The middle child in heart failure: heart failure with mid-range ejection fraction (40–50%)
The ‘middle child’, classically the second in a family of three siblings, often feels neglected. While lavish attention is generally paid to the first born and the baby, the middle child is typically squeezed between these two and has trouble finding his/her ‘niche’ in the family. In short, the older child gets all the awards, the younger gets all the love, and the middle gets nothing
The analogy within the heart failure (HF) family is striking: The big brother—HF with reduced ejection fraction (HFrEF)—was the first born in HF clinical trials, with the vast majority of early HF trials being restricted to patients with LVEF <35–40%.1, 2 This is the group for which we have accumulated the most therapeutic evidence, including effective pharmacological and device therapies that have led to impressive improvements in survival. The youngest sibling, HF with preserved EF (HFpEF; defined in current guidelines using an EF cut-off of ≥50%1, 2) was conceived as ‘diastolic HF’ barely two decades ago. While initially regarded by some as an ‘illegitimate’ child, this syndrome has grown to be accepted as true HF, recognized as affecting half the HF population, and projected to become the predominant form of HF in the future.3 While the attention given to HFpEF has increased exponentially, with more and larger trials being focused on this patient population, there is still no effective evidence-based therapy.4
The specific LVEF cut-off for HFpEF that has been advocated by guidelines, and modified slightly by clinical trials (which have mostly chosen 45% rather than 50%), has not been based on any real pathophysiologic or outcomes data advocating for one LVEF cut-off versus the other. However, by defining a cut-off for HFpEF that is higher than that used to define the HFrEF population, we have necessarily left a gap in the 40–50% middle range, leaving the ‘middle child’ of HF neglected. In the American College of Cardiology/American Heart Association guidelines,2 this range is referred to as an ‘intermediate group’, and dismissed with treatment for underlying risk factors/co-morbidities and with medical therapies similar to those used for HFrEF. Similarly, the European Society of Cardiology guidelines1 refer to HF with EF 35–50% as a ‘grey area’ to be regarded as mild systolic dysfunction (i.e. a lesser big brother).
We fully acknowledge, along with others,5 that LVEF is not an ideal parameter to stratify patients with HF. For one, there is inherent variability in the estimation of LVEF from echocardiography, which can potentially lead to misclassification. Other measures of systolic function such as myocardial deformation imaging may be better parameters.6 Yet, clinical trials historically using LVEF to stratify HF have clearly demonstrated its utility in determining outcome benefit of HF therapies: trials of renin–angiotensin–aldosterone system blockade showed improved survival in HFrEF but not in HFpEF. Any classification that can guide treatment may be deemed useful in clinical practice. Until we can effectively tease apart pathophysiological subtypes in HF using a different classification system of proven utility for clinical management and targeted therapy, we are left with our current system of using LVEF. The existence of an ‘evidence gap’ for the patients with mid-range LVEF occurred due to historical development of HF trials rather than due to a strong pathophysiological basis for a third entity in HF. Nonetheless, we refer to this neglected middle child of HF as HF with mid-range EF (HFmEF) herein. The recognition of this group of patients with mid-range EF does not imply that patients inevitably progress from HFpEF to HFmEF then HFrEF; in fact ample evidence exists that HFpEF and HFrEF are distinct syndromes with fundamental pathophysiological differences, and rarely transition from one to the other except in the presence of CAD.4 Our purpose is to summarize available data regarding this patient group and discuss the gaps in evidence that need to be addressed.
What do we know about the middle child of heart failure?
Prevalence of heart failure with mid-range ejection fraction
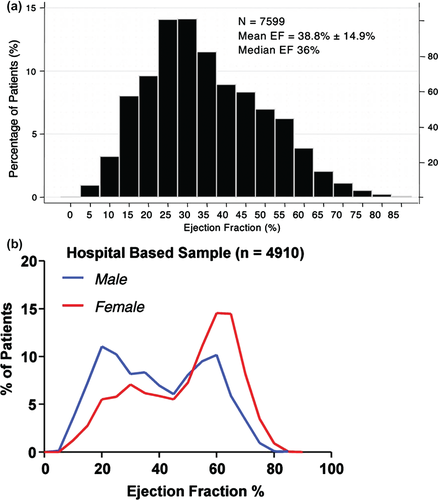
There are limited data regarding the prevalence of HFmEF, since most epidemiological HF studies stratified patients above and below EF 50%; most HFrEF clinical trials included patients with EF <35–40% exclusively; whereas HFpEF clinical trials such as Aldo-DHF7 and RELAX8 used an EF cut-off of 50%, while TOPCAT,9 PARAMOUNT,10 and I-PRESERVE11 used a cut-off of 45% in their inclusion criteria. Nonetheless, a handful of HF studies included a broad range of EF and reported the distribution of EF within their large study populations: in the CHARM Program,12 a unimodal bell-shaped distribution was observed across the range of EF, indicating a substantial proportion of patients in the ‘middle band’ of EF 40–50% (Figure 1A). In fact, 1295 of 7955 (17%) patients in CHARM had EF 43–52%. However, in the OPTIMIZE Registry of patients with acute HF,13 as well as the community-based study from Olmsted County,14 a bimodal distribution of EF was seen, with the nadir at EFs of 40–50%, thus suggesting that HFmEF was relatively uncommon compared with HFrEF or HFpEF in an unselected HF population (Figure 1B). Both community-based studies and clinical trials may be subject to bias.4 Importantly, in both distributions (Figure 1A and B) and in available cohorts (Table 1), a sizeable proportion had HFmEF—cumulatively estimated at 10–20% (14–17% in studies from Table 1) of all patients with HF, and representing a group that should not be neglected. The relatively few patients with HF and EF between 40% and 50% may be because most individuals with a mild reduction in EF do not have clinical HF.
| Study Group | CHARM | CHS | Chinese | ||||||
|---|---|---|---|---|---|---|---|---|---|
| HFrEFa | HFmEF | HFpEF | HFrEF | HFmEF | HFpEF | HFrEF | HFmEF | HFpEF | |
| EF cut-off | ≤40% | 43–52% | >52% | <45% | 45–54% | ≥55% | <40% | 40–55% | >55% |
| n | 4576 | 1295 | 1500 | 60 | 39 | 170 | 98 | 38 | 128 |
| Clinical characteristics | |||||||||
| Mean age, years | 65 | 66 | 67 | 74 | 73 | 75 | 62 | 66 | 72 |
| Men, % | 74 | 68 | 51 | 63 | 49 | 44 | 75 | 79 | 65 |
| Mean weight, kg | – | – | – | 75 | 77 | 76 | 68 | 70 | 70 |
| Mean BMI, kg/m2 | 28 | – | – | – | – | – | 24 | 25 | 25 |
| Mean heart rate, b.p.m. | 74 | 71 | 71 | – | – | – | 77 | 69 | 71 |
| Mean SBP, mmHg | 127 | 135 | 138 | 127 | 136 | 138 | 126 | 141 | 139 |
| Mean DBP, mmHg | 76 | 78 | 77 | 66 | 68 | 68 | 76 | 79 | 76 |
| Hypertension, % | 49 | 60 | 70 | 57 | 72 | 59 | 54 | 77 | 88 |
| CAD/MI, % | 67 – 75 | 72 | 62 | 78 | 69 | 58 | 51 | 65 | 63 |
| Diabetes, % | 29 | 27 | 28 | 23 | 36 | 27 | 41 | 48 | 33 |
| Atrial fibrillation, % | 26 | – | – | – | – | – | 10 | 8 | 26 |
| Echocardiographic characteristics | |||||||||
| LVEDVI, mL/m2 | – | – | – | – | – | – | 101 ± 28 | 82 ± 20 | 53 ± 16 |
| LVESVI. mL/m2 | – | – | – | – | – | – | 70 ± 23 | 45 ± 13 | 20 ± 8 |
| SVI, mL/m2 | – | – | – | – | – | – | 31 ± 8 | 38 ± 8 | 32 ± 9 |
| LV mass, g | – | – | – | 220 ± 77 | 175 ± 32 | 163 ± 37 | 271 ± 66 | 264 ± 74 | 215 ± 69 |
| Left atrial dimension, mm | – | – | – | – | – | – | 42 ± 5 | 41 ± 6 | 39 ± 5 |
| Mitral E velocity, cm/s | – | – | – | 77 ± 34 | 83 ± 30 | 78 ± 27 | 91 ± 27 | 75 ± 28 | 80 ± 24 |
| Mitral A velocity, cm/s | – | – | – | 82 ± 34 | 84 ± 33 | 77 ± 33 | 65 ± 30 | 82 ± 22 | 88 ± 26 |
| Mitral E/A ratio | – | – | – | 1.9 ± 4.9 | 2.6 ± 6.3 | 2.6 ± 8.6 | 1.9 ± 1.3 | 1.0 ± 0.7 | 1.1 ± 0.8 |
| Mitral e' velocity, cm/s | – | – | – | – | – | – | 6 ± 2 | 7 ± 2 | 8 ± 2 |
| Mitral s' velocity, cm/s | – | – | – | – | – | – | 6 ± 1 | 8 ± 2 | 9 ± 2 |
| Laboratory characteristics | |||||||||
| Haemoglobin, g/dL | – | – | – | – | – | – | 13.4 ± 3.1 | 14.5 ± 2.1 | 13.5 ± 2.4 |
| Urea, mmol/L | – | – | – | – | – | – | 8 ± 6 | 10 ± 11 | 8 ± 4 |
| Creatinine, µmol/L | – | – | – | 137 ± 8 | 103 ± 27 | 106 ± 44 | 89 ± 57 | 117 ± 94 | 110 ± 93 |
| BNP, pg/mL | – | – | – | – | – | – | 1487 ± 1242 | 783 ± 1058 | 500 ± 627 |
| NT-proBNP, pg/mL | – | – | – | – | – | – | 8110 ± 9867 | 5623 ± 10234 | 2037 ± 3484 |
| Medical therapy | |||||||||
| ACE inhibitor, % | 56 | 21 | 17 | 42 | 28 | 25 | 50 | 45 | 37 |
| ARB, % | – | – | – | – | – | – | 4 | 23 | 11 |
| Beta-blocker, % | 55 | 57 | 54 | 7 | 8 | 17 | 39 | 58 | 55 |
| Spironolactone, % | 20 | 11 | 12 | – | – | – | |||
| Diuretics, % | 88 | 64 | 63 | 78 | 74 | 59 | 67 | 56 | 37 |
| Digoxin, % | 53 | 32 | 23 | 52 | 46 | 41 | 46 | 23 | 10 |
| Calcium channel blocker, % | 13 | 26 | 37 | 30 | 46 | 31 | 26 | 39 | 57 |
| Lipid lowering, % | 41 | 43 | 40 | 2 | 3 | 5 | – | – | – |
| Device therapy | |||||||||
| Pacemaker, % | 9 | 7 | – | – | – | – | – | – | |
| Defibrillator, % | 4 | 0.8 | – | – | – | – | – | – | |
- BMI, body mass index; CHARM, Candesartan in Heart failure Assessment of Reduction in Mortality and morbidity12; CHS, Cardiovascular Health Study17; Chinese study from the People's Liberation Army General Hospital (Beijing, China)16; DBP, diastolic blood pressure; HFmEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; LVEDVI, left ventricular end-diastolic volume index; LVESVI, left ventricular end-systolic volume index; MI, myocardial infarction; SBP, systolic blood pressure; SVI, stroke volume index.
- a From Young et al. Circulation 2004;110:2618–2626.
Clinical characteristics of heart failure with mid-range ejection fraction
We summarized available data from studies comparing HFmEF with HFrEF and HFpEF (Table 1), acknowledging that slightly different LVEF cut-offs were used to define each group. Clinical characteristics of HFmEF were found to be intermediate between those of HFrEF and HFpEF. Patients with HFmEF are younger and more predominantly male compared with those with HFpEF. Several cardiovascular risk factors were shared among HFmEF, HFrEF, and HFpEF, but patients with HFmEF were more likely to have hypertension (60–77%) compared with those with HFrEF. A significant proportion of patients with HFmEF were diabetic (28–48%). Of note, patients with HFmEF were more likely to have ischaemic heart disease (65–72%) compared with those with HFpEF, and similar to those with HFrEF. The key role of ischaemic heart disease in determining the clinical course of HFpEF has recently been highlighted,15 where HFpEF patients with CAD had greater deterioration of LVEF and increased mortality during follow-up. It is therefore possible that HFmEF constitutes a subset of HFpEF enriched with CAD and representing an early or mild stage of HFrEF. These may be patients with limited or revascularized myocardial infarctions in an early phase of cardiac remodelling after an ischaemic event. Other potential aetiologies such as partially recovered or early stages of myocarditis or cardiomyopathy cannot be excluded.
Echocardiographic and laboratory characteristics of heart failure with mid-range ejection fraction
The intermediate phenotype of HFmEF between HFrEF and HFpEF was most notable in the echocardiographic and haemodynamic features examined in the Chinese cohort.16 LV eccentric remodelling was apparent compared with HFpEF, but less than in HFrEF. This is consistent with the notion that HFmEF may represent an early stage of HFrEF. Functional differences were also discernible, with decreased LV contractility and increased LV diastolic stiffness in HFmEF compared with HFpEF (Figure 2), as well as progressive arterial–LV coupling mismatch from HFpEF (1.3 ± 0.4) to HFmEF (1.9 ± 0.7) and HFrEF (2.6 ± 0.9), associated with progressive left atrial enlargement and increasing levels of circulating natriuretic peptides. Of note, the longitudinal changes in LV remodelling and potential reverse remodelling response to renin–angiotensin–aldosterone inhibitors and beta-blockers have not been studied specifically in HFmEF. Such information would be very useful for testing the hypothesis of whether HFmEF is truly an early manifestation of HFrEF.

Outcomes in heart failure with mid-range ejection fraction
In the Cardiovascular Health Study (Figure 3A),17 the mortality rate was intermediate between that of HFrEF and HFpEF: 115 deaths per 1000 person-years in HFmEF, compared with 154 and 87 deaths per 1000 person-years in HFrEF and HFpEF, respectively, and 25 deaths per 1000 person-years in controls without HF. Adjusted for covariates, the risk for all-cause mortality was significantly increased in HFmEF compared with controls. This is consistent with data in community-based studies of asymptomatic LV systolic dysfunction (Framingham Heart Study,18 Strong Heart Study,19 and Echocardiographic Heart of England Screening Study20) where participants with borderline EF of 40–50% (but without HF) were found to have a poorer prognosis compared with those with EF >50%.
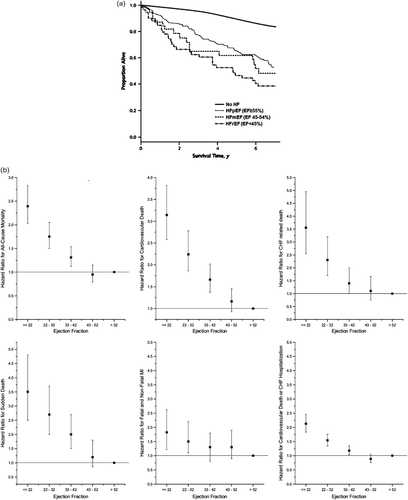
Among patients with stable chronic HF, a graded relationship between lower EF and higher risk of events has been demonstrated, with increased risk beginning at an EF <40%21 to 45%.12 In patients with HF in the CHARM Program (Figure 3B),12 the adjusted hazard ratio for all-cause mortality increased by 39% for every 10% reduction in EF below 45%; however, above an EF of ∼45%, all-cause mortality, cardiovascular death, and all components of cardiovascular death remained relatively stable with increasing EF. These data therefore suggest that in terms of outcomes, chronic stable HFmEF resembles HFpEF more than HFrEF. However, this may not apply to the acute HF hospitalization episode and does not imply that outcomes from therapies in HFmEF will necessarily be more similar to that of HFpEF. Indeed, more data are needed to discern if HFmEF patients derive benefits from disease-modifying agents known to improve outcomes in HFrEF. Such data would help to substantiate a recommendation to treat HFmEF as an early form of HFrEF.
How are we treating this middle child?
Medical therapy of heart failure with mid-range ejection fraction
Heart failure clinical trials have systematically either excluded HFmEF,7, 8 ‘embedded’ HFmEF within the HFpEF group,22 or ‘split’ HFmEF down the middle at an EF cut-off of 45%.9-11 Evidence specific for this group of patients is therefore lacking, and guidelines contain general recommendations to treat HFmEF similarly to HFrEF.1, 2 The lack of consensus and outcomes-proven therapies is reflected in the variability of medications currently prescribed for HFmEF in observational studies (Table 1).
Device therapy of heart failure with mid-range ejection fraction
Similarly, device therapy in HF has been focused on HFrEF. Indeed, CRT has been shown to be effective in reducing morbidity and mortality in patients with HFrEF (EF <35%), and wide QRS or LBBB. In the Multicenter Automatic Defibrillator Implantation Trial-Cardiac Resynchronization Therapy (MADIT-CRT) trial, device therapy was found to improve outcomes in HF patients with EF >30% (up to 42%).23 However a large gap in evidence still exists for the EF 40–50% group. The MIRACLE EF (ClinicalTrials.gov Identifier: NCT01735916) and MADIT-ASIA (ClinicalTrials.gov Identifier: NCT01872234) trials were designed to address patients with HFmEF (EF 35–50%), but both trials have been halted due to difficulties with enrolment.
How should we approach this middle child in future?
We have a few options for approaching HFpEF in future trials: we could (i) study HFmEF as a separate population; (ii) include HFmEF in HFpEF trials by lowering the EF criteria; (iii) include HFmEF in HFrEF trials by raising the EF criteria; or (iv) forget about EF cut-offs in umbrella programmes that cover the entire range of EF in HF, and look for heterogeneity in response by EF thereafter.
The first approach, while most scientifically desirable for its specific focus on HFmEF, may not be feasible given that the population of HFmEF is less prevalent than that of HFrEF or HFpEF and recruiting adequate numbers of patients will therefore be challenging, as evidenced by the recent termination of the MIRACLE EF and MADIT-ASIA trials which specifically included HF patients in the mid-range of EF.
Since current evidence-based therapy for HF includes all patients with LVEF <40%, it would not be desirable to repeat these previous HFrEF trials with a higher cut-off of LVEF <50%. On the other hand, despite prevailing notions of what constitutes ‘preserved’ in HFpEF, there is currently no approved therapy for any patients with HF with an LVEF >40%. We might thus argue that future trials in the higher EF ranges should be more inclusive, i.e. to include all patients with LVEF >40% in this broad category as an alternative to planning separate trials in the ‘middle’ range. However, given the imprecision of LVEF measurements, this strategy may result in the inclusion of patients with LVEF closer to 30–35%. The current trend of using a cut-off of 45% stems from efforts to avoid an ‘LVEF gap’ in trials studying both HFpEF and HFrEF (i.e. efforts to avoid excluding any patient with HF in broad HF trial programmes). Considering that patients with HFmEF may have distinct clinical, echocardiographic, and prognostic characteristics, this may not be an ideal approach either.
In the fourth approach, an EF-agnostic approach risks being underpowered in particular subgroups that are highly pertinent to clinical practice. Furthermore, this approach will only apply to therapies aimed at common mechanisms underlying both HFpEF and HFrEF.
Conclusions
The middle child of HF deserves attention: available data suggest that it constitutes a sizeable proportion (10–20%) of the HF population, has a unique clinical, echocardiographic, haemodynamic, and biomarker profile compared with HFrEF and HFpEF, and carries a poor prognosis. Large gaps in evidence regarding its treatment warrant further study.
Funding
C.S.P.L. is supported by a Clinician Scientist Award from the National Medical Research Council of Singapore.
Conflict of interest: C.S.P.L. has received research support from Boston Scientific, Medtronic, and Vifor Pharma, and has consulted for Bayer and Novartis. S.D.S. has received research support from Novartis and has consulted for Novartis and Bayer.




