Iron deficiency in a multi-ethnic Asian population with and without heart failure: prevalence, clinical correlates, functional significance and prognosis
Abstract
Aims
Current heart failure (HF) guidelines highlight the importance of iron deficiency (ID) in HF. Whether HF itself or age-related comorbidities contribute to ID is uncertain, and previous data were limited to Western populations. We aimed to study the prevalence, clinical correlates, functional significance and prognosis of ID in HF patients, compared with community-based controls in a multi-ethnic Southeast Asian population.
Methods and results
Iron status was assessed in 751 HF patients (age 62.0 ± 12.2 years, 75.5% men, 64.7% Chinese, 23.9% Malay, 10.2% Indian) and 601 controls (age 56.9 ± 10.4 years, 49.8% men, 70.9% Chinese, 21.5% Malay, 7.2% Indian). ID, defined as ferritin <100 µg/L or ferritin 100–300 µg/L and transferrin saturation (Tsat) <20%, was present in 39.3% of controls and 61.4% of HF [odds ratio (OR) 3.5, 95% confidence interval (CI) 2.5–4.9, adjusting for clinical covariates]. Independent correlates of ID in HF were Indian ethnicity (OR 2.4 vs. Chinese, 95% CI 1.2–5.0), female gender (OR 2.8, 95% CI 1.7–4.8), larger body mass index (OR 1.05/unit increase, 95% CI 1.01–1.1) and decreased left ventricular ejection fraction (OR 1.03/unit decrease, 95% CI 1.01–1.04). In a subset of 48 HF patients undergoing cardiopulmonary exercise testing, Tsat correlated with peak oxygen consumption (ρ = 0.53, P < 0.01), independent of baseline characteristics. The HF patients with Tsat <20% as well as anaemia showed the poorest event-free survival after adjusting for clinical covariates.
Conclusions
ID was highly prevalent and independently related to functional capacity and outcomes in our cohort. These findings suggest a pathophysiological role of ID in HF and support its importance as a therapeutic target in Southeast Asian patients with HF.
Introduction
The clinical and prognostic significance of iron deficiency (ID) in heart failure (HF) is now recognized in international HF guidelines.1, 2 Chronic HF is associated with both absolute and functional ID at least in part because of reduced iron absorption and the presence of a generalized inflammatory state.3 However, data on correlates of ID in patients with HF are scarce and it is uncertain if HF itself, or underlying comorbidities, contributes to ID. In a recent pooled analysis of 1500 Caucasian patients with chronic HF, the prevalence of ID was 50%. Independent associates of ID were: gender, New York Heart Association (NYHA) class, mean corpuscular volume and amino-terminal pro-brain natriuretic peptide (NT-ProBNP) levels.4 Existing reports have not included age- or comorbidity-matched controls without HF. Furthermore, previous data have been largely restricted to Western populations with scant data on ID in Asian populations.
Accordingly, we documented the prevalence, clinical correlates, functional significance and prognostic impact of ID in a multi-ethnic Asian population of patients with HF, compared with community-based controls without HF.
Methods
Study population
We studied a cross-sectional sample of participants from the Singapore HF Outcomes and Phenotypes (SHOP) study, which is a prospective longitudinal nation-wide case-control study that aims to establish prevalence, characteristics and outcomes of patients with HF in Singapore, compared with community-based participants. Details on the trial rationale and design have been published.5
Briefly, cases were consecutive patients with a primary diagnosis of HF, uniformly recruited in a compensated state after in-hospital stabilization and just before discharge, or when attending a hospital clinic during an outpatient follow-up visit for HF management within 6 months of an episode of HF decompensation. Trained cardiologists established the diagnosis of HF based on validated clinical criteria from the European Society of Cardiology (ESC),1 the ESC guidelines for the diagnosis of HF with preserved ejection fraction (HFPEF),6 and the Framingham criteria.7 Exclusion criteria were chosen based on study emphasis on HF-specific outcomes. We therefore excluded patients who had comorbid non-cardiac conditions (e.g. metastatic cancer) that would limit life expectancy to <1 year or cause confounding in assignment of aetiology for fluid overload (e.g. end-stage renal failure), as well as patients with specific aetiologies (e.g. congenital heart disease) who would be expected to follow a different natural history compared with a ‘typical’ HF patient.
Controls for our study included randomly sampled free-living adults ≥55 years old identified from the general community through an ongoing epidemiological study of aging in Singapore.8 These were residents (Singapore citizens and permanent residents) in contiguous precincts within five districts in the Southeast region of Singapore who were identified from a door-to-door census and did not have coronary artery disease or HF based on history, clinical, and echocardiographic examination.
The current study population comprised 751 patients with HF and 601 community-based controls. In both groups, Southeast Asian Chinese, Malay and Indian ethnicities were represented. Informed consent was obtained from all participants and the ethics committees of each participating institution approved the protocol.
Study procedures
All participants underwent thorough history and clinical evaluation, resting 12-lead electrocardiogram, blood sampling and comprehensive transthoracic Doppler echocardiography using standardized equipment (Vivid ultrasound systems, General Electric, Milwaukee, WI, USA) with adherence to a uniform image acquisition protocol. Complying with recommendations from the American Society of Echocardiography,9, 10 reporting doctors blinded to the participants' medical condition analysed the images at the Cardiovascular Imaging Core Laboratory, Investigational Medicine Unit, National University Health System, Singapore. The biplane method of disks was used to evaluate left ventricular ejection fraction (LVEF); a LVEF ≥50% was used to define patients with HFPEF. The simplified Modification of Diet in Renal Disease (MDRD) equation was used to calculate estimated glomerular filtration rate (eGFR).11
Measurement of haemoglobin and iron status
Blood samples were collected in ethylenediaminetetraacetic acid (EDTA) and serum tubes and placed on ice before transfer to the Department of Laboratory Medicine, National University Hospital, Singapore for analysis. Plasma was separated out after 10 min at 3500 g, and stored at –80°C before further assessment. We used haemoglobin concentrations of <12 g/dL in women and <13 g/dL in men as cut-offs for anaemia based on the World Health Organization (WHO) definition.12 Serum iron (laboratory reference values were 9.5–30.0 µmol/L for males and 8.8–27.0 µmol/L for females), ferritin (20–300 µg/L for males and 10–120 µg/L for females), and transferrin (200–300 mg/dL) were measured (Siemens Healthcare Diagnostics, Frimley, Camberley, UK). The serum iron and transferrin levels were used to calculate total iron binding capacity (TIBC), where TIBC (µmol/L) = 0.25 × transferrin (mg/dL); and transferrin saturation (Tsat), where Tsat (%) = [serum iron (µmol/L)/transferrin (mg/dL)] × 400.
ID was defined as serum ferritin <100 µg/L or ferritin 100–300 µg/L in combination with Tsat <20%. This definition matches that used in recent trials addressing ID in HF [e.g. the Ferinject Assessment in patients with IRon deficiency and chronic Heart Failure (FAIR-HF) study].13-15 Further to this, we defined functional ID (FID) as Tsat <20%.16
Cardiopulmonary exercise testing (CPET)
A subset of 48 patients with HF and 95 controls underwent maximal-effort recumbent cycle exercise testing using an incremental protocol on a cycle ergometer (Corival; Lode BV, Groningen, the Netherlands). The initial workload of 20 W was increased by 20 W every 3 min until exhaustion. Gas analysis measurements, including the volume of oxygen consumed (VO2), volume of carbon dioxide produced (VCO2) and minute ventilation (MV) were measured using spirometry on a breath-by-breath basis through the Medical Graphics Corporation (St Paul, MN, USA) Cardiorespiratory Diagnostic System (Breeze Suite CPX/D Ultima Series).
Outcomes
Our HF patients were classified into four groups: (i) neither FID nor anaemia, (ii) anaemia only, (iii) FID only, and (iv) FID and anaemia. These patients were followed up for a median of 10 months for HF readmissions and deaths.
Statistical methods
Normally distributed data are presented as mean ± standard deviation, whereas categorical variables are expressed in numbers and percentages. Participants with missing data accounted for <5% of the total sample size and were omitted from analysis. Unadjusted comparisons between the HF and control groups were performed using the Student t-test, Pearson chi-square test and Fisher's exact test as appropriate. The prevalence of ID was compared between HF and control groups, between HF with reduced ejection fraction (HFREF) and HFPEF, as well as between HF patients recruited as inpatients vs. outpatients. Adjustment for demographic and risk factors was performed in the statistical analysis to account for imperfect matching.
To evaluate the clinical correlates of ID, patients were stratified by group (HF vs. control) and covariates tested using multiple logistic regression, where the outcome of interest was the presence or absence of ID, and the covariates considered included age, gender, race, body mass index, renal status (where presence of kidney disease is defined as eGFR <60 mL/kg.1.73 m2), history of diabetes mellitus, history of hypertension, alcohol consumption and smoking status. Additional variables included in the analysis for the HF group were LVEF, history of admission for decompensated HF, the presence of coronary artery disease, previous myocardial infarction, NT-ProBNP level, and inpatient status at recruitment. In the participants who completed the CPET, we assessed the relationship between Tsat and peak VO2 using Spearman's correlation analysis and adjusted for age, gender, haemoglobin concentration and LV ejection fraction using a linear regression model. Risk factors for HF readmissions and deaths were assessed using a Cox proportional hazard regression and prognosis stratified by iron and anaemia status was presented via Kaplan–Meier survival curves.
Results
A total of 4485 patients were screened across all institutions during the study period of March 2011 to December 2013 and 4124 patients were eligible. A total of 808 were excluded because of specific subgroups of HF (e.g. valvular heart disease as a primary cause, isolated right HF caused by severe lung disease, congenital heart disease, etc.), 78 were excluded because of transient acute pulmonary oedema from acute coronary syndrome, 200 were excluded because of end-stage renal failure, 51 were excluded because of a limited life expectancy of <1 year, and 2164 were excluded because of a lack of informed consent, participation in another research trial, inability to comply with study protocol requirements, or refusal to participate. The remaining 823 patients satisfied inclusion and exclusion criteria and provided informed consent for participation. Of these, 72 patients were further excluded; these included 67 with iron panel results not available at time of analysis and five who withdrew from the study, resulting in a final study population of 751 HF patients.
Baseline characteristics
Baseline characteristics of the 751 HF patients and 601 controls are listed in Table 1. The HF group had a mean age of 62.0 ± 12.2 years. Seventy five per cent were male and the ethnic breakdown was 64.7% Chinese, 23.9% Malay, and 10.2% Indian. The 601 participants in the control group were younger, had an ethnic distribution comparable to the HF group, and 49.8% were male. A higher proportion of participants in the HF group had comorbidities, including hypertension, stroke and diabetes. Using haemoglobin cut-off levels of <12 g/dL and <13 g/dL for females and males, respectively, 47.4% of HF patients (308 participants) were found to be anaemic compared with 13% of controls (P < 0.01), and of these 65.3% were iron deficient. Participants in the HF group differed significantly from those in the control group in all measures of iron status, with lower values in all indices except for serum ferritin, which was significantly higher in the HF group. This is largely an effect of the larger number of HF patients with elevated ferritin >300 µg/L (21.8 vs. 15.1%, P < 0.01) compared with controls. In addition, patients with HF had lower eGFR as well as haemoglobin levels compared with community-based controls.
| HF (n = 751) | Controls (n = 601) | P-value | |
|---|---|---|---|
| Age/years (SD) | 62 ± 12.2 | 56.9 ± 10.4 | <0.01 |
| Gender | |||
| Male, n (%) | 563 (75.5) | 299 (49.8) | <0.01 |
| Female, n (%) | 183 (24.5) | 302 (50.2) | |
| Race | |||
| Chinese, n (%) | 482 (64.7) | 426 (70.9) | <0.01 |
| Malay, n (%) | 178 (23.9) | 129 (21.5) | |
| Indian, n (%) | 76 (10.2) | 43 (7.2) | |
| Others, n (%) | 9 (1.2) | 3 (0.5) | |
| Body mass index, kg/m2 | 26.1 ± 5.5 | 25 ± 4 | <0.01 |
| Systolic blood pressure, mmHg | 123.9 ± 22.4 | 131.7 ± 18.6 | <0.01 |
| Diastolic blood pressure, mmHg | 70.1 ± 12.4 | 75.9 ± 11.1 | <0.01 |
| Comorbidities | |||
| Hypertension, n (%) | 533 (71.5) | 189 (31.4) | <0.01 |
| Stroke, n (%) | 83 (11.1) | 2 (0.3) | <0.01 |
| Diabetes, n (%) | 407 (54.6) | 47 (7.8) | <0.01 |
| Peptic ulcer disease, n (%) | 21 (2.8) | 19 (3.2) | 0.8 |
| Anaemia, n (%) | 308 (47.4) | 35 (13) | <0.01 |
| Alcohol consumption, n (%) | 247 (33.1) | 155 (25.8) | <0.01 |
| Smoking, n (%) | 404 (54.1) | 98 (16.3) | <0.01 |
| Investigations | |||
| Serum creatinine (umol/L) | 120.3 ± 59 | 69.3 ± 19.8 | <0.01 |
| MDRD eGFR (ml/min.1.73 m2) | 71.2 ± 44.2 | 108.5 ± 34.3 | <0.01 |
| Haemoglobin (g/dL) | 12.9 ± 2.1 | 13.8 ± 1.6 | <0.01 |
| Haematocrit (%) | 38.6 ± 6.9 | 42.1 ± 10.5 | <0.01 |
| Red cell distribution width (%) | 14.8 ± 2.6 | 13 ± 1.8 | <0.01 |
| Plasma albumin (g/L) | 33.3 ± 5.4 | 42.1 ± 4.6 | <0.01 |
| Left ventricular ejection fraction (%) | 34.4 ± 15.9 | 64.2 ± 4 | <0.01 |
| Iron status | |||
| Serum iron (µmol/L) | 10.6 ± 6.7 | 16.9 ± 6.1 | <0.01 |
| Serum ferritin (µg/L) | 207.3 ± 210.9 | 179 ± 160 | <0.01 |
| Transferrin saturation (%) | 18.3 ± 11.3 | 28.5 ± 11.1 | <0.01 |
| Total iron binding capacity (µmol/L) | 59.2 ± 13.7 | 60.9 ± 9.9 | 0.01 |
| Transferrin (mg/dL) | 227.7 ± 52.8 | 234.1 ± 38 | <0.01 |
| Functional iron deficiency, n (%) | 479 (63.8) | 112 (18.6) | <0.01 |
| Serum ferritin range (µg/L) | |||
| <100 | 272 (36.2) | 208 (34.6) | <0.01 |
| 100–300 | 315 (41.9) | 302 (50.2) | |
| >300 | 164 (21.8) | 91 (15.1) |
- MDRD, Modification of Diet in Renal Disease; eGFR, estimated glomerular filtration rate.
Prevalence of ID
Association with HF status
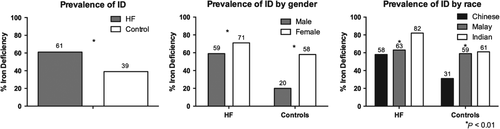
ID (defined using the common criteria from the FAIR-HF study) was present in 39.3% of controls and 61.4% of patients with HF (P < 0.001) (Figure 1). This difference remained significant even after adjusting for age, race, gender, body size, and comorbidities including presence of hypertension, diabetes, and renal status (OR 3.5, 95% CI 2.5–4.9, P < 0.001) (Table 2). Recognizing that the FAIR-HF study criteria may not apply to controls without HF, we also compared FID (defined as Tsat <20%) between HF and controls and found a higher prevalence of FID among patients with HF, even after adjusting for age, race, gender, body size and comorbidities including presence of hypertension, diabetes and renal status (OR 11.7, 95% CI 8.0–17.0, P < 0.001) (Table 2).
| (a) HF vs. controls | Multivariate OR | 95% CI | P value |
|---|---|---|---|
| ID | |||
| Unadjusted | 2.459 | 1.973–3.063 | <0.001 |
| Adjusted* | 3.471 | 2.475–4.869 | <0.001 |
| FID | |||
| Unadjusted | 7.689 | 5.967–9.908 | <0.001 |
| Adjusted* | 11.662 | 7.991–17.020 | <0.001 |
| (b) | HFPEF (n = 154) | HFREF (n = 596) | |
| NT-proBNP (pg/mL) | 2631 ± 4009 | 4780 ± 6320 | <0.001 |
| Transferrin saturation (%) | 17.9 ± 9.4 | 18.4 ± 11.8 | 0.6 |
| FID (%) | 94 (61) | 385 (64.6) | 0.3 |
- OR, odds ratio.
- * Adjusted for: age, gender, race, body mass index, estimated glomerular filtration rate <60 ml/kg/1.73 m2, diabetes, alcohol consumption, smoking and hypertension.
Association with gender
The prevalence of ID was higher in women compared with men in both HF (70.5% vs. 58.6%, P = 0.004) and control (57.9% vs. 20.4%, P < 0.001) groups (Figure 1). These gender differences persisted after adjusting for age, race, body mass index, comorbidities and alcohol and smoking status (OR for HF group 2.8, 95% CI 1.7–4.8, P < 0.001; OR for control group 5.8, 95% CI 3.7–9.3, P < 0.001) (Table 3).
| Variables | Multivariate OR | 95% CI | P-value | |
|---|---|---|---|---|
| HF | ||||
| Age (per 1 year) | 0.997 | 0.980 | 1.015 | 0.761 |
| Female gender | 2.826 | 1.668 | 4.789 | <0.001 |
| Race | ||||
| Malay vs. Chinese | 1.219 | 0.802 | 1.852 | 0.354 |
| Indian vs. Chinese | 2.427 | 1.190 | 4.950 | 0.015 |
| Body mass index (per 1 kg/m2) | 1.051 | 1.014 | 1.090 | 0.007 |
| MDRD eGFR <60 mL/kg.1.73 m2 | 1.223 | 0.845 | 1.770 | 0.285 |
| Diabetes | 1.419 | 0.997 | 2.021 | 0.052 |
| Alcohol consumption | 1.197 | 0.793 | 1.808 | 0.393 |
| Smoking | 1.209 | 0.789 | 1.852 | 0.384 |
| Hypertension | 0.823 | 0.555 | 1.223 | 0.335 |
| LVEF (per 1% decrease) | 1.025 | 1.012 | 1.039 | <0.001 |
| History of HF | 0.992 | 0.647 | 1.523 | 0.972 |
| Coronary artery disease | 1.175 | 0.769 | 1.796 | 0.456 |
| Previous myocardial infarction | 1.180 | 0.746 | 1.867 | 0.479 |
| NT-ProBNP | 1.000 | 1.000 | 1.000 | 0.741 |
| Inpatient recruitment status | 2.062 | 1.451 | 2.930 | <0.001 |
| Controls | ||||
| Age (per 1 year) | 0.955 | 0.936 | 0.975 | <0.001 |
| Female gender | 5.844 | 3.664 | 9.322 | <0.001 |
| Race | ||||
| Malay vs. Chinese | 2.115 | 1.268 | 3.529 | 0.004 |
| Indian vs. Chinese | 3.669 | 1.752 | 7.685 | 0.001 |
| Body mass index (per 1 kg/m2) | 0.962 | 0.913 | 1.014 | 0.146 |
| MDRD eGFR <60 mL/kg.1.73 m2 | 0.594 | 0.061 | 5.737 | 0.652 |
| Diabetes | 1.215 | 0.585 | 2.522 | 0.601 |
| Alcohol consumption | 1.113 | 0.666 | 1.862 | 0.682 |
| Smoking | 1.297 | 0.695 | 2.420 | 0.414 |
| Hypertension | 1.117 | 0.717 | 1.739 | 0.625 |
- OR, odds ratio; MDRD, Modification of Diet in Renal Disease; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; NT-proBNP, Amino-terminal pro-brain natriuretic peptide.
Association with ethnicity
The prevalence of ID differed by ethnicity in both the HF and control groups (P < 0.01 for both) with highest prevalence in Indian participants compared with Malays and Chinese in both groups (81.6 vs. 62.9 vs. 58.1% in HF, P = 0.001; 60.5 vs. 58.9 vs. 30.8% in controls, P < 0.001) (Figure 1). These ethnic differences persisted after adjusting for age, gender, body mass index, comorbidities and alcohol and smoking status (Indian vs. Chinese ethnicity: OR for HF group 2.4, 95% CI 1.2–5.0, P = 0.015; OR for control group 3.7, 95% CI 1.8–7.7, P = 0.001) (Table 3).
Subgroup analyses within the HF group
Within the HF group, 154 (20.5%) patients had HFPEF. There were no statistically significant differences in Tsat and proportion of FID between HFPEF and HFREF groups, although this may have been because of the relatively small number of patients with HFPEF.
Of the 751 patients in the HF group, 452 (60.2%) were recruited as inpatients at discharge and 299 (39.8%) were recruited as outpatients (all within 6 months of clinical decompensation). Baseline characteristics of patients in each group are summarized in the Supporting Information (Table S1). The prevalence of ID was higher among the inpatient group compared with the outpatient group (67.3 vs. 52.5%, P < 0.001). Similar to the HF group as a whole, there were notable sex and ethnicity associations with ID in both the inpatient and outpatient groups (Figure 2).

Clinical correlates of ID
Independent associations with ID in patients with HF were: Indian race (OR 2.4, 95% CI 1.2–5.0, P = 0.015 vs. Chinese), female gender (OR 2.8, 95% CI 1.7–4.8, P < 0.001), larger body mass index (OR 1.05 per 1 kg/m2 increase, 95% CI 1.01–1.1, P = 0.007) and decreased LVEF (OR 1.03 per 1% decrease, 95% CI 1.01–1.04, P < 0.001) (Table 3). Each factor remained statistically significant after adjusting for previous history of decompensated HF or myocardial infarction, presence of coronary artery disease, NT-ProBNP levels and recruitment status. Among controls, Indians (OR 3.7, 95% CI 1.8–7.7, P = 0.001 vs. Chinese) and younger women (OR 0.96 per year age increase, 95% CI 0.94–0.98, P < 0.001; OR for women 5.8, 95% CI 3.7–9.3, P < 0.001) were more likely to have ID.
Association of Tsat with exercise capacity
In 48 HF patients undergoing CPET, Tsat correlated with peak VO2 (Spearman's ρ = 0.53, P < 0.01). Following adjustment for age, gender, haemoglobin level and LVEF, Tsat remained independently associated with peak VO2 (P < 0.01). In contrast, ferritin levels did not correlate with peak VO2 (Spearman's ρ = 0.05, P = 0.71; adjusted P = 0.95). For the 95 control participants, neither Tsat (Spearman's ρ = 0.126, P = 0.22) nor ferritin (Spearman's ρ = 0.05, P = 0.63) was associated with exercise capacity.
Outcomes
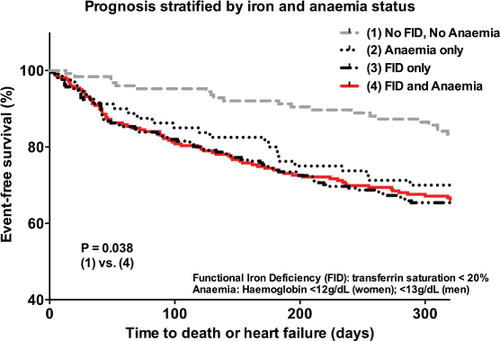
Over a median follow up of 10 months, there were 105 deaths and 140 HF readmissions, of which 35 deaths were a consequence of HF readmission, giving a total of 210 events. The HF patients with both FID and anaemia had the poorest event-free survival compared with those without FID or anaemia even after adjusting for possible confounding factors such as age, gender, ethnicity and comorbidities (P = 0.038). These findings are presented in a Kaplan–Meier survival curve in Figure 3. Inpatient status at recruitment was not associated with worse prognosis (OR 1.282, 95% CI 0.875–1.877, P = 0.202). In addition, there was no interaction between HF status (HFPEF vs. HFREF) and iron status on outcomes (P = 0.704).
Discussion
These first data on iron status in >1300 multi-ethnic Southeast Asian community-based controls and patients with HF demonstrate a remarkably high prevalence of ID with striking ethnic differences. ID was present in two out of five community-dwelling adults, and in the majority (61%) of patients with HF. Among those with HF, Tsat and proportion with FID were comparable in both HFPEF and HFREF groups. The higher prevalence of ID in HF could not be accounted for by age and comorbidities alone. Among ethnic groups, participants of Indian ethnicity had the highest rates of ID regardless of HF status at 82% in the HF group and 61% in community-based controls. The HF patients with anaemia in addition to Tsat <20% had the worst event-free survival compared with those with normal haemoglobin and iron indices. The high prevalence of ID in patients with HF, the independent effect of Tsat on exercise tolerance and prognostic impact suggests that ID may be an important therapeutic target in Southeast Asian patients with HF.
There is increasing awareness worldwide of the significance of ID in patients with HF. In Europe, prevalence rates ranging from 37% to 50%4, 14 have been reported. In the USA, a prospective study of community-dwelling adults with self-reported HF revealed a prevalence rate of 61.3%.17 At 61.4%, the prevalence of ID in patients with HF demonstrated in our study is one of the highest figures among current data and highlights the burden of this condition. Despite the relatively small number of patients with HFPEF in our study (n = 154, 20.5%), our preliminary results suggest that ID may be as highly prevalent in HFPEF as in HFREF, and warrants further study as a novel therapeutic target in HFPEF. Our study also brings to light the previously unrecognized burden of ID in participants without HF, with almost 40% of community-based controls being affected.
We identified gender and ethnicity as independent correlates of ID in patients with HF and in community-based controls. Our findings confirm those of Klip et al.,4 who showed that women were more likely than men to be iron deficient. With a mean age of 60 ± 12 years, the women we studied were mostly post-menopausal, making blood loss from menstruation a very unlikely cause of ID. Moreover, this would not explain the difference in proportion of anaemic females with HF compared with controls. Ethnic differences in iron status have not been widely studied to date and our novel finding of the high prevalence of ID in Indian participants, even after adjusting for clinical and statistical characteristics, suggests the need to explore alternative factors affecting the prevalence of ID. Such factors include dietary habits—there are high proportions of vegetarians among Indian communities as many Indian religions promote vegetarianism. A combination of tea consumption and vegetarianism may further promote ID as black tea has been shown to reduce iron absorption by more than 50% in participants with and without anaemia.18, 19 The potential contribution of genetic determinants of iron status also warrants further study.20
Notably, the mean age of the HF group in our study—62 years—is significantly lower than that of HF patients in Europe or the USA. This worrying trend of Asian patients with HF presenting at a younger age than their American or European counterparts, was also observed in the ADHERE International Asia Pacific Registry21 compared with the US-based ADHERE Registry and European Heart Failure Survey II results. These data highlight the large public health and economic burden of HF in Asia, and suggest that an ‘Asian phenotype’ of aggressive disease progression may exist—a key area of ongoing research.22
There were significant ethnic differences between the HF and control groups in our study. While the ethnic distribution of our control subjects reflects that of the general population of Singapore, there was over-representation of Malay and Indian ethnicities in the HF group. This has previously been attributed to higher rates of hypertension and diabetes among Malays and Indians compared with their Chinese counterparts.23
Ferritin levels were paradoxically higher in HF compared with controls, despite the higher prevalence of ID in HF than in controls, as ferritin is an acute-phase reactant elevated in inflammatory conditions such as HF. This phenomenon has similarly been described in patients with renal failure, where the inflammatory state inhibits the reticuloendothelial system and prevents iron from binding to transferrin. Tsat is relatively unaffected by these conditions and may prove to be a more reliable and specific measure of iron status than ferritin in HF.16
In our study, Tsat correlated with exercise capacity in HF patients whereas ferritin did not. These findings are in contrast with a previous study by Kasner et al.24 who investigated 26 HFPEF patients (15 of whom had FID) and showed no association between exercise capacity and both ferritin and Tsat. Differences in our study populations (stable HFPEF alone in the previous study vs. both HFPEF and HFREF within 6 months of acute decompensation in our current study) may explain these differences. We also found a relationship between Tsat and exercise capacity in patients with HF but not in controls. We postulate that our study may not have been adequately powered to detect a subtle relationship between Tsat and peak oxygen consumption in controls. Furthermore, as multiple factors beyond Tsat contribute to exercise capacity, we postulate that in the control population, compensatory mechanisms in otherwise healthy individuals mask the impact of reduced exercise capacity from low Tsat, whereas these mechanisms may be impaired in patients with HF, thus allowing the ‘unmasking’ of exercise intolerance.
We showed that iron and anaemia status independently correlated with poor prognosis measured by HF readmissions and mortality, reinforcing previous findings in a cohort of European patients with HF.4 This potent prognostic impact suggests a pathophysiological role of ID in HF and adds to recognition of its importance as a potential therapeutic target. Despite this increasing recognition, interventional trials of iron therapy in HF have been few, mixed, and involved relatively short follow-up periods.25 No studies in the literature appear to have proven that iron replacement improves survival in HF. In the largest trial to date, Anker et al.13 randomized 459 chronic HF patients to receive intravenous ferric carboxymaltose or placebo, and showed that intravenous iron improved symptoms, functional capacity, and quality of life in patients with HF. International guidelines now recommend assessing iron status in patients with HF: Class IC in the European Society of Cardiology guidelines26 and Grade B in the National Heart Foundation of Australia guidelines.27
This is the first study to systematically investigate iron status in a cohort of multi-ethnic Southeast Asian community-based controls and patients with HF. Underlying reasons for the high prevalence of ID and striking ethnic differences among Southeast Asian adults deserve further study. The high prevalence of ID in patients with HF, and the independent effect of Tsat on exercise tolerance and prognosis suggests that ID may be an important therapeutic target in Asian patients with HF.
Acknowledgments
We acknowledge Timothy Cushway (Vifor Pharma) for valuable advice and editorial support, as well as the research coordinators, nurses and sonographers who contributed to this paper.
Funding
This work was supported by the National Medical Research Council, Singapore [Centre Grant: R-172-003-219-511 and Clinician Scientist Award to C.S.L.] and an unrestricted research grant from Vifor Pharma.
Conflict of interest: C.S.L. has received research support from Boston Scientific, Medtronic, and Vifor Pharma, and has consulted for Bayer and Novartis. All the other authors have no conflict of interest to declare.




