, 32% to 39% in white women, and 24% to 46% in black women.46 In a recent study from the UK, linking electronic health records (from primary care, national registries and hospitals) of 1.25 million individuals, the overall chance that a 30-year-old person develops heart failure during the rest of his or her life is 5%. In hypertensive persons (systolic blood pressure > 140 mmHg), this chance is 7.8%.47 The discrepancies between these studies are likely caused by differences in the study population: the British study excluded people who had experienced any type of cardiovascular disease before the outcome of interest and therefore is expected to find lower estimates, especially since heart failure is strongly associated with age and pre-existing cardiovascular disease.
Heart failure in the young
Heart failure is primarily a disease of the elderly. However, recent studies have indicated that the heart failure burden in the young may be increasing. In a Danish national sample of hospitalized patients, the adjusted IRR of heart failure was 0.90 (95% CI 0.88–0.93) when comparing 2012 with 1995, indicating a substantial decline. However, the mean age at onset of heart failure declined as well, so that the proportion of patients with incident heart failure of 50 years or younger doubled, from 3% to 6%.26 In a Swedish study, linking national hospital discharge and death registries between 1987 and 2006, heart failure incidence increased from the first to the last 5-year period by 50% and 43%, among people aged 18–34 and 35–44 years, respectively.48 Reasons for the opposing trend in the younger population are uncertain, but links have been made with the relentless increase in the worldwide prevalence of obesity and obesity-related comorbidities, such as type 2 diabetes, hypertension, and atrial fibrillation, which also occur in younger patients.26 Alternatively, the increase may reflect a better survival of patients with congenital heart disease, an increase of patients with rare causes of heart failure (e.g. Chagas disease, rheumatic heart disease, amyloidosis) due to migration or simply result from improved registration. To our knowledge, none of these factors have been studied yet. If the increased incidence of heart failure in younger adults continues its current trend, a rise in the future burden of heart failure is to be expected: after all, years lived with heart failure-associated disability will grow as this population segment ages.
Sex differences in heart failure occurrence
While women have a significantly lower incidence rate of heart failure compared to men (at all age categories except >74 years),26 they still account for approximately half of the prevalent cases; notably HFpEF is more common in women.27, 49, 50 In the Swede-HF registry, women accounted for 55% of all HFpEF patients and only 29% of all HFrEF patients.51 Other community-based cohort studies also consistently report higher proportions of women in HFpEF populations, leading to the general idea that women may be more susceptible to HFpEF than men.34, 52 Although the incidence of all-type heart failure decreased for both sexes in Olmsted County, women showed a greater decline, mostly because women exhibited a much larger decline in the incidence of HFrEF than HFpEF (−61% vs. −27%), compared with men (−29% vs. −27%).34 The higher percentage of women with HFpEF in observational studies may, however, partly be the result of the age distribution of the population at risk, as women have a higher life expectancy.53, 54 In the Framingham Heart Study, female sex was not associated with an increased risk of HFpEF, but with a lower risk of HFrEF.31 In pooled data from the Cardiovascular Health Study and the Multi-Ethnic Atherosclerosis Study, the lifetime risk for HFrEF was higher in men than women (10.6% vs. 5.8%) whereas the lifetime risk for HFpEF was similar for both sexes.55
Women are more often obese than men, and the relationship between obesity and incident HFpEF seems stronger in women.56, 57 Similarly, in the Framingham Heart Study, frequency of heart failure was doubled in diabetic men (aged 45 to 74 years), whereas diabetic women had a fivefold increased risk.58 A recent meta-analysis of 47 cohort studies, including 12 million individuals, found that the relative risks for heart failure associated with type 2 diabetes were 1.95 (95% CI 1.70–2.22) in women and 1.74 (95% CI 1.55–1.95) in men, with a pooled men-to-women ratio of the relative risks of 1.09 (95% CI 1.05–1.13), indicating a significant difference.59 Women with type 2 diabetes show more pronounced adverse left ventricular remodelling (oriented toward concentric hypertrophy), and have a lower quality of life and worse outcomes compared with type 2 diabetic men, even when they have a normal body mass index (BMI) and glucose levels in the ‘pre-diabetes’ range.60, 61
A cohort study from the UK including 88 416 individuals with incident heart failure between 1998 and 2017 found that age-adjusted first-year rates of hospitalization increased by 28% for both all-cause admissions and heart failure admissions, with the highest annual increases in women. The reasons for this disparity between the sexes are not clear, but may include later presentation of severe heart failure and a higher comorbidity burden in women, and lack of therapeutic options in HFpEF.62 First-year mortality rates were higher in men than in women in 1998 to 2001, but the gap decreased by 2015, due to declining mortality rates in men and stable rates in women.62
The global burden of heart failure
There is a concerning lack of epidemiological data from countries outside Europe and North America, especially from lower and middle-income countries, even though these are estimated to carry 80% of the cardiovascular disease burden.63 Available data on worldwide prevalence are shown in Figure 1.17, 25, 29, 39, 64-71 To date, there are no population-based studies estimating prevalence and incidence in Northern and sub-Saharan Africa. From scarce literature, heart failure prevalence in Asia seems to be rather similar compared to Western countries, ranging between 1% and 1.3%, although most studies rely on administrative data. A single population-based echocardiographic study investigating HFpEF in Northern China, found a prevalence of 3.5%.64 The prevalence in Australia is 1% to 2% based on national surveys,65 although echocardiographic and biomarker studies showed that the prevalence in Indigenous communities is 5.3%, despite a lower mean age. Over 60% of the cases found through screening were previously unrecognized66 (Figure 1).
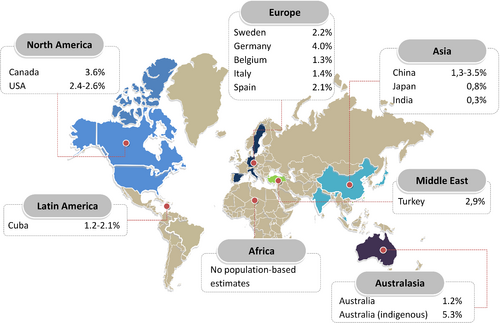
Enrolment of heart failure patients from the Middle-East and Asia-Pacific regions in trials increased from 2.0% in 2005 to 12.8% in 2011, while the contribution of patients from North America and Western Europe declined simultaneously.72 This shift has advanced our understanding of regional differences in heart failure phenotypes. In the Prospective Comparison of ARNI [Angiotensin Receptor–Neprilysin Inhibitor] with ACEI [Angiotensin-Converting–Enzyme Inhibitor] to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial, for example, HFrEF patients from Asia-Pacific regions and Latin-America were 10 years younger compared to European and Northern-American patients.73 Similarly, Asian HFpEF patients in the Asian Sudden Cardiac Death in Heart Failure (ASIAN-HF) registry are also a decade younger than HFpEF patients in the Olmsted County cohort, and have a relatively high comorbidity burden (Table 3).33, 34, 72, 74-78 Particularly the diabetes burden is high in Asia, in spite of a much lower prevalence of overweight/obesity (21.6–26.2% in Southeast Asia compared to 69.6% in the USA).79 In Southeast Asia, a unique lean diabetic phenotype makes up for ∼20% of all heart failure cases and is associated with higher rates of all-cause mortality and hospitalization.80 Infectious diseases including rheumatic fever and HIV remain important causes of heart failure in developing countries across the globe.81, 82 Chagas cardiomyopathy, a preventable parasitic disease, is responsible for about half of all heart failure cases in Latin America.83, 84 Because infections occur at all ages, heart failure in these regions is not primarily a disease of the elderly. In fact, about half of all patients hospitalized in sub-Saharan Africa are under 55 years old.85 Developing countries struggle under a double disease burden as heart failure numbers also continue to rise, due to diseases associated with a Western-type lifestyle, such as diabetes, obesity and ischaemic heart disease.86
| ESC-HF-LT75 | IMPROVE-HF76 | ASIAN-HF77 | INTER-CHF78 | |||||
|---|---|---|---|---|---|---|---|---|
| Eastern Europe | Western Europe | Southern Europe | North-America | Asia-Pacific | Africa | Middle-East | Latin-America | |
| Patients (n) | 1290 | 514 | 4248 | 15 177 | 5276 | 1294 | 1000 | 869 |
| Age (years) | 64 | 62 | 66 | 68 | 60 | 53 | 56 | 67 |
| Male sex (%) | 73 | 73 | 71 | 71 | 78 | 52 | 72 | 61 |
| Diabetes mellitus (%) | 31 | 22 | 33 | 34 | 40 | 17 | 57 | 21 |
| Ischaemic aetiology (%) | 47 | 33 | 41 | 65 | 50 | 20 | 50 | 25 |
| Hypertensive aetiology (%) | N/A | N/A | N/A | N/A | N/A | 35 | 10 | 21 |
| Mortality at 1 year (%) | 8 | 8 | 7 | N/A | 12 (11–13)a | 34 | 9 | 9 |
| Heart failure hospitalization at 1 year (%) | 13 | 16 | 10 | N/A | 15 (14–16)a | N/A | N/A | N/A |
- ASIAN-HF, Asian Sudden Cardiac Death in Heart Failure Registry; ESC-HF-LT, European Society of Cardiology Heart Failure Long-Term Registry; IMPROVE-HF, Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting; INTER-CHF, International Congestive Heart Failure; N/A, not available.
- a All-cause mortality/hospitalization per 100 person-years.
- Adapted from Tromp et al.72
In a laudable effort, the Prospective Urban Rural Epidemiology (PURE) cohort study, involving more than 150 000 adults in three high-, ten middle-, and four low-income countries, quantified worldwide incidence and mortality rates of cardiovascular disease. The incidence of heart failure per 1000 person-years according to income status, estimated after 9.5 years of follow-up, was similar in low-, middle- and high-income countries, despite a higher risk factor burden in high-income countries.87, 88 PARADIGM-HF showed regional differences in heart failure hospitalization rates, which were highest in North-American patients (11 per 100 person-years, 95% CI 9–13) and lowest in Latin-American patients (5 per 100 person-years, 95% CI 5–6).72, 89 Lower hospitalization rates do not reflect lower disease severity, however, as patients from Latin-America also have a high risk of mortality, significantly higher than patients from Western Europe, for example.72, 89 Instead, links can be made with social economic determinants: an inverse relation exists between out-of-pocket costs of health care and hospitalization rates.72
Aetiology of heart failure
Because heart failure can be viewed as the chronic stage of any disease leading to cardiac functional impairment, assigning a specific cause to heart failure in an individual is challenging. Multiple causes often co-exist and most comorbid conditions do not occur independently of heart failure, but share a set of risk factors, have a role in syndrome pathogenesis or function as a perpetuating factor. The majority of patients with heart failure exhibit multi-morbidity and the number of patients with three or more chronic comorbidities increased from 68% in 2002 to 87% in 2014.27 Comorbidity is associated with increased severity of heart failure symptoms and corresponds to a poor quality of life and a worse prognosis.90, 91
Cardiovascular risk factors
Several population-based cohorts have examined common factors that predispose to heart failure, most notably coronary artery disease, hypertension, diabetes, obesity and smoking (online supplementary Table S1). Except for smoking, the burden of risk factors in patients with heart failure is increasing over time, significantly so for diabetes, obesity and hypertension.26, 92 A Belgian study in primary care patients aged 45 years or older showed that the age-adjusted prevalence of cardiovascular risk factors in absence of heart failure symptoms (also known as ‘stage A heart failure’), increased from 27% in 2000 to 35% in 2015 for both men and women.29
Estimates of the prevalence of ischaemic heart disease among heart failure patients vary considerably and are, to some extent, contradictory. Among all Danish adults with a first-time in-hospital diagnosis of heart failure, prevalence of ischaemic heart disease increased from 32% to 45% between 1995 and 2012 (P < 0.0001).26 In contrast, a Swedish study using data from the national hospital discharge and death registries found decreasing trends in the incidence of heart failure after MI (with 4% decrease per year in risk of heart failure, adjusted for age, sex and comorbidities) between 1993 and 2004.93 These discrepancies likely result from differences in population and diagnostic ascertainment, as these studies used non-validated administrative criteria for heart failure diagnosis as well as coronary artery disease diagnosis. In Olmsted County, the proportion of ischaemic HFrEF declined from 39.8% in 2000 to 29.4% in 2010.34 During the same time period, the proportion of ischaemic HFpEF increased from 29.0% to 32.6%. This shift in the contribution of coronary heart disease to the respective types of heart failure parallels the trends observed in the epidemiology of MI, which suggest that both the incidence of hospitalized MI and the severity of MI are decreasing94, 95; a change presumably attributable to improved primary and secondary prevention, a reduction in smoking (including second- and third-hand smoke) and a swifter access to emergency percutaneous coronary interventions. However, ischaemic heart disease will likely remain a major contributor to heart failure incidence, as an ironic success of improved survival after MI and the ageing of the general population.
Hypertension is another potent risk factor. A recent analysis of 123 health surveys from 12 high-income countries showed that hypertension awareness, treatment, and control have improved substantially since 1980, but control rates seemed to have plateaued in the past decade, at levels below 70% even in the countries with the highest rates of control.96 The presence of hypertension increased from 54% to 76% in incident cases of heart failure, between 2002 and 2014.27 In incident heart failure, higher baseline systolic and diastolic levels are associated with a higher rate of adverse events.97
After decades of observing a steadily increasing incidence rate of diagnosed diabetes, U.S. national surveillance data now show a sustained decline.98 However, this may simply be the result of depletion of the population at risk due to successful screening and detection in the previous years.99 Diabetes remains highly prevalent and is present in approximately 40% of HFrEF patients and 45% of HFpEF patients.100 Diabetes and obesity affect left ventricular function also in absence of coronary artery disease and hypertension.1, 101 Importantly, the relationship between diabetes and heart failure seems to be bidirectional.102 Because both type 2 diabetes and heart failure can result in reduced exercise tolerance, and because having a sedentary lifestyle can mask heart failure symptoms, patients with diabetes who develop heart failure are at risk of remaining unrecognized. In a Dutch study of 581 patients aged >60 years with type 2 diabetes, in whom the diagnosis of heart failure was not known, opportunistic screening with questionnaires, physical examination, electrocardiogram and echocardiography revealed ‘new’ heart failure in 27.7% of the participants (4.8% HFrEF and 22.9% HFpEF).103
The rate of obesity is fast on the rise, and the cumulative effects of years lived with excess fat have been linked to the risk of heart failure.26, 104 By 2025, 20% of the world population will be obese (BMI ≥ 30 kg/m2).105 In the general population, there is a direct relationship between body mass and parameters of diastolic dysfunction106 and patients with HFpEF tend to be more obese than their HFrEF counterparts.107 Because obesity is often accompanied by reduced exercise tolerance and supressed levels of natriuretic peptides, and is associated with ventricular enlargement even in the absence of heart failure, diagnosing heart failure in obese patients is particularly difficult.108 Obesity is an independent risk factor, but once the heart failure diagnosis is established higher BMI is associated with lower mortality—the so-called obesity paradox.109
Inflammation
Inflammation plays a key role in the development of both main types of heart failure.110, 111 In HFrEF, cardiac myocytes are directly damaged during the initial cardiac insult (i.e. myocardial infection or ischaemia) and the resulting inflammation triggers eccentric cardiac remodelling. Whether inflammation also perpetuates chronic heart failure is not entirely clear, but studies demonstrating elevations of pro-inflammatory biomarkers (such as tumour necrosis factor-α, interleukin-1, interleukin-6, galectin 3) during heart failure progression suggest that it does.112
Heart failure with preserved ejection fraction, on the other hand, is a slowly progressive condition often without a clear index event. There is controversy on whether HFpEF should be regarded as a single entity or a condition comprising multiple diseases with unique underlying pathophysiological pathways.113 One major paradigm suggests there may be a single common mechanism responsible: a chronic pro-inflammatory state, caused by the plethora of comorbidities that typically affect HFpEF patients, resulting in endothelial inflammation and reduced formation of nitric oxide. Reduced bio-availability of nitric oxide is linked to myocardial and vascular stiffness through the nitric oxide- sensitive protein kinase G/cyclic guanosine monophosphate (cGMP) pathway. This unifying hypothesis is supported by tissue studies of HFpEF patients showing reduced levels of cGMP.114 Metabolic disorders and obesity also promote expansion of the epicardial adipose tissue and secretion of adipocytokines, which causes further inflammation and fibrosis of the underlying myocardium. Based on proteomic analyses, there seems to be a strong relation between inflammatory biomarkers, HFpEF and extracellular matrix reorganization.115 This affects not only the left ventricle, but also the left atrium, and atrial fibrillation may often be the first sign of HFpEF especially in obese or diabetic patients.116 The inflammatory paradigm is somewhat weakened by the fact that trials investigating therapies that stabilize vascular inflammation (such as phosphodiesterase-5 inhibitors) in HFpEF have thus far yielded negative results.113
Elevated concentrations of pro-inflammatory biomarkers are common in both forms of heart failure and associated with disease severity and mortality.117-120 Prediction of new-onset heart failure and discrimination between subtypes using these biomarkers remains problematic: some studies have found pro-inflammatory cytokines (interleukin-6 and tumour necrosis factor-α) to be predictive of both types of heart failure,121 whereas others have found only modest associations of C-reactive protein with incident HFrEF but not HFpEF.122
Socioeconomic status
Socioeconomic status is a well-recognized risk factor for incident cardiovascular diseases. In the UK, prevalence of heart failure standardized by age and sex was 2.0% in the most socioeconomically deprived vs. 1.2% in the least deprived (adjusted rate ratio 1.57, 95% CI 1.55–1.58) based on hospital and primary care data.27 A recent meta-analysis of 11 studies found that low socioeconomic status assessed by all common measures (education, income, occupation and area) independently increases risk of incident heart failure by 62%, overall.123 Although heart failure incidence declined evenly across all socioeconomic strata in the UK, age at first diagnosis differed significantly between the highest and lowest socioeconomic quintiles, and this gap in age widened over time.27
There are various possible mechanisms that may explain the higher incidence rates in the socioeconomically most deprived. Lower socioeconomic status is associated with a higher prevalence of unfavourable behavioural risk factors, including physical inactivity, poor diet, smoking, and medication non-adherence.124 The Framingham Study found a graded inverse association between inflammatory biomarkers and socioeconomic status,125 possibly as the result of stress-induced autonomic and neuroendocrine activation found in socioeconomically deprived.126, 127 Lastly, health care access and utilization decrease with socioeconomic status, even in countries with universal health care systems.128
Risk factors and comorbidities in heart failure with reduced versus preserved ejection fraction
A pooled analysis of four prospective community based cohorts, including nearly 30 000 individuals and 1800 incident HFpEF (LVEF > 45%) and HFrEF (LVEF ≤ 45%) diagnoses, found that very few risk factors and comorbidities differentially predict heart failure subtypes.54 Age was associated with a greater risk of incident HFpEF, whereas smoking status, left ventricular hypertrophy on the electrocardiogram and left bundle branch block were more strongly associated with incident HFrEF (P for comparison ≤0.02). A history of MI was associated with a higher risk of future HFrEF than HFpEF.54 Still, coronary artery disease is prevalent in up to 64% of patients with HFpEF and HFmrEF undergoing coronary angiography129 and is associated with poor outcomes. A recent study suggests that obesity and obesity-related cardiometabolic traits (including insulin resistance) are more strongly associated with the risk of incident HFpEF than HFrEF, especially in women.56 The prevalence of comorbidities has previously been reported to be higher in patients with HFpEF, with on average one more comorbidity than those with HFrEF.130 This is consistent with the idea that the inflammatory state induced by chronic obstructive pulmonary disease, renal impairment and other comorbidities is predictive of incident HFpEF, but not of HFrEF.1, 121, 131 The higher number of comorbidities found in patients with HFpEF may (partly) be driven by age, although a cohort study investigating the occurrence of eight non-cardiac comorbidities according to LVEF showed that the prevalence of all comorbidities except chronic kidney disease remained higher in patients with HFpEF after age adjustment.107 In contrast, in a recent Italian community-based cohort of 2314 chronic heart failure patients, prevalence of 15 non-cardiac comorbidities was similar across all LVEF subtypes, except for hypertension and obesity which were more prevalent in HFpEF.132 A higher number of non-cardiac comorbidities was associated with a higher risk of death (hazard ratio 1.25, 95% CI 1.10–1.26) and hospitalization (hazard ratio 1.17, 95% CI 1.12–1.23). Remarkably, the risk of adverse outcomes increased with the number of comorbidities irrespective of LVEF.132
Prognosis of heart failure
Prognosis has improved significantly since clinical trials came out 30 years ago showing for the first time that the grim outcome of heart failure patients can be considerably altered. There is no doubt however that prognosis remains poor and quality of life remains severely reduced. In age- and risk factor-adjusted models, incident heart failure conferred a fivefold increased risk of death.133 However, to translate the natural history of heart failure as an individual disease into the prognosis of the diseased individual is difficult, and physicians are understandably reluctant to communicate explicit quantitative information on prognosis to their patients. Many prognostic markers of death and hospitalization have been identified, but the clinical value of prognostic models is limited and individual risk stratification remains challenging.
Mortality of heart failure
Estimates of the mortality of heart failure vary considerably, depending on study design, baseline risk of the population studied, heart failure criteria and LVEF cut-off values and the introduction of bias through exclusion of patients with missing LVEF values. The high mortality rates in observational studies contrast with those in clinical trial populations, which generally include ‘stable’ outpatients, younger and with less comorbidities, yielding a lower mortality rate.134
A recent meta-analysis including over 1.5 million all-type heart failure patients, estimated the 1, 2, 5 and 10-year survival to be 87%, 73%, 57% and 35%, respectively.135 Mortality was lowest in studies conducted in secondary care, which was attributed to higher reported prescription rates of heart failure medication. Alternatively, patients in primary care and nursing homes may simply be older and more frail. Not surprisingly, studies applying screening methods found the highest survival rates.135
Hospitalization is an event with clear prognostic value. In a recent report, long-term outcomes among almost 40 000 patients [aged ≥65 years, stratified into HFrEF (46%), HFmrEF (8%) and HFpEF (46%)] hospitalized with heart failure in the Get With The Guidelines-Heart Failure cohort were explored; a very high 5-year mortality rate of 75% was found, regardless of LVEF.136 In a study of 2.1 million inhabitants of the UK, assessing the prognostic burden based on level of care, patients with heart failure newly recorded in primary care and no prior hospital admission had a 5-year mortality rate of 56%, compared to 78% in patients who were hospitalized for heart failure but did not have a primary care record.137 In general, the survival curve drops down fastest during the initial weeks after hospital admission, declining more gradually thereafter. In-hospital mortality rates thus vary widely and their value is unclear as length and continuation of hospital stay may in part depend on whether or not the patient is thought to have reached the palliative stage. Estimates of 30-day mortality, which is less susceptible to bias, range from 5% to 20% and depend strongly on age at admission.20, 21, 138
Observational studies report the risk of death to be as high, or nearly as high, in patients with HFpEF compared to HFrEF.33, 34, 136 This may in part be due to limited power. The large Meta-analysis Global Group in Chronic Heart Failure (MAGGIC) study, pooling data from 31 observational studies and clinical trials, showed that patients with HFpEF were at a 32% lower (adjusted) risk of death compared to their HFrEF counterparts (22% lower adjusted risk after exclusion of clinical trials).139
Trends in mortality of heart failure
Approximately 20% more people survive at both 1-year and 5-year follow-up today compared to 1950–1970. Heart failure survival improved substantially until 1990, but only modestly from 1990 onwards.135 In Olmsted County, mortality rates have remained stable during the last decade, reflecting the transition from HFrEF to HFpEF, for which effective evidence-based strategies are still largely lacking, and the increased burden of comorbidities.34
Causes of death
Because heart failure is a syndrome that can be viewed as the chronic stage of any underlying disease or condition leading to cardiac impairment, estimating the number of deaths attributable to heart failure as the actual cause of death is difficult. Death by heart failure will often be assigned to whatever is thought to be the most likely cause.
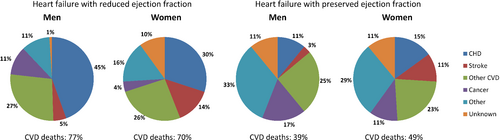
Previously, the majority of patients with heart failure died of cardiovascular causes. In the Framingham Heart Study, causes of death were adjudicated by an expert panel for 463 participants who died between 1974 and 2004 and for whom LVEF and detailed death reports were available (Figure 2).140 Approximately 66% died of cardiovascular causes (70% in HFrEF, 45% in HFpEF patients). Over time, the proportion of cardiovascular deaths seems to be decreasing: in a Spanish cohort following up 1876 patients with LVEF <50%, non-cardiovascular deaths accounted for 17.4% of deaths in 2002, increasing to 65.8% in 2018, mainly due to an increase in cancer deaths.141
In a recent analysis of 12 clinical trials (1995–2014), sudden (or unexpected) cardiac death occurred in 8.9% of patients with HFrEF (LVEF ≤ 40%). There was a decline in the risk of sudden death of 44% over the 19 years studied (hazard ratio 0.56, 95% CI 0.33–0.93; P = 0.03).142 Although for a long time patients with HFpEF were thought to be at low risk for sudden cardiac death, recent studies have shown a considerable increased risk. In HFpEF patients in the Irbesartan in Patients with Heart Failure and Preserved Ejection Fraction (I-PRESERVE) trial and the Aldosterone Antagonist Therapy for Adults With Heart Failure and Preserved Systolic Function (TOPCAT) trial (Americas region included only), 20% to 26% of the deaths were classified as (arrhythmic) sudden cardiac death.143, 144
Hospitalizations
Heart failure hospitalizations represent 1% to 2% of all hospital admissions145 and heart failure is the most common diagnosis in hospitalized patient aged >65 years.17, 146 After the initial diagnosis, the average heart failure patient is hospitalized about once a year.147 In Olmsted County, hospitalization was common at a mean rate of 1.34 per person-year between 2000 and 2010 for both HFpEF and HFrEF and 63% of hospitalizations was for non-cardiovascular causes.34 Because the case mix of heart failure is changing, with a larger proportion of patients with HFpEF compared to HFrEF, the proportion of heart failure hospitalizations for HFpEF increases as well. In the Get With The Guidelines-Heart Failure cohort, the proportion of patients admitted for acute decompensated heart failure who had HFpEF, increased from 33% in 2005 to 39% in 2010.148
Heart failure is, of any diagnosis, associated with the highest 30-day readmission rate (around 20–25%).149-152 Approximately half of the patients will be admitted at least once within 1 year after diagnosis,147, 150, 153, 154 20% will be readmitted again within that same year,62 and over 80% will be readmitted within 5 years.136 Readmissions are often due to reasons other than heart failure, reflecting the high comorbidity burden in these patients. Only around 35% of 30-day readmissions are for heart failure and about half are for cardiovascular causes in general.147, 151, 152
During the '90s, a peak in the number of hospitalizations for heart failure was observed in Sweden,48 Scotland,21 the Netherlands,155 Italy,156 and the USA,157 followed by a decline.18, 21, 26, 157-159 In Denmark, the standardized hospitalization rate (per 100 000 persons) decreased between 1983 and 2012 by 25% for women (from 192 to 144) and by 14% for men (from 217 to 186).160 A similar decline was observed in the USA,161, 162 although a recent report from the ARIC study surveillance data showed a substantial increase in rates of hospitalization for acute decompensated heart failure, primarily HFpEF, between 2005 and 2014. The increase was most prominent in black women (+4.3% annual percentage change) and black men (+3.7%).163 Socioeconomically deprived heart failure patients are at substantially higher risk of all-cause hospitalization (least vs. most affluent group in the UK, IRR 1.34, 95% CI 1.32–1.35).62 Absolute numbers of hospital admissions for heart failure are projected to increase by about 50% over the next 25 years, due to a growing and aging population.164
Conclusion and future directions
The heart failure epidemic is changing. Although age-adjusted incidence has stabilized and seems to be declining, heart failure remains a serious clinical and public health problem as the total number of patients living with heart failure is increasing, reflecting the chronic course of the disease as well as population growth and aging.
The burden of risk factors and comorbidities is high and increasing, especially in the elderly. MI is playing a less prominent role in heart failure aetiology, while obesity is on the rise and projected to play an increasingly important part. The potential gains of reducing risk factors and improving primary prevention and treatment adherence will likely overshadow the effect of any new therapeutic strategy. Care programmes should focus on managing multi-morbidity and chronicity, as individuals will be living with heart failure longer than ever before.
The case mix of heart failure is evolving as well and a larger proportion of patients presenting with heart failure have a preserved ejection fraction. Therapies to effectively reduce mortality in these patients are only beginning to emerge. The decrease in heart failure-related mortality that resulted from progress in preventive and therapeutic management of predominantly HFrEF during the last decades has reached a plateau while hospitalizations remain frequent. Better understanding of the causes of hospital admission and readmission in patients living with heart failure will be needed to improve outcomes.
Lastly, very little is known about heart failure epidemiology in low-income nations. Scarce literature suggests that the prevalence is rapidly increasing in these regions. Low-income nations are disproportionally affected by preventable causes of heart failure, such as rheumatic heart disease and hypertension, which should urge us even more to direct research there.
Funding
This work was supported by the Dutch Heart Foundation (CVON2017-11).
Conflict of interest: none declared.




