Coronary artery disease and 10-year outcome after hospital admission for heart failure with preserved and with reduced ejection fraction
Abstract
Aims
The prognostic impact of coronary artery disease (CAD) in heart failure is debated. Whereas causes of death have been well described in patients with cardiomyopathy, little is known about how CAD influences causes of death in heart failure with preserved ejection fraction (HFPEF). We undertook a 10-year study and analysed causes of death in relation with CAD in HFPEF and in heart failure with reduced ejection fraction (HFREF).
Methods and Results
Our prospective analysis included 591 consecutive patients (320 HFPEF and 271 HFREF) hospitalized for the first time for heart failure during 2000 and followed for 10 years. History of CAD was documented in 25% of HFPEF and 39% of HFREF patients (P < 0.001). Overall, CAD was independently predictive of all-cause and cardiovascular death. CAD had powerful prognostic impact in HFREF [adjusted hazard ratio (HR) 1.60 (1.19–2.15) for all-cause death, and adjusted HR 2.01 (1.38–2.92) for cardiovascular death]. In HFPEF, the association between CAD and cardiovascular death was no longer observed after adjustment [adjusted HR 1.01 (0.69–1.50)]. In HFREF, CAD was associated with increased risk of heart failure-related (adjusted HR 2.03 (1.21–3.43)] and myocardial infarction-related fatal events [adjusted HR 3.84 (1.16–12.7)], while HFPEF patients with CAD appeared at greater risk of sudden death [adjusted HR 2.22 (1.05–4.95)].
Conclusion
The prognostic impact of CAD is different in HFPEF compared with HFREF. Patients with HFPEF and CAD are at high risk of cardiovascular death, especially sudden death.
Introduction
Coronary artery disease (CAD) is the major cause of heart failure (HF) in contemporary Western populations.1 Although CAD has traditionally been reported to adversely impact long-term outcome in heart failure with reduced ejection fraction (HFREF),2-4 recent data are less convincing in this regard.5-7 The excess mortality associated with CAD in a post-hoc analysis of the BEST (Beta-blocker Evaluation of Survival Trial) trial6 could not be demonstrated in patients with systolic HF included in the EVEREST (Efficacy of Vasopressin Antagonist in Heart Failure Outcome Study with Tolvaptan) trial.7 In addition to differences in study design and clinical characteristics, the definition of CAD might explain heterogeneous CAD-related outcomes across studies. Furthermore, the strength of the association between CAD and post-discharge mortality in patients with acute HF seems to be hampered by previous coronary revascularization, which might confer a survival advantage in this setting.5 However, it remains unclear whether systematic assessment of coronary anatomy could provide better explanation for the high mortality and rehospitalization rates in patients who survive a hospital admission for acute HF.1, 8, 9
Heart failure with preserved ejection fraction (HFPEF) is a frequent condition with a notoriously difficult diagnosis, poor prognosis, and no effective treatment.10 Although still matter of debate, the outcome of HFPEF seems better than that of HFREF.11 The reported prevalence of CAD or myocardial ischaemia in HFPEF studies ranges from 0% to 67% depending on the characteristics of the populations.9, 12 Few studies have reported on the association between CAD and survival in HFPEF13, 14 and there are no studies describing the prognostic impact of documented CAD in patients admitted to hospital for new-onset HF. Survival data from the Coronary Artery Surgery Study (CASS)13 obtained in the 1970s show that the extent of angiographically confirmed CAD is adversely affecting prognosis in patients with symptoms of HF and ejection fraction (EF) ≥45%. A report from the Duke Cardiovascular Databank published in 2000 links multivessel CAD with increased mortality in patients with HF symptoms and EF >40%.14 It has been suggested that the frequency of CAD is directly related to the rate of cardiovascular (CV) deaths in different studies15 with higher proportion of non-cardiac deaths in cohorts with a low prevalence of CAD.16, 17
The ETICS (Epidémiologie et Thérapeutique de l'Insuffisance Cardiaque dans la Somme) registry was designed to prospectively follow a large cohort of patients admitted to hospital with new-onset HF during 2000. In the current analysis we aimed to study the influence of a baseline documented diagnosis of CAD on 10-year outcome (overall and cause-specific mortality) in patients with preserved EF and in patients with reduced EF.
Methods
Population and study protocol
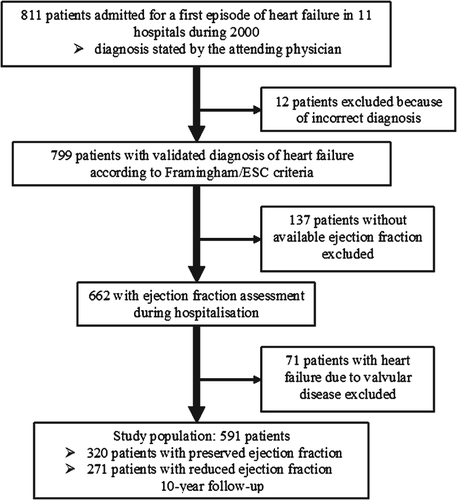
The ETICS registry has been described previously.18 Briefly, all consecutive patients >20 years old, admitted to hospital during the year 2000 for new-onset HF in the 11 establishments of the Somme department, France, were prospectively enrolled. Patients admitted for worsening chronic HF and patients admitted primarily for acute myocardial infarction were excluded. However, patients in whom the diagnosis of acute coronary syndrome was not suspected at admission but confirmed subsequently based on electrocardiogram (ECG) changes and biomarkers were kept in the analysis. The diagnosis of HF was first established by the attending physician in 811 patients, and then validated by two independent cardiologists in 799 patients using the Framingham criteria amended by the European Society of Cardiology.19 Patients in whom EF was not assessed during the index hospitalization were excluded. Furthermore, patients in whom valvular heart disease was the underlying cause of HF were also excluded. The study population therefore included 591 patients (Figure 1). A cut-off of 50% was used to distinguish HFPEF from HFREF.18, 20 EF was preserved in 320 patients and reduced in 271.
Clinical data, including medical history and cardiovascular risk factors were recorded. Complementary investigations included laboratory tests, ECG, chest X-ray on admission, echocardiography, and in some patients coronary angiography. The performance of coronary angiography during hospitalization was left at the choice of the attending physician. EF was determined during hospitalization by echocardiography and/or left ventriculography.
Coronary artery disease was defined by the presence of documented history of acute coronary syndromes, significant CAD previously confirmed by coronary angiography (reduction of the normal diameter ≥50% for the left main coronary artery and of ≥70% for the right coronary, left anterior descending, and circumflex arteries), or history of coronary revascularization before the index admission for HF.
Prognosis
The vital status was obtained either by a consultation with the general practitioner or the referring cardiologist, or by consulting the civil registry. Ten-year follow-up was 97% complete. Cause of death was ascertained by hospital records, death certificates, and autopsy records or by contacting the patients' physician or the referring cardiologist. Records of death were analysed by two independent physicians in a blinded fashion. If reviewers did not agree, a third physician made the final decision regarding a particular event. The cause of death was classified in one of the following six categories: sudden death, HF-related death, acute myocardial infarction death, other CV death, non-cardiovascular (non-CV) death and unknown cause of death.
Sudden death was defined as an unexpected death in a patient who was previously stable and included observed arrhythmic deaths and sudden deaths not attributable to myocardial infarction or other identifiable causes. Death was considered to be HF-related when preceded by marked aggravation of HF symptoms without response to therapy. Acute myocardial infarction death was defined as death occurring after a definite acute myocardial infarction or death with autopsy evidence of recent myocardial infarction. Other CV deaths were considered deaths by CV causes that did not respond to the previous criteria (cerebrovascular events, renal and abdominal vascular events, aortic dissection and pulmonary embolism).21, 22
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) and were compared between groups by Student t-tests. Categorical variables were summarized by frequency per cents and analysed with chi-square tests.
The risk of all-cause mortality and cause-specific mortality was compared in patients with and without CAD. Analyses of mortality were performed by means of Cox proportional hazards models using a competing risks approach. Each cause of death was separately analysed in a Cox model while patients who died of other causes were censored (as non-events) at the time of death.22, 23 For multivariable analyses, we adopted an epidemiological approach without the use of model-building techniques. To determine the independent association of CAD with overall and cause-specific mortality, we adjusted for covariates selected a priori, considered of potential prognostic impact on the basis of clinical judgment. These covariates were: age, sex, New York Heart Association (NYHA) class, history of hypertension, diabetes mellitus, cancer, stroke, chronic obstructive pulmonary disease, atrial fibrillation at admission, serum sodium, estimated glomerular filtration rate, and admission systolic blood pressure. We tested the proportionality assumption for each variable in single-variable analyses using the test proposed by Grambsch and Therneau,24 with a P-value <0.10 indicating non proportionality. In addition, we performed a competing risk regression based on the proportional subhazards method described by Fine and Gray,25 which is implemented in STATA (version 12; StataCorp LP, College Station, TX, USA) under the stcrreg command. In secondary multivariable analyses we used the 45% cut-off to define HFPEF. All-cause survival curves were plotted using the Kaplan–Meier one-failure function and were compared using log-rank tests. Cumulative incidence curves were used to depict cause-specific mortality and corresponding P values were calculated using the proportional subhazards method by Fine and Gray. For all tests, a two tailed P-value ≤0.05 was considered statistically significant. Statistical analysis was performed with SPSS 13.0 statistical software (SPSS Inc., Chicago, IL, USA) and STATA (version 12, StataCorp LP).
The study complied with the Declaration of Helsinki and was approved by local institutional review boards. Informed consent was obtained from all patients. The database was approved by the French Computers and Privacy Commission.
Results
Baseline characteristics
The study population comprised 591 patients (323 males and 268 females). History of CAD was present in 187 patients (32%). Of these, 155 patients (83%) had a previous documented acute coronary syndrome, 30 (16%) had previous coronary bypass grafts and 45 (24%) had previous percutaneous coronary interventions. Patients with CAD (Table 1) were more often males and more had frequently diabetes mellitus, peripheral artery disease, and signs of pulmonary congestion. On admission, EF and estimated glomerular filtration rate were significantly lower in patients with CAD than in patients without CAD (Table 1). Atrial fibrillation was recorded more often in patients without CAD. Among the 591 patients, only eight had an implantable cardioverter–defibrillator. With regard to medical treatment prescribed at discharge in patients who survived the index hospitalization (n = 559), the CAD group had higher prescription rates of angiotensin-converting enzyme inhibitors, beta-blockers, statins, and antiplatelet agents.
| Coronary artery disease | P value | ||
|---|---|---|---|
| No, n = 404 | Yes, n = 187 | ||
| Age (years) | 73.2 ± 12.4 | 74.7 ± 10.9 | 0.18 |
| Systolic blood pressure (mmHg) | 150.8 ± 31.0 | 144.9 ± 32.4 | 0.04 |
| Female gender | 201 (49.8%) | 67 (35.8%) | 0.002 |
| Smoking | 148 (36.6%) | 84 (44.9%) | 0.06 |
| New York Heart Association class III–IV | 385 (95.3%) | 178 (95.2%) | 0.95 |
| Diabetes mellitus | 96 (23.8%) | 63 (33.7%) | 0.010 |
| History of hypertension | 257 (63.6%) | 114 (61.0%) | 0.54 |
| History of stroke | 17 (4.2%) | 14 (7.5%) | 0.1 |
| Peripheral artery disease | 46 (11.4%) | 47 (25.1%) | <0.001 |
| Chronic obstructive pulmonary disease | 88 (21.8%) | 38 (20.3%) | 0.69 |
| Cancer | 42 (10.4%) | 18 (9.6%) | 0.77 |
| Atrial fibrillation at admission | 166 (41.1%) | 33 (17.6%) | <0.001 |
| Left bundle branch block | 58 (14.4%) | 27 (14.4%) | 0.98 |
| Cardiomegaly | 297 (73.5%) | 128 (68.4%) | 0.20 |
| Pulmonary congestion on X-ray | 195 (48.3%) | 133 (71.1%) | <0.001 |
| Ejection fraction (%) | 51.4 ± 16.4 | 46.2 ± 14.7 | <0.001 |
| Serum sodium (mEq/L) | 137.6 ± 4.3 | 137.2 ± 4.5 | 0.35 |
| Glomerular filtration rate (mL/min.1.73 m2) | 61.6 ± 24.1 | 56.0 ± 22.4 | 0.008 |
| Angiotensin-converting enzyme inhibitorsa | 226 (57.4%) | 113 (68.5%) | 0.01 |
| Beta-blockersa | 71 (18.0%) | 72 (43.6%) | <0.001 |
| Aldosterone antagonistsa | 112 (28.4%) | 37 (22.4%) | 0.14 |
| Statinsa | 38 (9.6%) | 75 (45.5%) | <0.001 |
| Aspirina | 102 (25.9%) | 130 (78.8%) | <0.001 |
- Data are mean values ± standard deviations for continuous variables and absolute numbers with frequency percents for categorical variables.
- a Treatment in patients alive at discharge (n = 559).
More than half of the study population had preserved EF (320 patients had HFPEF and 271 HFREF). Among the 187 patients with CAD, previous documented acute coronary syndromes were more frequently recorded in HFREF than in HFPEF (51% vs. 32%, P = 0.03). In contrast, the proportion of patients with previous myocardial revascularization tended to be higher in HFPEF (coronary bypass grafts: 9.6% vs. 6.4%; P = 0.20 and percutaneous coronary interventions: 14% vs. 10%, P = 0.14). In HFPEF, the precipitating causes of hospital admission for HF were: arrhythmias in 30% of patients, acute coronary syndromes in 14%, worsening renal function in 12%, uncontrolled hypertension in 10%, respiratory infections in 9%, and ‘other’ or ‘unknown’ in the remaining 25% of cases. Among patients with HFREF, the precipitating causes of hospital admission were: acute coronary syndromes (30%), arrhythmias (20%), worsening renal function (13%), respiratory infections (4%), uncontrolled hypertension (3%), and ‘other’ or ‘unknown’ (30%). Compared with HFREF, HFPEF admission to hospital appeared to be triggered more frequently by arrhythmia and less often by acute coronary syndromes (both P < 0.01). The characteristics of the two EF groups according to the presence or absence of CAD followed the characteristics of the overall population and are displayed in Table 2.
| HFPEF | HFREF | |||||
|---|---|---|---|---|---|---|
| No CAD, n = 239 | CAD, n = 81 | P value | No CAD, n = 165 | CAD, n = 106 | P value | |
| Age (years) | 75.6 ± 10.4 | 77.9 ± 7.8 | 0.07 | 69.8 ± 14.1 | 72.2 ± 12.2 | 0.16 |
| Systolic blood pressure (mmHg) | 154.9 ± 30.9 | 152.0 ± 35.7 | 0.51 | 144.9 ± 30.3 | 139.6 ± 28.6 | 0.15 |
| Female gender | 136 (56.9%) | 35 (43.2%) | 0.03 | 65 (39.4%) | 32 (30.2%) | 0.12 |
| Smoking | 73 (30.5%) | 30 (37.0%) | 0.28 | 75 (45.5%) | 54 (50.9%) | 0.38 |
| New York Heart Association class III–IV | 225 (94.1%) | 79 (97.5%) | 0.23 | 160 (97.0%) | 99 (93.4%) | 0.16 |
| Diabetes mellitus | 57 (23.8%) | 32 (39.5%) | 0.007 | 39 (23.6%) | 31 (29.2%) | 0.30 |
| History of hypertension | 185 (77.4%) | 58 (71.6%) | 0.29 | 72 (43.6%) | 56 (52.8%) | 0.14 |
| History of stroke | 12 (5.0%) | 5 (6.2%) | 0.69 | 5 (3.0%) | 9 (8.5%) | 0.05 |
| Peripheral artery disease | 21 (8.8%) | 19 (23.5%) | 0.001 | 25 (15.2%) | 28 (26.4%) | 0.02 |
| Chronic obstructive pulmonary disease | 52 (21.8%) | 16 (19.8%) | 0.70 | 36 (21.8%) | 22 (20.8%) | 0.84 |
| Cancer | 33 (13.8%) | 6 (7.4%) | 0.13 | 9 (5.5%) | 12 (11.3%) | 0.08 |
| Atrial fibrillation at admission | 104 (43.5%) | 15 (18.5%) | <0.001 | 62 (37.6%) | 18 (17.0%) | <0.001 |
| Left bundle branch block | 18 (7.5%) | 9 (11.1%) | 0.32 | 40 (24.2%) | 18 (17.0%) | 0.16 |
| Cardiomegaly | 159 (66.5%) | 51 (63.0%) | 0.56 | 138 (83.6%) | 77 (72.6%) | 0.03 |
| Pulmonary congestion on X-ray | 99 (41.4%) | 55 (67.9%) | <0.001 | 96 (58.2%) | 78 (73.6%) | 0.01 |
| Ejection fraction (%) | 63.1 ± 8.2 | 60.3 ± 8.1 | 0.007 | 34.4 ± 8.5 | 35.5 ± 8.0 | 0.29 |
| Serum sodium (mEq/L) | 137.5 ± 4.3 | 137.7 ± 4.3 | 0.63 | 137.7 ± 4.3 | 136.8 ± 4.7 | 0.11 |
| Glomerular filtration rate (mL/min.1.73 m2) | 61.5 ± 24.2 | 53.9 ± 20.2 | 0.01 | 61.6 ± 24.1 | 57.7 ± 23.9 | 0.19 |
| Angiotensin-converting enzyme inhibitorsa | 100 (42.9%) | 45 (57.7%) | 0.02 | 126 (78.3%) | 68 (78.2%) | 0.98 |
| Beta-blockersa | 44 (18.9%) | 38 (48.7%) | <0.001 | 27 (16.8%) | 34 (39.1%) | <0.001 |
| Aldosterone antagonistsa | 49 (21.0%) | 16 (20.5%) | 0.92 | 63 (39.1%) | 21 (24.1%) | 0.02 |
| Statinsa | 24 (10.3%) | 38 (48.7%) | <0.001 | 14 (8.7%) | 37 (42.5%) | <0.001 |
| Aspirina | 65 (27.9%) | 62 (79.5%) | <0.001 | 37 (23.0%) | 68 (78.2%) | <0.001 |
- Data are mean values ± standard deviations for continuous variables and absolute numbers with frequency percents for categorical variables.
- CAD, coronary artery disease.
- a Treatment in patients alive at discharge (n = 559).
Short-term outcome
During the index hospitalization, 32 patients died (23 patients with HFREF and nine patients with HFPEF). The presence of CAD was associated with higher in-hospital mortality rate in HFREF (5.5% vs. 2.7%, P = 0.002). In HFPEF, the presence of CAD did not significantly influence in-hospital mortality (1.9% vs. 0.8%, P = 0.57). While in HFPEF, 1-year all-cause mortality was not influenced by CAD (28% for CAD vs. 21% for non-CAD, P = 0.23), in HFREF, CAD was associated with excess 1-year all-cause mortality (38% for CAD vs. 17% for non-CAD, P < 0.001). In HFPEF, on multivariable analysis, CAD did not increase the risk of 1-year all-cause mortality [adjusted HR 1.19 (0.69–2.07)] and 1-year CV mortality [adjusted HR 1.22 (0.62–2.40)]. In contrast, in HFREF CAD was independently predictive of 1-year all-cause and CV mortality [adjusted HR 2.22 (1.33–3.73) for all-cause death and adjusted HR 2.61 (1.43–4.76) for CV death]. On multivariable analysis, patients with CAD and HFREF were at higher risk of 1-year all-cause death [adjusted HR 1.74 (1.01–3.03)] and 1-year CV death [adjusted HR 1.95 (1.03–3.69)] compared with patients with CAD and HFPEF.
Overall long-term prognostic impact of CAD
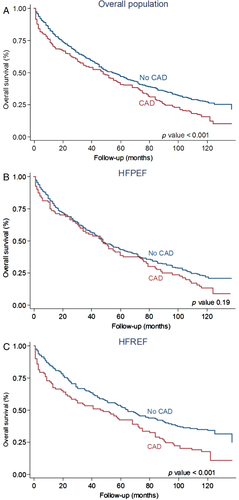
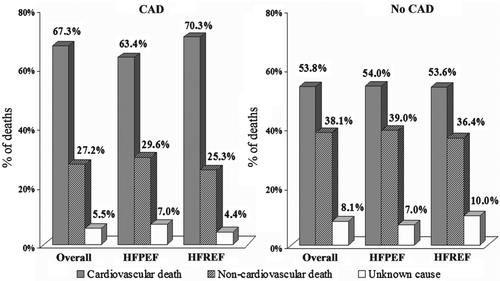
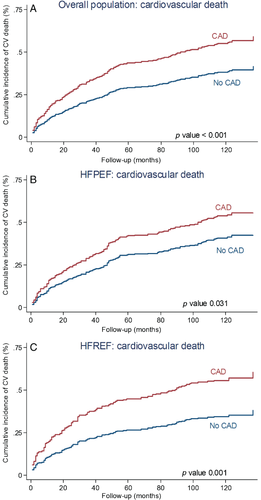
During the 10-year follow-up, 459 patients (78%) died. The 10-year all-cause mortality was significantly higher in patients with CAD than in patients without CAD (87% vs. 74%, P < 0.001; Figure 2). The strong association between CAD and all-cause death was still observed on multivariable analysis (Table 3). Causes of death were CV in 269 patients, non-CV in 157 patients and unknown in 33 patients (Figure 3). The cumulative incidence of CV death was greater in patients with CAD than in non-CAD patients (Figure 4). On multivariable analysis, CAD was independently predictive of CV death (Table 3).
| Univariate analysis | Multivariable analysisa | |||
|---|---|---|---|---|
| HR (95% CI) | p | HR (95% CI) | P | |
| Overall population | ||||
| All-cause mortality | 1.44 (1.19–1.74) | <0.001 | 1.27 (1.03–1.55) | 0.02 |
| Cardiovascular mortality | 1.78 (1.39–2.27) | <0.001 | 1.52 (1.17–1.97) | 0.002 |
| Sudden death | 2.74 (1.63–4.62) | <0.001 | 2.15 (1.23–3.76) | 0.008 |
| Heart failure-related death | 1.46 (1.02–2.10) | 0.04 | 1.29 (0.88–1.89) | 0.19 |
| Myocardial infarction | 2.66 (1.23–5.74) | 0.01 | 2.42 (1.08–5.43) | 0.03 |
| Other cardiovascular deaths | 1.46 (0.84–2.51) | 0.17 | 1.25 (0.70–2.24) | 0.44 |
| Non-cardiovascular mortality | 1.05 (0.74–1.48) | 0.81 | 1.02 (0.69–1.44) | 0.98 |
| Unknown cause of death | 0.97 (0.45–2.09) | 0.94 | 0.76 (0.33–1.74) | 0.51 |
| HFPEF | ||||
| All-cause mortality | 1.20 (0.91–1.58) | 0.19 | 0.93 (0.69–1.26) | 0.65 |
| Cardiovascular mortality | 1.41 (0.99–2.00) | 0.06 | 1.01 (0.69–1.50) | 0.94 |
| Sudden death | 3.36 (1.66–6.81) | 0.001 | 2.22 (1.05–4.95) | 0.03 |
| Heart failure-related death | 0.72 (0.38–1.35) | 0.30 | 0.57 (0.29–1.11) | 0.10 |
| Myocardial infarction | 1.61 (0.48–5.36) | 0.43 | 1.15 (0.31–4.31) | 0.84 |
| Other cardiovascular deaths | 1.62 (0.83–3.15) | 0.15 | 1.09 (0.52–2.32) | 0.81 |
| Non-cardiovascular mortality | 0.91 (0.56–1.48) | 0.70 | 0.77 (0.46–1.29) | 0.33 |
| Unknown cause of death | 1.20 (0.43–3.37) | 0.73 | 1.04 (0.34–3.21) | 0.95 |
| HFREF | ||||
| All-cause mortality | 1.77 (1.34–2.34) | <0.001 | 1.60 (1.19–2.15) | 0.002 |
| Cardiovascular mortality | 2.26 (1.58–3.22) | <0.001 | 2.01 (1.38–2.92) | <0.001 |
| Sudden death | 2.22 (1.03–4.81) | 0.04 | 1.78 (0.78–4.08) | 0.17 |
| Heart failure-related death | 2.33 (1.43–3.81) | 0.001 | 2.03 (1.21–3.43) | 0.008 |
| Myocardial infarction | 3.90 (1.31–11.6) | 0.02 | 3.84 (1.16–12.7) | 0.03 |
| Other cardiovascular deaths | 1.35 (0.52–3.48) | 0.54 | 1.64 (0.54–4.96) | 0.38 |
| Non-cardiovascular mortality | 1.31 (0.79–2.20) | 0.30 | 1.52 (0.86–2.69) | 0.15 |
| Unknown cause of death | 0.73 (0.23–2.31) | 0.59 | 0.48 (0.12–1.92) | 0.31 |
- CI, confidence interval; HFPEF, heart failure with preserved ejection fraction; HFREF, heart failure with reduced ejection fraction; HR, hazard ratio.
- a Adjusted for: age, sex, New York Heart Association class, history of hypertension, diabetes mellitus, cancer, stroke, chronic obstructive pulmonary disease, atrial fibrillation at admission, serum sodium, estimated glomerular filtration rate, and systolic blood pressure at admission.
CAD and long-term outcome in HFPEF
During the 10-year follow-up, in HFPEF there were more events in the CAD group than in the non-CAD group but the difference did not reach statistical significance (88% vs. 78%; P = 0.19; Figure 2). On multivariable analysis CAD was not independently predictive of all-cause death (Table 3). With regard to cause-specific mortality, there were 146 CV deaths, 94 non-CV patients, and in 18 patients the cause of death remained unknown (Figure 3). The HFPEF patients with CAD had greater cumulative incidence of CV death compared with non-CAD patients (Figure 4). After adjustment for covariates, the prognostic impact of CAD was no longer observed (Table 3). Cumulative incidence curves (Figure 5) demonstrated a greater frequency of sudden death among HFPEF patients with CAD compared with non-CAD patients while the proportion of HF-related death was comparable between CAD and non-CAD patients. On multivariable analysis, CAD remained independently predictive of sudden death [adjusted HR 2.22 (1.05–4.95); Table 3).
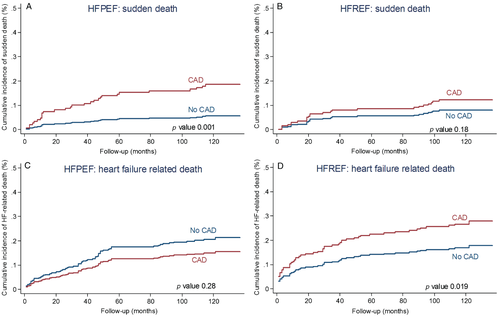
CAD and long-term outcome in HFREF
In HFREF, 10-year event-rate was significantly greater in the CAD group than in the non-CAD group (86% vs. 67%, P < 0.001; Figure 2). On multivariable analysis, CAD was independently predictive of all-cause death (Table 3). In the HFREF group, 123 patients died of CV causes, 63 deaths were non-CV, and in 15 cases the causes of death were unknown (Figure 3). Cumulative incidence of CV death was significantly greater in patients with CAD than in patients without CAD (Figure 4). Multivariable analysis confirmed that CAD was independently predictive of CV death in HFREF (Table 3). The HFREF patients with CAD had greater cumulative incidence of HF-related death (Figure 5) and acute myocardial infarction death than patients without CAD. After adjustment for covariates of prognostic impact, CAD was still predictive of HF-related death and acute myocardial infarction death (Table 3).
Long-term prognostic impact of CAD in HFPEF vs. HFREF
Among patients with CAD, the 10-year event-rate was practically identical in the two EF groups (88% in HFPEF and 86% in HFREF; P = 0.72). On multivariable analysis, patients with CAD and HFREF were at higher risk of all-cause death than patients with CAD and HFPEF [adjusted HR 1.48 (1.05–2.07)]. The frequency of 10-year CV death was comparable in CAD patients with HFPEF and with HFREF (56% vs. 60%, P = 0.51). After adjustment for covariates, patients with CAD and HFREF had a greater risk of CV death compared with patients with CAD and HFPEF [adjusted HR 1.68 (1.11–2.54)].
Secondary analyses
When the 45% cut-off was used to define HFPEF, results remained essentially the same. In HFREF, CAD was independently predictive of 10-year all-cause death [adjusted HR 1.88 (1.34–2.62)] and 10-year CV death [adjusted HR 2.42 (1.58–3.70)]. No association between CAD and increased risk of all-cause and CV death was observed in HFPEF [adjusted HR 0.96 (0.71–1.28) for 10-year all-cause death and adjusted HR 1.04 (0.74–1.48) for 10-year CV-death]. With regard to cause-specific mortality, in HFREF CAD increased the risk of HF-related death [adjusted HR 2.12 (1.18–3.83)] and acute myocardial infarction death [adjusted HR 4.43 (1.28–15.3)] while in the HFPEF group CAD increased the risk of sudden death [adjusted HR 1.91 (1.06–4.07)].
Discussion
To the best of our knowledge, this prospective study is the first to investigate the influence of a documented history of CAD on 10-year outcome after a first hospital admission for HF with preserved and with reduced EF. Our results confirm that CAD is associated with substantial long-term mortality in patients with HF. In the presence of documented CAD, the vast majority of our patients died of cardiovascular causes, and this proportion was comparable between HFPEF and HFREF. However, after taking into account other variables with prognostic impact, CAD remained independently predictive of CV mortality in HFREF but not in HFPEF. Furthermore, the relationship between CAD and causes of death was different in patients with preserved and with reduced EF. In the presence of CAD, sudden death was the leading cause of death in HFPEF, while most patients with CAD and HFREF died of refractory HF. Finally, after adjustment for other important variables, CAD remained independently predictive of sudden death in HFPEF. In HFREF, CAD showed an independent association with HF-related death and with acute myocardial infarction death.
The prevalence of CAD in different cohorts of HF patients is extremely variable depending on the characteristics of the populations and the criteria used for the definition of CAD.9, 15 Randomized HF clinical trials7, 21 report higher frequency of CAD compared with epidemiological studies.16 In our cohort, documented CAD was present in 25% of patients with HFPEF and in 39% of patients with HFREF—figures that are very similar with those reported by Henkel et al.16 In contrast, with our findings, the frequency of CAD was slightly higher among patients with HFPEF included in the Irbesartan in Heart Failure with Preserved Ejection Fraction Study (I-Preserve)21 and reached 65% in the angiographic study reported by O'Connor et al.14 Our study used a clinical definition of CAD that probably led to an underestimation of the true proportion of patients with coronary disease. However this definition is, in our opinion, the most straightforward and was validated by previous reports.5, 7, 16
Classical studies conducted in HFREF which included angiographic criteria for CAD have demonstrated a strong link between CAD and mortality.3, 26 However, in recent analyses, documented CAD did not have an impact on mortality,5, 7 but increased the risk of readmission for HF.7 These latter studies had relatively short follow-up. Despite these discordances, a systematic search for CAD is performed in routine clinical practice in patients admitted to hospital for new-onset HF with reduced EF or in patients with low EF who experience frequent readmissions. On the other hand, the influence of CAD on mortality in patients admitted to hospital for HFPEF has not been well studied.13, 14, 27 A report from the CASS published in 1991 showed that the extent of angiographically confirmed CAD had definite prognostic impact in patients with symptoms of HF and EF ≥45%.13 In patients with HF symptoms and EF >40% who underwent cardiac catheterization at a single tertiary centre between 1984 and 1996, multivessel CAD was associated with increased mortality.14 Recently, Mentz et al.27 have demonstrated that in patients with HFPEF, angina pectoris predicted higher revascularization rates but was not associated with increased risk of death, myocardial infarction, stroke, or rehospitalization. Our study shows that CAD-related mortality is high in both HFPEF and HFREF (more than 60% of deaths). Whereas in HFREF CAD showed a strong impact on 10-year CV mortality on both univariate and multivariable analysis, in HFPEF the excess CV death rate associated with CAD was no longer observed after taking into account other important variables associated with outcome. This lack of independent predictive power is not surprising given the important differences in baseline characteristics between CAD and no-CAD groups. Along with a possible underestimation of the true frequency of CAD among patients with HFPEF, these differences might have dampened the association between CAD and CV death.
Our study adds to the growing literature describing the causes and modes of death in HFPEF and provides for the first time for HFPEF cause-specific mortality in relation with documented CAD. The proportion of CV deaths in HFPEF varies between 50% and 70% and is, in general, higher in randomized trials21, 28 and lower in epidemiological studies.16, 29, 30 In accordance with previous data, our results show that a majority of patients with HFPEF die of CV causes. However, when looking exclusively at patients with documented CAD, the risk of all-cause death and CV death appears greater in patients with HFREF than in patients with HFPEF. Interestingly, in the Olmsted County registry, the percentage of fatal events attributable to coronary deaths was also higher in patients with HFREF than in patients with HFPEF.16 Furthermore, causes of death in patients with HFPEF are rarely described in the literature. In the I-Preserve trial, the leading mode of death was CV in 60% of cases.21 Adabag et al.29 report, in a large retrospective study, a high proportion of non-CV deaths and a relatively low proportion of sudden death and HF-related death in patients with HFPEF. Finally, Grigorian-Shamagian et al.30 suggest that patients admitted to hospital for HF with preserved or with reduced EF have the same spectrum of causes of death, with differences in the dynamics of each cause of death during follow-up. We observed in both HFPEF and HFREF that the distribution of death causes is significantly influenced by the presence of CAD. Whereas patients with HFREF and CAD had a greater cumulative incidence of HF-related and myocardial infarction-related fatal events compared with those without CAD, in patients with HFPEF CAD was associated with greater cumulative incidence of sudden death compared with non-CAD. The association between CAD and the risk of sudden death in HFPEF was still present after adjustment for covariates.
Our findings have potential clinical implications. We have demonstrated that among patients with HFPEF, the burden of CV fatal events is high and that the presence of CAD might be associated with an increased risk of sudden cardiac death. Given the small population size, these results need to be confirmed by future carefully designed studies. Considering the lack of effective therapeutics in this subset of patients with HF, and the questionable value of various stress tests,9 coronary angiography should be discussed in the absence of contraindications in patients with preserved EF admitted to hospital for worsening HF. This might lead to a better understanding of factors that trigger decompensation.
Limitations
Our study did not include all patients with HF from the Somme department during 2000, as less symptomatic patients are not always admitted to hospital. However, restricting the analysis to patients hospitalized for a first episode of HF presumably resulted in a more homogeneous study population. Our patients were enrolled from community hospitals, private clinics, and the university hospital in the Somme department, which should eliminate referral bias of hospital-based studies conducted exclusively in tertiary centres. Despite the fact that physicians who examined the death records had large experience in HF research, and that in cases of disagreement all records were carefully examined by an independent third reviewer who gave the final classification, it is possible that some causes of death may have been misclassified. However, the evenly distributed proportion of unknown causes of death among HFPEF and HFREF patients is reassuring in this regard. The use of a clinical definition for CAD might have led to an underestimation of the true frequency of CAD in the study population. Although current guidelines31 request evidence of diastolic dysfunction to confirm the diagnosis of HFPEF, we did not collect indexes of diastolic function. An overwhelming proportion of patients with HFPEF in the present study had severe symptoms at the time of hospital admission and it was previously shown that such patients have almost always abnormal left ventricular end-diastolic pressures.32 Nevertheless, we cannot rule-out the possibility that a small number of patients with other causes of dyspnoea may have been misclassified as HFPEF. We acknowledge that the time-span of coronary disease and the number of coronary events and revascularizations represent different degrees of risk for HF patients. This information was not collected for our cohort of patients admitted to hospital for a first episode of HF. We could not analyse rehospitalizations for HF during follow-up, because this item was not recorded during follow-up. Finally, we acknowledge that the multiple analyses conducted in the present study might have increased the likelihood of the type I error.
Conclusion
Documented CAD is associated with high event-rate after a first hospital admission for HF. Coronary artery disease patients with HFPEF and with HFREF are at a high risk of dying of CV causes. The CAD-related cause-specific mortality shows important differences between HFPEF and HFREF. While in HFREF CAD is associated with HF-related and myocardial infarction-related fatal events, in HFPEF it is predictive of greater risk of suddendeath.
Funding
This work was supported by a grant from the French Ministry of Health, Paris, France.
Conflict of interest: none declared




