Long-term outcomes of cardiac resynchronization therapy by left ventricular ejection fraction
Abstract
Aims
Despite our prior report suggesting heart failure (HF) risk reduction from cardiac resynchronization therapy with defibrillator (CRT-D) in mild HF patients with higher left ventricular ejection fraction (LVEF > 30%), data on mortality benefit in this cohort are lacking. We aimed to assess long-term mortality benefit from CRT-D in mild HF patients by LVEF > 30%.
Methods and results
Among 1274 patients with mild HF and left bundle branch block enrolled in MADIT-CRT, we analysed long-term effects of CRT-D vs. implantable cardioverter defibrillator (ICD) therapy only, and reverse remodelling to CRT-D (left ventricular end-systolic volume percent change ≥ median at 1 year), on all-cause mortality and HF for the LVEF ≤ 30% and LVEF > 30 subgroups using Kaplan–Meier and Cox analyses. During long-term follow-up, CRT-D vs. ICD was associated with reduction in all-cause mortality in both patients with LVEF > 30% and LVEF ≤ 30% [hazard ratio (HR) 0.47, 95% confidence interval (CI) 0.25–0.85, P = 0.036 vs. HR 0.69, 95% CI 0.49–0.98, P = 0.013, interaction P = 0.261]. The efficacy of CRT-D vs. ICD only to reduce HF was similar in those with LVEF above and below 30% (HR 0.36, 95% CI 0.35–0.61, P < 0.001 vs. HR 0.46, 95% CI 0.35–0.61, P < 0.001; interaction P = 0.342). Patients with CRT-D-induced reverse remodelling had significant mortality reduction when compared to ICD, with either LVEF > 30% or LVEF ≤ 30% (HR 0.17 and 0.39), but no mortality benefit was seen in patients with less reverse remodelling. HF events, however, were reduced in both CRT-D-induced high and low reverse remodelling vs. ICD only, in both LVEF subgroups.
Conclusions
In MADIT-CRT, left bundle branch block patients with higher LVEF (> 30%) derive long-term mortality benefit from CRT-D when exhibiting significant reverse remodelling.
Clinical Trial registration: ClinicalTrials.gov ID NCT00180271, NCT01294449, and NCT02060110
Introduction
Cardiac resynchronization therapy (CRT) is an effective treatment modality to reduce heart failure (HF) and mortality in HF patients with left ventricular ejection fraction (LVEF) < 30%, advanced HF, and a wide QRS.1, 2 The Multicenter Automatic Defibrillator Implantation Trial - Cardiac Resynchronization Therapy (MADIT-CRT) suggested benefit of CRT with defibrillator (CRT-D) reducing HF events in patients with New York Heart Association (NYHA) class I or II HF, extending the indication of CRT-D to patients with mild HF.3-5
We previously reported that in MADIT-CRT, the echocardiography core laboratory identified one-third of our enrolled patients with LVEF > 30%, beyond the eligibility criteria, and these patients with LVEF > 30% derived benefit from CRT-D with reduction in HF events.6 Mortality reduction was not seen during short-term follow-up.6 It is not known however whether patients with LVEF > 30% exhibit sustained long-term CRT-D benefit with reduction in HF events, or gain mortality benefit from CRT-D during long-term follow-up.
We also demonstrated that CRT-D-induced left ventricular reverse remodelling at 1 year appeared greater in patients with LVEF > 30%,6 but it has not been investigated whether differential reverse remodelling translates in differences in long-term outcomes of HF or death for patients with LVEF ≤ 30% vs. LVEF > 30%.
Therefore, the aim of our study was (i) to determine the effects of CRT-D compared to implantable cardioverter defibrillator (ICD) therapy only on all-cause mortality and HF events for patients with LVEF ≤ 30% and LVEF > 30% during long-term follow-up, and (ii) to evaluate the effects of left ventricular reverse remodelling to CRT-D on long-term outcomes of death or HF in patients with LVEF ≤ 30% and LVEF > 30%, enrolled in MADIT-CRT.
Methods
Study population
The design, protocol and results of the MADIT-CRT study have been published previously.3, 4 A total of 1820 patients, from 110 hospital centres from North America and Europe, meeting enrolment criteria of ischaemic cardiomyopathy (NYHA functional class I or II) or non-ischaemic cardiomyopathy (NHYA functional class II only), physician-reported LVEF < 30%, and a prolonged QRS duration > 130 ms were randomized to receive CRT-D or ICD therapy in a 3:2 ratio. All eligible patients met the guideline criteria for ICD.7 Patients were excluded for various reasons as previously described.3 The study was in compliance with the Declaration of Helsinki and all enrolling sites had the protocol being approved by the local institutional review board. All patients provided informed consent before enrolment in the study, and patients were consented again before entering the long-term follow-up registry.
While the MADIT-CRT inclusion criteria specified patients with LVEF < 30% as evaluated by centres prior to enrolment, all patients additionally underwent central echocardiographic analysis of LVEF in the study core laboratory of Brigham and Women's Hospital, Boston, Massachusetts. During core laboratory assessment, a substantial proportion of patients were identified as having LVEF > 30%, beyond the eligibility criteria.
Patients were excluded from the present study if they had non-left bundle branch block (LBBB) electrocardiographic morphology (n = 537), as previous studies suggested that non-LBBB patients receive no benefit from CRT-D. We also excluded patients with no baseline LVEF measurement available (n = 10). As such, the present study sample comprised 1274 (70%) of the 1820 patients enrolled in MADIT-CRT, 760 (60%) of which were randomized to CRT-D therapy. Analysis was performed on an intention-to-treat basis, patients with crossover were treated as if in their original randomization arm.
Data acquisition and patient follow-up
As previously published, the MADIT-CRT trial was carried out from 22 December 2004 through 22 June 2009.4 Post-trial follow-up was conducted for all 1691 surviving study participants until 10 September 2010 as mandated by the Food and Drug Administration (FDA). After 10 September 2010, long-term follow-up data collection was performed with ongoing patient follow-up conducted at 48 of the 88 U.S. centres that agreed to participate in the long-term data collection requested by the FDA for patients enrolled in the United States, and at 23 of the 24 non-U.S. centres, involving a total of 854 patients, as reported previously.5 Sites participating in the long-term registry had the long-term follow-up protocol approved by their local institutional review boards, and patients in the long-term follow-up registry provided informed consent.
During the in-trial period, 96 patients crossed over from ICD to CRT-D therapy (5.3%), and 87 crossed over from CRT-D to ICD-only therapy (4.8%). Subsequently, during the two post-trial phases, there were 25 (3%) and 2 (0.2%) additional crossovers between the two treatment arms. This analysis was an intention-to-treat analysis, crossovers were not taken into account. The median follow-up of the long-term study was 5.6 years.
Echocardiography methods
Echocardiograms were obtained at baseline prior to device implantation and at 1 year according to a pre-specified study-specific protocol. The recordings were analysed at the independent echocardiography core laboratory at the Brigham and Women's Hospital, Boston, Massachusetts (S.D.S.). Echocardiography core laboratory investigators were blinded to treatment assignment and clinical outcome in the study.
Left ventricular volumes were measured by Simpson's disk method in the apical four- and two-chamber views and LVEF was calculated according to the established American Society of Echocardiography protocols.8 The coefficients of variation for end-diastolic volume, end-systolic volume, and LVEF were 5.2%, 6.2%, and 5.5%, respectively, as reported previously.9
Left ventricular mechanical dyssynchrony was measured using B-mode speckle tracking software (TomTec Imaging Systems, Unterschleissheim, Germany) as reported previously.10 Left ventricular mechanical dyssynchrony was determined as the standard deviation of regional time-to-peak transverse strain, measured during systole in the 12 anatomic wall segments (septum, lateral, anterior and inferior walls, and basal, mid and apical segments) of the left ventricle.
Left ventricular reverse remodelling to CRT-D was defined as a greater than 35% reduction (median value for LBBB) in left ventricular end-systolic volume (LVESV) between enrolment and 1-year echocardiogram (calculated as the difference between the baseline and 1-year LVESV, divided by the baseline LVESV).
Definitions and endpoints
Patients with baseline LVEF measurements were divided into two groups based on the echocardiography core laboratory assessment: LVEF ≤ 30% and LVEF > 30%.11
The primary endpoint of this study was long-term all-cause mortality. Secondary endpoint was HF alone. In both the MADIT-CRT in-trial and long-term follow-up study, physicians who were not blinded to treatment assignments determined the diagnosis of HF based on signs and symptoms consistent with congestive HF requiring diuretics in the outpatient or inpatient setting. However, adjudication of the endpoints in the in-trial follow-up was conducted by an independent mortality committee and HF committee unaware of treatment assignments based on pre-specified criteria. In the long-term follow-up, central adjudication of HF events and death was not carried out.
When assessing outcomes by the degree of left ventricular reverse remodelling (LVESV percent reduction from baseline) to CRT-D at 1 year, patients were divided into two groups using the median value of LVESV percent reduction: (i) CRT-D patients with LVESV percent reduction ≥ 35% (high responders), (ii) CRT-D patients with LVESV percent reduction < 35% (low responders). This analysis was performed for the subgroup of patients with LVEF ≤ 30% and LVEF > 30%. In this analysis, patients randomized to ICD only served as the control group.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation. Categorical data are summarized as frequencies and percentages. Baseline clinical characteristics were compared between the LVEF subgroups of LVEF ≤ 30% and LVEF > 30%, using non-parametric Wilcoxon rank-sum test for continuous variables and χ2 test or Fisher exact test for dichotomous variables.
Cumulative probability of long-term death, and HF alone by treatment arm within each LVEF group was displayed using Kaplan–Meier survival analysis with comparisons of cumulative event rate curves according to the log-rank test. Cumulative probability of death, and HF was plotted for CRT-D high responders, CRT-D low responders, and ICD-only patients, within LVEF ≤ 30% and LVEF > 30%.
Multivariate Cox proportional hazards regression analysis was used to evaluate the long-term risk of all-cause mortality, and HF alone by CRT-D vs. ICD only. Proportionality assumption testing was performed, using interaction testing with follow-up time. Cox models were adjusted for covariates chosen by best subset regression modelling, with the stipulation that all variables reached statistical significance of < 0.05. Separate models were fit for the endpoint of death, and HF. For death, models were adjusted for age, creatinine > 1.4 mg/dL, ischaemic aetiology, baseline LVESV, worst NYHA class >III 3 months prior to enrolment. For HF alone, models were adjusted for race, age, coronary artery bypass grafting, heart rate at baseline, QRS duration > 150 ms, systolic blood pressure, and baseline LVESV. Interaction with assigned treatment LVEF group was utilized to assess the modifying effect of LVEF.
Uni- and multivariate landmark analyses starting follow-up at 1 year was performed to assess the relationship between the 1-year reverse remodelling and clinical outcomes for patients with LVEF > 30% and LVEF ≤ 30%. Cox models were adjusted for the covariates described above.
All statistical tests were two-sided and a P-value of < 0.05 was considered statistically significant. Analyses were carried out with SAS software (version 9.4, SAS Institute, Cary, NC, USA).
Results
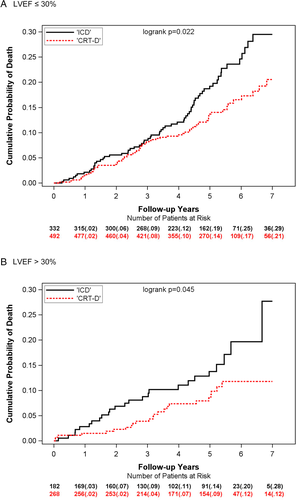
Of the 1274 LBBB patients included in this study, baseline LVEF assessment identified 824 (65%) patients with higher LVEF ≤ 30% and 450 (35%) patients with LVEF > 30% (Table 1). During long-term follow-up (median 5.6 years), 136 (16.5%) patients died with LVEF ≤ 30% (ICD n = 66, 20% CRT-D n = 70, 14%), and 48 (11%) patients died with LVEF > 30% (ICD n = 25, 14% CRT-D n = 23, 9%). Analysis was carried out as intention-to-treat.
| Clinical characteristics | LVEF ≤ 30% (n = 824) | LVEF > 30% (n = 450) | P-value |
|---|---|---|---|
| Age at enrolment, years | 63.7 ± 11.0 | 65.2 ± 10.6 | 0.027 |
| Female sex | 229 (28) | 163 (36) | 0.002 |
| CRT-D treatment | 492 (60) | 268 (60) | 0.958 |
| Ischaemic cardiomyopathy | 372 (45) | 188 (42) | 0.247 |
| Worst NYHA class >II (>3 months prior) | 98 (12) | 37 (9) | 0.044 |
| Hospitalization in prior year | 360 (45) | 201 (45) | 0.960 |
| Prior CHF hospitalization | 327 (40) | 160 (36) | 0.104 |
| Diabetes | 250 (30) | 133 (30) | 0.779 |
| Hypertension | 512 (62) | 292 (65) | 0.372 |
| ACE inhibitor or ARB | 793 (96) | 431 (96) | 0.686 |
| Aldosterone antagonists | 291 (35) | 137 (30) | 0.079 |
| Amiodarone | 58 (7) | 21 (5) | 0.093 |
| Beta-blocker excluding sotalol | 768 (93) | 429 (95) | 0.127 |
| Diuretic | 578 (70) | 291 (65) | 0.045 |
| QRS, ms | 165.0 ± 20.1 | 159.5 ± 17.1 | <0.001 |
| Heart rate, b.p.m. | 68.5 ± 10.9 | 67.7 ± 10.9 | 0.180 |
| BUN, mg/dL | 21.5 ± 8.9 | 21.3 ± 8.7 | 0.615 |
| Creatinine, mg/dL | 1.15 ± 0.31 | 1.12 ± 0.33 | 0.008 |
| SBP, mmHg | 121.7 ± 16.5 | 124.5 ± 18.2 | 0.010 |
| LVEF, % | 26.9 ± 2.6 | 32.2 ± 1.9 | <0.001 |
| LVEDV indexed by BSA, mL/m2 | 133.0 ± 32.8 | 113.6 ± 19.5 | <0.001 |
| LVESV indexed by BSA, mL/m2 | 97.6 ± 26.1 | 77.1 ± 14.0 | <0.001 |
| LAV indexed by BSA, mL/m2 | 50.4 ± 9.7 | 41.1 ± 7.8 | <0.001 |
| LV mass indexed by BSA, g/m2 | 113.0 ± 19.3 | 101.5 ± 14.6 | <0.001 |
| Baseline dyssynchrony, ms | 195 ± 61 | 185 ± 64 | 0.021 |
- Values are given as n (%) of patients, or mean ± standard deviation.
- ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BSA, body surface area; BUN, blood urea nitrogen; CHF, congestive heart failure; CRT-D, cardiac resynchronization therapy with defibrillator; LAV, left atrial volume; LV, left ventricular; LVEDV, left ventricular end-diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume; NYHA, New York Heart Association; SBP, systolic blood pressure.
Effects of CRT-D on long-term all-cause mortality by left ventricular ejection fraction subgroups
CRT-D was associated with a significantly lower rate of all-cause mortality compared to ICD only in patients with LVEF ≤ 30%, with 7-year cumulative probabilities of 21% vs. 29% (Figure 1A). In patients with LVEF > 30%, CRT-D vs. ICD was similarly associated with significantly lower probability of all-cause mortality with 7-year cumulative probabilities of 12% vs. 29% (Figure 1B). Multivariate analyses confirmed these findings, demonstrating a significant, 53% reduction in the risk of death for patients with LVEF > 30% (P = 0.013), and significant, 31% decrease in the risk of death for patients with LVEF ≤ 30% with CRT-D vs. an ICD-only (P = 0.036) (Table 2). The treatment-LVEF-group-interaction P-value was non-significant, suggesting similar treatment effect for patients with LVEF ≤ 30% and LVEF > 30%.
| Endpoint | Hazard ratio | 95% CI | P-value | Interaction P-value |
|---|---|---|---|---|
| Death | 0.261 | |||
| CRT-D vs. ICD only for LVEF ≤ 30% | 0.69 | 0.49–0.98 | 0.036 | |
| CRT-D vs. ICD only for LVEF > 30% | 0.47 | 0.25–0.85 | 0.013 | |
| Heart failure alone | 0.342 | |||
| CRT-D vs. ICD only for LVEF ≤ 30% | 0.46 | 0.35–0.61 | <0.001 | |
| CRT-D vs. ICD only for LVEF > 30% | 0.36 | 0.23–0.55 | <0.001 |
- CI, confidence interval; CRT-D, cardiac resynchronization therapy with defibrillator; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction.
- For death, models were adjusted for age, creatinine > 1.4 mg/dL, ischaemic aetiology of cardiomyopathy, baseline left ventricular end-systolic volume, worst New York Heart Association class >III 3 months prior to enrolment.
- For heart failure alone, models were adjusted for race, age, coronary artery bypass grafting, heart rate at baseline, QRS duration > 150 ms, systolic blood pressure, and baseline left ventricular end-systolic volume.
Effects of CRT-D on long-term heart failure by left ventricular ejection fraction subgroups
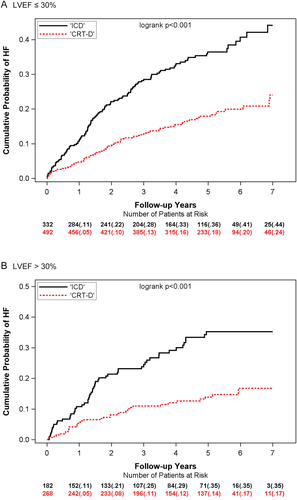
For HF alone, similar benefit of CRT-D was observed for both LVEF ≤ 30% and LVEF > 30%. Kaplan–Meier analyses showed a reduction in 7-year cumulative probability of HF events for CRT-D vs. ICD-only in patients with LVEF ≤ 30% and for patients with LVEF > 30% (P <0.001) (Figure 2). Multivariate analyses showed a significant risk reduction in HF events with CRT-D vs. ICD for both LVEF subgroups with hazard ratios (HR) of 0.46 and 0.36 for LVEF ≤ 30% and LVEF > 30% (P <0.001; interaction P = 0.342) (Table 2).
Long-term effects of CRT-D induced reverse remodelling by left ventricular ejection fraction subgroups
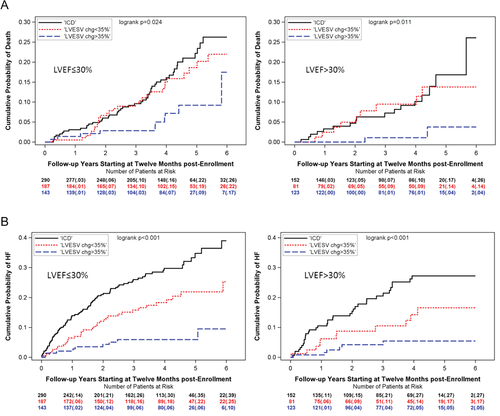
Kaplan–Meier analyses in the LVEF ≤ 30% and LVEF > 30% patient groups revealed differences in all-cause mortality for CRT-D high responders (LVESV percent reduction ≥ 35%) vs. CRT-D low responders (LVESV percent reduction < 35%) vs. ICD only, with only CRT-D high responders displaying lower rates of mortality (P = 0.024 for LVEF ≤ 30%, P = 0.011 for LVEF > 30%) (Figure 3A). Cox models showed a significant risk reduction in mortality for CRT-D high responders vs. ICD only, for both LVEF subgroups (HR 0.48 and 0.26, P < 0.05). There was, however, no difference in the risk of all-cause mortality between CRT-D low responders and ICD-only patients, in patients with either LVEF ≤ 30% or LVEF > 30% (Table 3). CRT-D high responders had a lower risk of mortality when compared to CRT-D low responders.
| Hazard ratio | 95% CI | P-value | |
|---|---|---|---|
| Death | |||
| LVEF ≤ 30% | |||
| CRT LVESV change <35% vs. ICD | 0.82 | 0.52–1.31 | 0.410 |
| CRT LVESV change ≥35% vs. ICD | 0.39 | 0.20–0.78 | 0.007 |
| CRT LVESV change ≥35% vs. CRT LVESV change < 35% | 0.48 | 0.23–0.98 | 0.045 |
| LVEF > 30% | |||
| CRT LVESV change <35% vs. ICD | 0.63 | 0.27–1.48 | 0.287 |
| CRT LVESV change ≥35% vs. ICD | 0.17 | 0.04–0.72 | 0.017 |
| CRT LVESV change ≥35% vs. CRT LVESV change < 35% | 0.26 | 0.06–1.25 | 0.093 |
| Heart failure alone | |||
| LVEF ≤ 30% | |||
| CRT LVESV change <35% vs. ICD | 0.56 | 0.38–0.84 | 0.005 |
| CRT LVESV change ≥35% vs. ICD | 0.23 | 0.11–0.45 | <0.001 |
| CRT LVESV change ≥35% vs. CRT LVESV change < 35% | 0.40 | 0.19–0.84 | 0.015 |
| LVEF > 30% | |||
| CRT LVESV change <35% vs. ICD | 0.44 | 0.22–0.88 | 0.020 |
| CRT LVESV change ≥35% vs. ICD | 0.17 | 0.07–0.44 | <0.001 |
| CRT LVESV change ≥35% vs. CRT LVESV change < 35% | 0.39 | 0.14–1.14 | 0.086 |
- CI, confidence interval; CRT, cardiac resynchronization therapy; ICD, implantable cardioverter defibrillator; LVEF, left ventricular ejection fraction; LVESV, left ventricular end-systolic volume.
- For death, models were adjusted for age, creatinine > 1.4 mg/dL, ischaemic aetiology of cardiomyopathy, baseline LVESV, worst New York Heart Association class >III 3 months prior to enrolment.
- For heart failure alone, models were adjusted for race, age, coronary artery bypass grafting, heart rate at baseline, QRS duration > 150 ms, systolic blood pressure, and baseline LVESV.
The probability of HF events was lower for CRT-D high responders as well as for CRT-D low responders as compared to ICD only, for both LVEF ≤ 30% and LVEF > 30% (P <0.001) (Figure 3B). Multivariate analyses confirmed that both CRT-D high responders and low responders had a significantly lower risk of HF compared to ICD only, in both patients with LVEF ≤ 30% and LVEF > 30% (HR 0.56, 0.23, 0.44, 0.17, all P < 0.05). CRT-D high responders had lower risk of HF when compared to low responders (Table 3).
Sensitivity analyses
We analysed CRT-D vs. ICD effects by LVEF 30% in all patients including non-LBBB, and similar CRT-D benefits were revealed for HF alone, while CRT-D-induced risk reduction in mortality was not evident in either LVEF group (data not shown). When assessing outcomes by three LVEF groups (LVEF < 25, 25–30%, > 30%), reduction in mortality and HF events by CRT-D was similar in the LVEF < 25%, and LVEF 25–30% subgroups, further justifying combining them (data not shown). A formal three-way interaction analysis was performed between treatment, LVEF, and left ventricular reverse remodelling, and it was not found to be statistically significant.
Discussion
Our study demonstrates that mild HF patients with LVEF > 30% derive long-term mortality benefit from CRT-D as compared to ICD alone, similar to patients with LVEF < 30%. This mortality benefit, however, is confined to patients with a higher degree of CRT-D-induced left ventricular reverse remodelling. Long-term sustained reduction in HF events from CRT-D was evident in both patients with LVEF ≤ 30% and LVEF > 30%, independent of CRT-D-induced left ventricular reverse remodelling.
There have been a few prior studies suggesting that patients with advanced HF and higher LVEF might derive benefit from CRT with clinical improvement and left ventricular reverse remodelling.12-15 Limited data are available regarding CRT-D benefit in mild HF patients with higher ejection fraction. Our prior MADIT-CRT substudy was one of the first to suggest benefit from CRT-D in mild HF patients with LVEF > 30%. We have shown reductions in the combined endpoint of HF or death, and reductions in left ventricular volumes; however, there was no effect seen on mortality during the short-term follow-up of MADIT-CRT.6 Nevertheless, this report launched other investigations to assess outcomes in mild HF patients with higher ejection fraction.16 Linde et al.15 reported from the REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction (REVERSE) study that the 177 out of 608 (29%) patients with higher LVEF exhibited left ventricular reverse remodelling and clinical symptom reduction along with a lower risk for HF or death when CRT was on. However, they did not investigate the effects of CRT-D therapy on all-cause mortality alone, and the follow-up of their study was short (12 months in North America and 24 months in the United States). We extended findings from both of these studies6, 16 with new observations, most importantly by demonstrating mortality benefit in mild HF patients with higher LVEF, and suggesting that the mortality benefit is only seen with sufficient amount of left ventricular reverse remodelling.
The benefit of CRT in patients with low ejection fraction has been well-established,1, 2 and it was linked to immediate improvement in cardiac index,17 filling time, myocardial performance index,18 and reduction in functional mitral regurgitation.19 Such changes were accompanied by left ventricular reverse remodelling, a reversal of the HF-induced left ventricular dilatation.17 Newer studies now also provide molecular mechanism of these favourable changes, indicating restoration of left ventricular cardiomyocyte diameter, suppression of collagen deposition, and most importantly, down-regulation of transforming growth factor (TGF)-β1 cytokines linked to lower levels of the matrix glycoprotein, osteopontin, required for the activation of fibroblasts upon TGF-β1 stimulation.20 As we know from large clinical trials, however, LVEF is linked to HF risk as a continuum up to an LVEF of 45%21; therefore it is conceivable that the aforementioned benefits would be present in those with a higher ejection fraction, as indicated in our current analysis.
While prior studies primarily used 15% reduction in LVESV as a response definition, those studies assessed echocardiography response earlier, at 6 months. In MADIT-CRT, we assessed echocardiographic reverse remodelling at 12 months, and CRT-derived reverse remodelling is greater at 12 months than at 6 months. This has been shown in the REVERSE study, demonstrating in serial echocardiograms of CRT patients that there is an increasing degree of left ventricular reverse remodelling at 1 year, up to 2 years, and then the reverse remodelling plateaus. This explains why we have seen a median reduction of 35% in LVESV at 1 year in LBBB patients. We have also previously reported that reverse remodelling was found to be greater in patients with LBBB as compared to non-LBBB.22 And while 35% reduction in LVESV that we use in this study might not be generalizable to other studies with echocardiography performed at 6 months, using the median reduction in LVESV in those studies might similarly serve as a useful cut-off.
Importantly, the benefit of CRT-D in MADIT-CRT was only seen in patients with LBBB, not in those with non-LBBB.22 This is in line with other studies, meta-analyses, and current guidelines that distinguish indication for CRT by LBBB and QRS duration. Namely, there is a class I CRT indication for patients with LVEF < 35%, LBBB, NYHA class II–IV symptoms, and QRS at least 150 ms, while there is a class IIa CRT indication when QRS is 120–149 ms. Echocardiography can also play an important role not only selecting patients for CRT, but also identifying non-responders early, and aid physicians to intervene to improve outcomes. In addition, our current data can serve as hypothesis generating for new randomized trials assessing the role of CRT in patients with LVEF > 30%.
Interestingly, the mortality benefit was confined to CRT-D patients with high left ventricular reverse remodelling at 1 year compared to ICD only, a finding that was consistent in both patients with LVEF ≤ 30% and LVEF > 30%. This finding is in alignment with another study by Gold et al.,23 reporting significant improvement in mortality in patients with 15% reduction in LVESV index, at 6 months after CRT-D implantation. While we suggest a greater degree of left ventricular reverse remodelling to derive mortality benefit, this difference might likely arise from the different time points at which CRT-D response was assessed in the two studies (12 months in MADIT-CRT, and 6 months in REVERSE).
Our findings have clinical relevance suggesting that patients with mild HF and higher ejection fraction could be considered for implantation of CRT-D not only to reduce their morbidity burden through reduction in HF events, but also to improve survival. Thus, early prevention of HF progression in mild HF patients with higher LVEF clearly translates into mortality benefit. However, despite the favourable findings from our retrospective analyses, more research is warranted to investigate the role of CRT in patients with higher LVEF, preferably in randomized controlled clinical trials. One such effort has been the MIRACLE EF study by Linde et al.25 that had to be terminated early due to poor patient recruitment. It remains a challenge to identify patients with higher LVEF eligible for CRT who are not typically seen by electrophysiologists. Interdisciplinary team efforts of cardiologists, HF specialist, and electrophysiologists might facilitate identification of patients with higher LVEF potentially eligible for CRT.
We acknowledge certain limitations of our study. First, this is a post-hoc analysis of MADIT-CRT, and it was not a pre-specified analysis. Additionally, MADIT-CRT had not been intended to enrol patients with LVEF > 30%, but still a relatively large proportion of patients had LVEF > 30%. However, the patient group with an LVEF > 30% had a narrow standard deviation for LVEF (1.9%) and, therefore, most patients presented with an LVEF 30–40%. A large number of centres from the United States did not participate in the long-term follow-up registry, introducing a selection bias. Furthermore, if we used an echocardiography core lab for LVEF assessment before enrolment, patients with LVEF > 30% would not have been enrolled in the trial. In addition, a large number of 1-year echocardiograms were not available and some patients died before 1 year of follow up. In addition, we do not have longitudinal echocardiography data in MADIT-CRT available. Nevertheless, this is a large cohort of patients with higher LVEF > 30% treated with CRT-D with long-term follow-up data available. While in-trial HF and death events were centrally adjudicated, long-term death and HF events in MADIT-CRT were not adjudicated.
Conclusions
In a large cohort of mild HF patients, those with LVEF > 30% derive long-term benefit from CRT with sustained reductions in HF events and significant reduction in all-cause mortality. The mortality benefit, however, is confined to patients with significant CRT-D-induced left ventricular reverse remodelling. Follow-up echocardiography could play a major role to predict long-term mortality benefit from CRT-D.
Funding
This work included data from MADIT-CRT, a study that was supported by a research grant from Boston Scientific, St. Paul, Minnesota, to the University of Rochester School of Medicine and Dentistry.
Conflict of interest: V.K. reports research grants from Boston Scientific and Zoll Inc., and consultant fees from Biotronik and Zoll Inc. The other authors have no conflicts of interest to disclose.




