Upgrades from a previous device compared to de novo cardiac resynchronization therapy in the European Society of Cardiology CRT Survey II
Abstract
Background
To date, there are no data from randomized controlled studies on the benefit of cardiac resynchronization therapy (CRT) when implanted as an upgrade in patients with a previous device as compared to de novo CRT. In the CRT Survey II we compared the baseline data of patients upgraded to CRT (CRT-P/CRT-D) from a previous pacemaker (PM) or implantable cardioverter-defibrillator (ICD) to de novo CRT implantation.
Methods and results
In the European CRT Survey II, clinical practice data of patients undergoing CRT and/or ICD implantation across 42 European Society of Cardiology (ESC) countries were collected between October 2015 and December 2016. Out of a total of 11 088 patients, 2396 (23.2%) were upgraded from a previous PM or ICD and 7933 (76.8%) underwent de novo implantation. Compared to de novo implantations, upgraded patients were older, more often male, more frequently had ischaemic heart failure aetiology, atrial fibrillation, reduced renal function, worse heart failure symptoms, and higher N-terminal pro-B-type natriuretic peptide levels. Upgraded patients were more often PM-dependent and less frequently received CRT-D. Total peri-procedural, in-hospital complications and length of hospital stay were similar. Upgraded patients were less frequently treated with heart failure medication at discharge.
Conclusion
Despite a lack of evidenced-based data, close to one quarter of all CRT implantations across 42 ESC countries were upgrades from a previous PM or ICD. Despite older age and worse symptoms, the CRT implantation procedures in upgraded patients were equally frequently successful and complications similar to de novo implantations. These results call for more studies.
Introduction
Cardiac resynchronization therapy (CRT) is an established therapy for patients with heart failure (HF) with a reduced left ventricular ejection fraction (LVEF) and electrical dyssynchrony.1-4 Prospective randomized trials on CRT have consistently demonstrated reductions in HF-related hospitalization and mortality rates among patients with a broad spectrum of symptomatic HF5-7 and in HF patients expected to be dependent of right ventricular (RV) pacing.8 Despite class IA recommendation in international guidelines,9, 10 therapy implementation has been slow and unevenly distributed across Europe.11
In the first CRT survey conducted between 2008 and 2009 in 13 European Society of Cardiology (ESC) countries, we established similar benefits by CRT in patient groups outside of randomized controlled trials (RCTs) such as >75 years or requiring an upgrade from a previous pacemaker (PM) or implantable cardioverter-defibrillator (ICD) who constituted one fourth of all patients.12-14 This means that doctors selected patients for CRT in the belief that the benefits will be similar in patients who fulfil class IA recommendations to weaker or absent recommendations. Although nearly 10 years have passed, there still has not been a RCT with the focus on studying CRT benefits of patients upgraded to CRT vs. not being upgraded illustrating the need for clinical trials.
In the European CRT Survey II,15 data on clinical practice of CRT and/or ICD implantation across 42 ESC countries were collected between October 2015 and December 2016 with regard to patient characteristics, implantation procedures and complications with the aim to compare quality control and identify obstacles for therapy implementation in a large contemporary patient cohort. In this paper, we compare baseline patient data in patients undergoing upgrading to CRT compared to those subject to de novo CRT implantation.
Methods
Structure and recruitment, data collection, management and analysis
The rationale15 and baseline results16 of the CRT Survey II have been published previously. This survey was designed as a joint initiative of the European Heart Rhythm Association (EHRA) and the Heart Failure Association (HFA). EHRA and HFA co-coordinated the survey with sponsorship from five device companies and from several drug and diagnostic companies (see Acknowledgements). The centres were asked to enrol consecutive new CRT implantations and upgrades from a previous PM or ICD to CRT whether or not they were successful between October 2015 and December 2016. Generator replacements were not included.
Of 47 ESC countries identified from the EHRA Whitebook,11 42 agreed to participate. A total of 288 implanting centres recruited patients. In order to achieve a cohort representative for each country, we aimed to include at least 10% of all CRT implantations performed in the respective geographical region during the survey time.
The web-based electronic case record form (eCRF) used for data collection was developed by the Institut for Herzforschung Ludwigshafen (IHF).17 It included anonymized data on patient characteristics, clinical assessment, indications for CRT, including reason for upgrades, implant procedures as well as peri-procedural and in-hospital complications and adverse events during index hospitalization. The eCRF was reviewed by ESC data protection consultants to ensure patient anonymity. This, together with the fact that the survey did not include follow-up data after discharge, obviated the necessity for formal institutional review board approval in most countries. Most centres were simply required to notify their local or national ethics committee of their participation in the survey.
Statistical analysis
All data analyses were performed by IHF using SAS®, release 9.3 (SAS Institute Inc., Cary, NC, USA) on a Microsoft® Windows® 7 Enterprise platform. All percentages are relative to the total number of patients with available information. Binary, categorical, and ordinal parameters were summarized by means of absolute and percentage numbers within the various categories. Numerical data were summarized by means of standard statistics (i.e. number of available data, mean, standard deviation, median, lower and upper quartile, i.e. interquartile range, 5th and 95th percentile). A P-value <0.05 was considered statistically significant. Non-parametric tests were used when appropriate. No imputation for missing data was made.
Results
A total of 11 088 patients were recruited from 42 countries. These patients constituted 11% of expected implantation rate during the enrolment period. Most countries (34/42) included at least 10% of the expected total number of implants for that country. The majority of CRT implantations (82%) were performed in either university or teaching hospitals.
For the purpose of this analysis, 757 patients were excluded due to missing data concerning reason for an upgrade to CRT. Thus, the total cohort in the present study was 10 331 patients. Of these, 2398 (23.2%) were upgraded from a previous PM (60.9%) or ICD (39.8%) and 7933 (76.8%) underwent de novo implantation (Table 1).
| Upgrades PM/ICD (n = 2398) | De novo (n = 7933) | P-value | |
|---|---|---|---|
| Demographics | |||
| Age, years (IQR) | 72 (64–78) | 69 (62–76) | <0.0001 |
| Age > 75 years | 37.9% (909/2398) | 30.2% (2398/7930) | <0.0001 |
| Female sex | 18.8% (451/2398) | 26.1% (2065/7927) | <0.0001 |
| Currently enrolled in a trial | 7.1% (169/2395) | 8.6% (680/7915) | 0.01665 |
| Primary aetiology | |||
| Ischaemic | 48.2% (1147/2380) | 43.7% (3448/7892) | 0.046 |
| Non-ischaemic | 43.5% (1035/2380) | 51.3% (4052/7892) | |
| Other | 8.3% (198/2380) | 5.0% (392/7892) | |
| Previous history | |||
| Myocardial infarction | 40.3% (957/2375) | 35.2% (2777/7886) | <0.0001 |
| PCI/CABG | 43.1% (1023/2372) | 37.8% (2979/7884) | <0.0001 |
| Valvular disease | 32.2% (764/2371) | 25.3% (1998/7883) | <0.0001 |
| Valvular surgery | 36.5% (337/923) | 28.7% (739/2571) | 0.0001 |
| Hypertension | 65.5% (1550/2366) | 63.6% (5003/7870) | 0.08456 |
| Diabetes | 32.2% (763/2373) | 31.2% (2463/7885) | 0.39902 |
| COPD | 11.6% (275/2372) | 12.0% (949/7885) | 0.56049 |
| Anaemia | 16.8% (398/2371) | 14.1% (1115/7881) | 0.00150 |
| GFR <60 mL/kg/min | 40.9% (971/2375) | 28.3% (2226/7869) | <0.00001 |
| HF hospitalization <1 year | 47.2% (1119/2371) | 47.3% (3730/7881) | 0.90895 |
| Atrial fibrillation | 54.5% (1292/2369) | 36.6% (2887/7884) | <0.00001 |
| Paroxysmal | 31.7% (409/1292) | 36.3% (1049/2887) | |
| Persistent | 21.7% (281/1292) | 22.8% (658/2887) | |
| Permanent | 45.8% (592/1292) | 40.4% (1165/2887) | |
| Missing | 0.8% (10/1292) | 0.5% (15/28879) | |
| Previous device* | |||
| PM | 60.9% (1460/2398) | N/A | |
| ICD | 39.8% (955/2398) | N/A | |
- CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; GFR, glomerular filtration rate; HF, heart failure; ICD, implantable cardioverter-defibrillator; IQR, interquartile range; N/A, not applicable; PCI, percutaneous coronary intervention; PM, pacemaker.
- * More than one option could be chosen.
Patient characteristics
The baseline patient characteristics are presented in Table 1. Upgraded patients were 3 years older than de novo patients with a larger proportion (35%) having ≥75 years of age. Overall, there were few women in the survey. Moreover, only 18.8% of upgraded patients were women compared to 26.1% in the de novo recipients. Upgraded patients more often had a history of ischaemic heart disease, valvular heart disease or cardiac surgery, and more often had glomerular filtration rate < 60 mL/kg/min. Moreover, they more often had atrial fibrillation (AF). Nearly half of both groups had a previous HF hospitalization within the last year. A minority of both groups participated in clinical trials. Patient characteristics of those excluded from the analysis did not differ significantly from the analysed patients, except that were more often PM-dependent (41.5%), had high-degree atrioventricular (AV) block (35.3%) and more often AF (27.1%).
Pre-implantation clinical evaluation and ECG (Table 2)
In accordance with the greater presence of co-morbidities in the upgraded group, New York Heart Association (NYHA) functional class and N-terminal B-type natriuretic peptide (NT-proBNP) levels were both higher in upgraded patients indicating a worse HF disease state. Severity and presence of mitral regurgitation was also greater in upgraded patients. Significantly more patients were in AF in the upgraded cohort. High-degree AV block was present in 44.2% of upgraded patients compared to 10% in the de novo cohort, and 51.9% in the upgrade cohort were PM-dependent. Accordingly, a PM indication and expected dependence of RV pacing was a more common reason for upgrades, whereas HF with wide QRS was the most common reason for de novo CRT implantation. Notably, PM indication with an expected dependence on RV pacing was the main reason for CRT upgrade in 39% of upgraded patients compared to 17.4% of patients subject to de novo implantation. The paced QRS duration had a median value of 180 ms and an intrinsic value of 160 ms. The 1344 patients in the upgraded cohort had significantly longer intrinsic QRS durations than de novo patients. Left (LBBB) and right bundle branch block (RBBB) were more frequent bundle branch block morphologies in de novo and of indeterminable morphology in the upgraded group. An AV nodal ablation was planned in 52.4% and performed in 47.6% of AF patients subject to upgrades. Mechanical dyssynchrony was amongst indications for CRT implantation in >10% of patients in both groups. However, mechanical dyssynchrony was the sole indication for CRT only in 52 in the de novo CRT and 36 in the upgraded group.
| Upgrades PM/ICD (n = 2398) | De novo (n = 7933) | P-value | |
|---|---|---|---|
| NYHA class | <0.00001 | ||
| I | 2.5% (60/2357) | 3.5% (275/7829) | |
| II | 33.0% (778/2357) | 38.4% (3009/7829) | |
| III | 59.1% (1392/2357) | 53.9% (4223/7829) | |
| IV | 5.4% (127/2357) | 4.1% (322/7829) | |
| BMI, kg/m2, median (IQR) | 27 (25–31) (n = 2291) | 27 (24–30) (n = 7551) | 0.26781 |
| Systolic BP, mmHg, median (IQR) | 120 (110–134) (n = 2326) | 123 (110–138) (n = 7722) | <0.00001 |
| Diastolic BP, mmHg, median (IQR) | 70 (65–80) (n = 2324) | 72 (66–80) (n = 7721) | 0.00053 |
| Echocardiography | |||
| LVEF,% | 30 (22–34) (n = 2342) | 29 (23–33) (n = 7806) | 0.3882 |
| LVEF ≥35% | 14.1% (331/2342) | 11.5% (901/7806) | |
| LVEDD, mm, median (IQR) | 63 (57–69) (n = 1862) | 63 (58–69) (n = 6293) | 0.09855 |
| Mitral regurgitation | 0.00756 | ||
| Mild | 42.9% (940/2189) | 47.7% (3443/7219) | |
| Moderate | 29.1% (637/2189) | 26.0% (1875/7219) | |
| Severe | 7.9% (174/2189) | 6.6% (1423/7219) | |
| None | 20.0% (1935/2189) | 19.7% (1423/7219) | |
| Laboratory, median (IQR) | |||
| NT-proBNP, pg/mL | 2811 (1264–6818) (n = 848) | 2228 (984–5131) (n = 2492) | <0.00001 |
| BNP, ng/L | 479 (164–1159) (n = 283) | 430 (153–1127) (n = 1016) | 0.44957 |
| Hb, g/dL | 13 (12–15) (n = 2249) | 138 (12–15) (n = 7408) | 0.50769 |
| SCr, µmol/L | 108 (88–139) (n = 1356) | 98 (82–126) (n = 4273) | <0.00001 |
| Pre-implantation ECG | |||
| Heart rate, b.p.m., median (IQR) | 70 (65–76) | 71 (61–82) | <0.00001 |
| Atrial rhythm | |||
| Sinus rhythm | 49.7% (1170/2354) | 75.8% (5937/7829) | |
| Atrial fibrillation | 34.4% (810/2354) | 22.9% (1791/7829) | |
| Atrial paced | 10.3% (243/2354) | 0.1% (9/7829) | |
| Other | 5.6% (131/2354) | 1.2% (92/7829) | |
| PR interval, ms, median (IQR) | 180 (157/215) | 180 (160/210) | 0.47623 |
| AV block II/III,% | 44.2% (1028/2327) | 10.0% (770/7728) | <0.00001 |
| Paced QRS duration, ms, median (IQR) | 180 (160–200) | 178 (135–191) | 0.05004 |
| Intrinsic QRS duration, ms, median (IQR) | 160 (140–180) | 160 (140–171) | 0.00002 |
| < 120 | 9.2% (123/1344) | 7.3% (563/7738) | |
| 120–130 | 6.0% (80/1344) | 5.2% (401/7738) | |
| 130–150 | 16.3% (219/1344) | 19.1% (1479/7738) | |
| 150–180 | 39.1% (526/1344) | 48.7% (3772/7738) | |
| > 180 | 29.5% (396/1344) | 19.7% (1523/7738) | |
| Intrinsic QRS morphology | |||
| LBBB | 58.9% (1368/2323) | 77.7% (6976/7822) | <0.00001 |
| RBBB | 4.9% (137/2323) | 7.3% (6569/7822) | 0.00005 |
| Indeterminable | 17.1% (397/2323) | 8.0% (626/7822) | <0.00001 |
| Normal | 7.3% (170/2323) | 7.3% (568/7822) | 0.92658 |
| Missing | 11.9% (277/2323) | 0.1% (4/7822) | |
| PM-dependent | 51.9% (1213/2338) | 0.4% (30/7767) | <0.00001 |
| AV nodal ablation* | <0.00001 | ||
| Performed | 47.6% (78/164) | 12.9% (81/627) | |
| Planned | 52.4% (86/164) | 87.1% (546/627) | |
| Indication for CRT† | |||
| HF wide QRS | 52.1% (1240/2381) | 63.1% (4964/7870) | <0.00001 |
| HF or LV dysfunction and an ICD indication | 42.0% (1000/2381) | 49.9% (3926/7870) | <0.00001 |
| PM indication and expected to be dependent of RV pacing | 39.0% (929/2381) | 17.4% (1368/7870) | <0.00001 |
| Mechanical dyssynchrony | 12.8% (305/2381) | 11.1% (872/7870) | 0.02036 |
| Other | 6.7% (159/2381) | 3.0% (240/7870) | 0.02036 |
- AV, atrioventricular; BMI, body mass index; BP, blood pressure; BNP, B-type natriuretic peptide; CRT, cardiac resynchronization therapy; Hb, haemoglobin; IQR, interquartile range; HF, heart failure; ICD implantable cardioverter-defibrillator; LBBB, left bundle branch block; LV, left ventricular; LVEF, left ventricular ejection fraction; LVEDD, left ventricular end-diastolic diameter; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PM, pacemaker; RBBB, right bundle branch block; RV, right ventricular; SCr, serum creatinine.
- * AV nodal ablation only regards patients with atrial fibrillation.
- † More than one indication could be given.
Cardiac resynchronization therapy implantation and peri-procedural complications (Table 3)
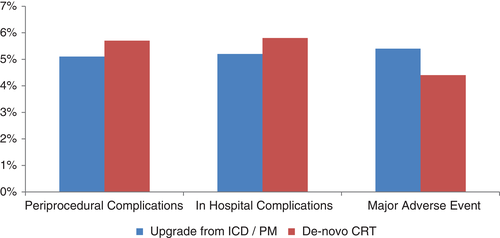
Admission for CRT was elective in most cases, more commonly in the upgraded cohort. Only one fourth of patients were referred from other centres. Implantations were most often performed by electrophysiologists. The implantation was successful in 97.1% of the upgraded and 97.3% of the de novo patients. Overall, about one third of patients received a CRT-P and the rest CRT-D. CRT-P was more often selected for the upgraded cohort than for the de novo group. The median procedure time was 90 min in both groups with slightly longer fluoroscopy time in the de novo implantations reflecting that more than one lead was implanted. Overall, multipolar left ventricular (LV) leads were more common than bipolar leads, although upgraded patients more commonly received bipolar LV lead and less often multipolar leads. LV lead optimization by electrical delay was used in 66.5% of upgraded compared to 59.4% of de novo patients. Optimization by measurement of the paced QRS duration was utilized in 52.4% of upgraded compared to 62% of de novo patients, indicating that attempts were indeed being made to optimize LV lead position by inexpensive methods. The peri-procedural complication rate (Figure 1) was 5.1% in the upgraded and 5.7% in the de novo cohort. Bleeding was more common in the upgraded group and pneumothorax in the de novo group. About one third in both cohorts experienced coronary sinus dissection. Very few patients had pericardial tamponade in both groups.
| Upgrades PM/ICD (n = 2398) | De novo (n = 7933) | P-value | |
|---|---|---|---|
| Admission | |||
| Elective admission | 81.6% (1936/2374) | 76.5% (6035/7884) | <0.0001 |
| Referral | 22.4% (532/2373) | 26.3% (2065/7927 | 0.00013 |
| Successful implantation | 97.1% (2349/2419) | 97.3% (7780/7993) | 0.54406 |
| Unsuccessful implantation | 2.9% (70/2419) | 2.7% (213/7993) | |
| Type of device | <0.00001 | ||
| CRT-P | 35.3% (828/2343) | 28.6% (2224/7766) | |
| CRT-D | 64.7% (151572343) | 71.4% (5542/7766) | |
| Operator | 0.00318 | ||
| Electrophysiologist | 80.0% (1873/2341) | 77.2% (5996/7770) | |
| HF physician | 4.9% (115/2341) | 5.0% (388/7770) | |
| Invasive cardiologist | 10.2% (238/2341) | 12.5% (971/7770) | |
| Surgeon | 4.0% (94/2341) | 3.9% (305/7770) | |
| Other | 0.9% (21/2341) | 1.4% (110/7770) | |
| Prophylactic antibiotics | 98.9% (2291/2316) | 98.6% (7588/7696) | 0.23263 |
| Procedure time, min, median (IQR) | 90 (65–125) | 90 (70–120) | |
| Fluoroscopy time, min, median (IQR) | 13 (7–23) | 14 (8–22) | |
| LV lead type | <0.00001 | ||
| Unipolar | 0.6% (14/2313) | 0.6% (44/7651) | |
| Bipolar | 45.2% (1046/2313) | 40.0% (3059/7651) | |
| Multipolar | 54.2% (1253/2313) | 59.4% (4548/7651) | |
| LV position optimized | 0.00529 | ||
| Electrical delay such as QLV | 66.5% (457/687) | 59.4% (1483/2495) | |
| Paced QRS duration | 52.4% (3357/681) | 62.0% (1548/2496) | |
| Other | 22.8% (155/680) | 24.8% (619/2491) | |
| Peri-procedural complications | 5.1% (125/2430) | 5.7% (461/8020) | 0.25683 |
| Death | 0.0% | 1.7% (8/461) | |
| Any bleeding | 29.6% (37/125) | 14.1% (65/461) | |
| Requiring intervention | 41.7% (15/36) | 26.2% (17/65) | |
| Pocket haematoma | 75.0% (27/36) | 84.6% (55/65) | |
| Pneumothorax | 8.8% (11/125) | 21.3% (98/461) | |
| Haemothorax | 2.4% (3/125) | 1.3% (6/461) | |
| Coronary sinus dissection | 33.6% (42/125) | 34.9% (161/461) | |
| Pericardial tamponade | 2.4% (3/125) | 5.0% (23/461) | |
| Other | 30.4% (38/125) | 24.9% (115/461) |
- CRT-D, cardiac resynchronization therapy-defibrillator; CRT-P, cardiac resynchronization therapy-pacemaker; IQR, interquartile range; HF, heart failure; ICD, implantable cardioverter-defibrillator; LV, left ventricular; PM, pacemaker.
Post-CRT implantation data including adverse events, discharge status and follow-up (Table 4)
Almost all patients in both groups were discharged alive after a median hospital stay of 3 days. However, there were 8 deaths in the upgraded cohort and 32 in the de novo cohort. The reasons for death were cardiovascular in all patients. Major adverse events occurred in 5.4% of upgraded and 4.4% de novo CRT recipients, with arrhythmia being the most common. The difference between CRT-paced and RV-paced QRS duration was –40 ms. For patients with pre-procedural intrinsic rhythm, the corresponding delta was –20 ms. AV and ventriculo–ventricular optimization were very frequently undertaken in both cohorts. Device-based software was used to optimize programming in 31.1% of upgraded patients compared to 37.6% of the de novo patients. Regarding the planned follow-up after discharge, more than 85% of both cohorts were scheduled for follow-up at the implantation centre and remote monitoring was planned in 30%.
| Upgrades PM/ICD (n = 2398) | De novo (n = 7933) | P-value | |
|---|---|---|---|
| Post-implant ECG | |||
| Paced QRS duration, ms, median (IQR) | 140 (129–160) | 134 (120–150) | <0.00001 |
| Paced-intrinsic | –20 (–40 to 0) | –20 (–40 to –3) | 0.00027 |
| CRT-paced | –40 (–59 to –20) | N/A | |
| Device programming | |||
| AV optimization | 52.4% (1206/2300) | 59.3% (4533/7642) | <0.0001 |
| VV optimisation | 51.3% (1178/2296) | 57.8% (4414/7631) | <0.00001 |
| Device optimization | 31.1% (712/2286) | 37.6% (2846/7565) | 0.00064 |
| Discharge state | |||
| Alive | 99.7% (2348/2356) | 99.6% (7790/7821) | 0.69566 |
| Dead | 0.3% (8/2356) | 0.4% (32/7821) | |
| Total length of hospital stay, days, median (IQR) | 3 (2–6) | 3 (2–7) | 0.11462 |
| Major adverse events after the procedure | 5.4% (129/2398) | 4.4% (352/7933) | 0.05496 |
| Myocardial infarction | 0.1% (2/2347) | 0.1% (4/7801) | |
| Stroke | 0.1% (3/2347) | 0.0% | |
| Infection | 0.6% (13/2347) | 0.5% (38/7801) | |
| Worsening HF | 0.9% (21/2347) | 0.7% (53/7801) | |
| Worsening renal function | 1.1% (26/2347) | 0.9% (67/7801) | |
| Arrhythmias | 1.3% (46/2347) | 1.2% (140/7801) | |
| Other | 2.0% (46/2347) | 1.8% (140/7801) | |
| Planned follow-up | 97.7% (2347/2398) | 98.4% (7803/7933) | 0.11043 |
| Implanting centre | 85.1% (1997/2398) | 87.2% (6803/7803) | |
| Other hospital | 7.5% (177/2398) | 8.1% (629/7803) | |
| Private cardiologist | 6.3% (148/2347) | 5.0% (394/7803) | |
| Primary care | 1.0% (24/2398) | 0.8% (65/7803) | |
| CRT/pacemaker clinic | 11.0% (257/2398) | 10% (781/7803) | |
| HF management clinic | 2.7% (63/2347) | 2.5% (193/7803) | |
| Other | 0.3% (7/2347) | 0.3% (21/7893) | |
| Remote monitoring planned | 30.1% (704/2339) | 30.2% (2334/7727) | 0.92100 |
| Drug therapy at discharge | |||
| ACEi/ARB | 83.7% (1925/2299) | 87.4% (6692/7659) | <0.00001 |
| Beta-blocker | 88.6% (2046/2308) | 89.2% (6859/7692) | 0.48089 |
| MRA | 60.1% (1377/2292) | 64.6% (4934/7637) | 0.00008 |
| Digoxin | 11.6% (266/2290) | 9.8% (744/7611) | 0.01073 |
| Ivabradine | 3.9% (90/2284) | 6.2% (473/7616) | 0.00004 |
| Ca2+-channel-blocker | 7.6% (507/2286) | 15.7% (1198/7617) | 0.03055 |
| Amiodarone | 22.2% (507/2286) | 15.7% (1198/7617) | <0.00001 |
| Oral anticoagulant | 61.1% (1904/2303) | 41.8% (3198/7642) | <0.00001 |
| Warfarin | 72.4% (1016/1404) | 68.7% (2198/3198) | 0.01338 |
| Dabigatran | 6.3% (89/1404) | 6.8% (218/3198) | 0.54982 |
| Rivaroxaban | 11.0% (154/1404) | 13.4% (429/3198) | 0.02162 |
| Apixaban | 10.1% (142/1404) | 10.6% 338/3198) | 0.64184 |
| Edoxaban | 0.2% (3/1404) | 0.5% (15/3198) | 0.20128 |
| Antiplatelet agent | 36.4% (874/2396) | 46.7% (3706/7933) | <0.00001 |
- ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; AV, atrioventricular; CRT, cardiac resynchronization therapy; HF, heart failure; ICD, implantable cardioverter-defibrillator; IQR, interquartile range; MRA, mineralocorticoid receptor antagonist; N/A, not applicable; PM, pacemaker; VV, ventriculo–ventricular.
Heart failure medications at discharge included loop diuretics in >80% and beta-blockers (89%). Angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) were more commonly prescribed in de novo (87.4%) than in upgraded (83.7%) patients as were mineralocorticoid receptor antagonists (MRAs) (60.1% in upgrades compared to 64.6% in de novo patients). As expected from the much larger proportion of AF in the upgraded cohort, these patients were significantly more often anticoagulated (61.1%) compared to de novo (41.8%), most frequently with warfarin. In contrast, antiplatelet therapy was more common in the de novo cohort including dual or triple antiplatelet therapy.
Comparison of patients upgraded to a CRT-P or CRT-D (Table 5)
Of the 2398 upgrade patients, 1506 were upgraded to a CRT-D and 818 to a CRT-P. We further separated our comparison to patients upgraded from a previous PM to CRT-P, from a previous PM to CRT-D, and from a previous ICD to CRT-D. For this analysis we excluded 105 patients because of more than one previous device (either a PM or an ICD) ticked in the eCRF, a non-successful CRT implantation or missing information with regard to type of previous device.
| Upgrade from PM to CRT-P (n = 802) | Upgrade from PM to CRT-D (n = 595) | Upgrade from ICD to CRT-D (n = 896) | P-value* | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years (IQR) | 76 (69–81) | 72 (65–76) | 67 (60–74) | <0.00001 |
| Age ≥ 75 years | 57.0% (457/802) | 34.3% (204/595) | 22.7% (203/896) | |
| Female sex | 28.7% (230/802) | 17.1% (102/595) | 12.1% (108/896) | <0.00001 |
| Currently enrolled in a trial | 5.1% (41/801) | 9.6% (57/594) | 6.6% (59/895) | 0.00437 |
| Primary aetiology | ||||
| Ischaemic | 31.9% (254/795) | 49.5% (293/592) | 61.6% (548/890) | <0.00001 |
| Non-ischaemic | 52.6% (418/795) | 43.6% (258/592) | 35.5% (316/890) | |
| Other | 15.5% (123/795) | 6.9% (41/592) | 2.9% (26/890) | |
| Previous history | ||||
| Myocardial infarction | 25.0% (198/792) | 38.4% (227/591) | 54.9% (488/889) | <0.00001 |
| PCI/CABG | 28.3% (224/791) | 43.4% (256/590) | 56.3% (500/888) | <0.00001 |
| Valvular disease | 34.4% (273/793) | 32.1% (189/589) | 30.5% (270/885) | 0.22868 |
| Valvular surgery | 39.1% (129/330) | 45.5% (102/224) | 29.4% (96/327) | 0.00037 |
| Hypertension | 68.0% (538/791) | 67.6% (398/589) | 61.8% (546/884) | 0.01237 |
| Diabetes mellitus | 31.1% (247/794) | 32.9% (194/589) | 31.8% (282/887) | 0.76978 |
| COPD | 11.2% (89/793) | 13.1% (77/589) | 11.4% (101/887) | 0.51762 |
| Anaemia | 17.2% (136/793) | 16.6% (98/589) | 16.3% (144/885) | 0.88992 |
| GFR <60 mL/kg/min | 41.8% (331/792) | 33.4% (198/592) | 43.7% (388/887) | 0.00024 |
| HF hospitalization <1 year | 45.6% (361/792) | 44.0% (259/589) | 50.3% (446/886) | 0.03386 |
| Atrial fibrillation | 57.6% (456/792) | 56.9% (335/589) | 49.4% (437/884) | <0.00001 |
| Paroxysmal | 27.4% (125/456) | 29.6% (99/335) | 38.9% (170/437) | |
| Persistent | 20.4% (93/456) | 20.3% (68/335) | 26.1% (114/437) | |
| Permanent | 51.8% (236/456) | 49.0% (164/335) | 34.6% (151/437) | |
| Missing | 0.4% (2/456) | 1.2% (4/335) | 0.5% (2/437) | |
| Pre-implantation evaluation | ||||
| NYHA class | ||||
| I | 2.9% (23/791) | 2.9% (17/586) | 1.9% (17/881) | 0.00002 |
| II | 27.9% (221/791) | 38.7% (227/586) | 33.9% (299/881) | |
| III | 62.3% (493/791) | 55.8% (327/586) | 58.1% (512/881) | |
| IV | 6.8% (54/791) | 2.6% (15/586) | 6.0% (53/881) | |
| Pre-implantation ECG atrial rhythm | ||||
| Sinus rhythm | 41.8% (329/787) | 39.6% (233/588) | 65.2% (574/881) | <0.00001 |
| Atrial fibrillation | 40.3% (317/787) | 35.7% (210/588) | 27.1% (239/881) | |
| Atrial paced | 13.0% (102/787) | 15.6% (92/588) | 4.3% (38/881) | |
| PR interval, ms, median (IQR) | 176 (142–200) | 169 (150–200) | 198 (164–228) | <0.00001 |
| Intrinsic QRS, ms, median (IQR) | 160 (136–180) (n = 313) | 160 (140–180) (n = 271) | 160 (140–180) (n = 709) | 0.51657 |
| LVEF, %, median (IQR) | 30 (25–36) | 30 (25–33) | 25 (20–30) | <0.00001 |
| Peri-procedural complications | 4.4% (36/810) | 6.0% (36/596) | 4.9% (44/904) | 0.38528 |
| Death | 0.0% (0/36) | 0.0% (0/36) | 0.0% (0/44) | |
| Bleeding | 30.6% (11/36) | 36.1% (13/36) | 25.0% (11/44) | |
| Requiring intervention | 36.4% (4/11) | 50.0% (6/12) | 45.5% (5/11) | |
| Pocket haematoma | 63.6% (7/11) | 83.3% (10/12) | 72.7% (8/11) | |
| Pneumothorax | 5.6% (2/36) | 8.3% (3/36) | 13.6% (6/44) | |
| Haemothorax | 2.8% (1/36) | 2.8% (1/36) | 2.3% (1/44) | |
| Coronary sinus dissection | 33.3% (12/36) | 25.0% (9/36) | 31.8% (14/44) | |
| Pericardial tamponade | 2.8% (1/36) | 0.0% (0/36) | 4.5% (2/44) | |
| Other | 33.3% (12/36) | 33.3% (12/36) | 29.5% (13/44) |
- CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; CRT-D, cardiac resynchronization therapy-defibrillator; CRT-P, cardiac resynchronization therapy-pacemaker; GFR, glomerular filtration rate; IQR, interquartile range; HF, heart failure; ICD, implantable cardioverter-defibrillator; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PM, pacemaker.
- * For statistical significance the non-parametric Kruskal–Wallis test was used when appropriate.
Patients upgraded from a PM to a CRT-P had a median age of 73.7 years and were older compared to patients upgraded to CRT-D. Moreover, 57% were older than 75 years and 28.7% were women. A non-ischaemic aetiology was the most common HF aetiology. These patients were in worse NYHA class, and AF was more common than for patients upgraded from an ICD to CRT-D. Patients upgraded from a PM to a CRT-D had a median age of 70 years and 34.3% were older than 75 years with balanced distribution of HF aetiology. Previous valve surgery was more common in this group than in the other groups. Patients upgraded from an ICD to a CRT-D had a median age of 66.5 years and less often were women. They more often had ischaemic HF aetiology, previous myocardial infarction, a history of coronary interventions, and more often had chronic kidney disease. The median LVEF was lower in this group and a previous HF hospitalization more common. As expected, high-degree AV block and PM dependence was more common in patients upgraded from a PM than in those upgraded from an ICD. Although intrinsic QRS duration did not differ, patients upgraded from an ICD more commonly had a long PR interval. Peri-procedural complications did not differ significantly between upgraded groups but those upgraded from a PM to a CRT-D more often developed bleeds requiring an intervention. Such patients also tended to have more postoperative complications and major adverse events reflecting the more complex procedure of addition of an ICD lead in addition to a LV lead. The median length of stay was longer in patients upgraded to CRT-D than to CRT-P. Regarding medication at discharge, patients upgraded from a PM to CRT-P received less HF medication and less often were planned to be followed by remote monitoring.
Comparison of upgraded patients with intrinsic rhythm with upgraded pacemaker-dependent patients (online supplementary Table S1)
Of upgraded patients, 1125 had intrinsic rhythm and 1213 were PM-dependent. PM-dependent patients were older and had ischaemic heart disease (41.9%) and non-ischaemic disease (46.2%) as primary aetiology, whereas ischaemic heart disease was a more common aetiology (54%) in non-PM-dependent patients. Thus, a prior myocardial infarction or coronary intervention was also more common in this cohort. Importantly, a history of valve surgery, in particular aortic valve replacement, was much more common in PM-dependent patients, possibly reflecting damage to the conduction system during valve surgery. Mitral valve replacements were more common in non-PM-dependent patients. As expected, second–third degree AV block was predominantly found in PM-dependent patients. The PR interval was longer in non-PM-dependent patients and the median LVEF significantly lower.
Benchmarking
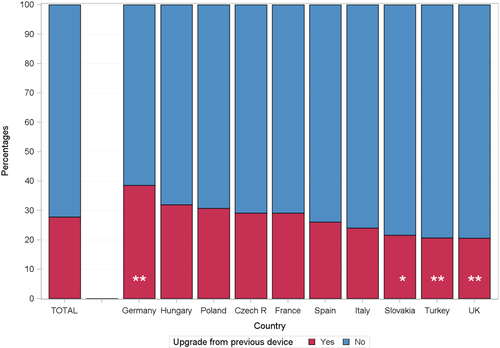
The device choice in upgraded patients amongst the 10 largest enrolment countries in the survey is shown in Figure 2. Slovakia and France chose a CRT-P in about half of the patients, whereas Turkey only implanted CRT-D devices. In Slovakia, 39% in upgraded vs. 43.5% in de novo CRT recipients had ischaemic HF aetiology and 35.4% in the upgraded group and 38.1% in the de novo group had a previous myocardial infarction. In France, 42.4% of upgraded vs. 36.2% in de novo CRT recipients had ischaemic HF aetiology and only 25.9% in the upgraded group and 22.4% in the de novo group had a previous myocardial infarction. In contrast, in Turkey, 56% in the upgraded group and 51.3% in the de novo group had ischaemic HF aetiology and 50% in the upgraded group and 41.5% in the de novo group had a previous myocardial infarction. Moreover, patients in Slovakia were on average 68.8 years old, in France 73.6 years old, and in Turkey 61.1 years old amongst upgrades. For the de novo group, Slovakian patients were 67.1 years old, French patients 71.0 years old, and Turkish 64.2 years old. Whether the younger age and more ischaemic heart disease in the Turkish cohort compared to Slovakia and France is the only explanation for primarily choosing CRT-D is not clear.
Discussion
Cardiac resynchronization therapy introduced in the late 1990s has been a valuable contribution to HF management. The evidence remains strongest in patients with LVEF <35%, sinus rhythm, with wide QRS and/or LBBB.9, 10 In recent years it has also been established that CRT is not of value in narrow QRS (≤130 ms) and may even be harmful.18 However, not much new evidence has emerged concerning upgrades from a previous PM or ICD to a CRT. Our results indicate that clinicians disregard this gap of evidence and continue to upgrade a large number of patients. Upgrades constituted 28% of the cohort16 and the same percentage in the previous survey.12 This proportion may indicate the firm belief by clinicians that CRT will be beneficial in these patient populations despite the absence of strong guideline indications.
In contrast to CRT Survey I, there was no follow-up in the present survey. This simplification was intentional to enable many more countries to join and to collect a larger cohort representative of all ESC geographies. Therefore, this second and much larger survey provides a valuable source of clinical information across the ESC countries and provides feedback on adherence to existing guidelines, which in turn supports the development of future guidelines.
In this survey, patients were older than in RCTs. They were rarely referred from another hospital, in particular for upgrades, indicating limited access to CRT outside of teaching or university hospitals. There were very few women overall in the survey and especially among those upgraded to CRT. This probably indicates under-implementation of therapy overall and in particular in women, which may call for specific actions. However, the true proportion of women with an indication for CRT may be influenced by the fact that women more often have HF with preserved ejection fraction.19 Previous studies indicate that CRT implementation is slow and that seeing a cardiologist or being treated at a HF clinic predicts being considered for CRT,20 thus underlining the need for HF specialist access across Europe. Our findings indicate that such access may be suboptimal. As expected, upgraded patients were also older and had more co-morbidities. Amongst patients upgraded, those upgraded from a PM to CRT-P were 7.2 years older than those upgraded from an ICD to CRT-D and more often women. CRT-P recipients less often had underlying ischaemic heart disease, which in part could explain this choice. Notably, upgraded patients who were PM-dependent had often had a previous aortic valve replacement, which may be indicative of previous conduction system damage. We do not have information if or to which extent the use of transcatheter aortic valve procedures contributed to this observation. Overall, guideline-indicated HF medication was suboptimal but comparable to that in a recent European HF registry.21 For example almost 60% of upgraded compared to 64% of patients with presumably severe HF undergoing de novo CRT implantation were discharged with MRAs. Upgraded patients were somewhat less frequently taking ACE inhibitors or ARBs and were more often taking oral anticoagulants.
A relatively large percentage (17.4%) of the de novo cohort received a CRT because they were expected to depend on RV pacing. This may reflect adoption of the ESC HFA 2016 guidelines,10 which state that CRT rather than RV pacing is recommended for patients with HF with reduced LVEF regardless of NYHA class or atrial rhythm, who have an indication for ventricular pacing due to high-degree AV block in order to reduce morbidity (class I recommendation, level of evidence A).7 This recommendation was published in the middle of the CRT Survey II, which may have influenced our results. Guideline criteria for CRT are based on symptoms, LVEF ≤35%, QRS duration and morphology and emphasize that HF medication should be optimized before considering CRT. In CRT Survey II, QRS duration was ≤120 ms in 9.2% of patients upgraded to CRT and 7.3% of those in the de novo cohort, which is clearly outside of guideline recommendations. Some patients in the upgraded (6%) and in the de novo cohort (5.2%) had a QRS duration 120–130 ms reflecting the 2013 EHRA guideline recommendation but not the 2016 ESC HFA guidelines that were published during the conduct of CRT Survey II.10 It cannot be ruled out that some of these patients were scheduled for an AV nodal ablation, which justified the short QRS duration at the time of implantation. Moreover, our results indicate that mechanical dyssychrony still remains amongst the selection criteria of physicians for CRT, though it is seldom the sole indication. Nonetheless, following the results of EchoCRT,18, 22, 23 these most recent guidelines indicate that CRT is contraindicated in patients with QRS <130 ms and that mechanical dyssynchrony is not an indication (class III recommendation, level of evidence A). In the first survey, there was similar 1-year mortality rates in patients upgraded compared to de novo CRT implantations. Most of the RCTs on CRT did not include patients with a previous device, except for the MUSTIC-AF24 and RAFT trials.7 In the small number of patients studied in these trials, all of whom had AF, only a small or no benefit was shown in patients upgraded from a previous PM25 or from a PM or ICD.26 The 2013 ESC EHRA guidelines on pacing and CRT gave a class I recommendation, level of evidence B9 for device upgrade for patients with persistent symptoms due to HF, but the most recent 2016 ESC HFA guidelines gave upgrades a class IIb recommendation, level of evidence B,10 underlining the lack of scientific evidence.
Chronic RV pacing prolongs QRS duration and induces ventricular dyssynchrony. Previous studies have indicated that extent of RV pacing is linked to the subsequent development of HF in patients paced due to sinus node disease,25 or in ICD recipients with previous LV dysfunction in the DAVID trial.26 The relationship between extent of previous RV pacing defined as >40% and response to upgrading to CRT has been previously studied.27, 28 Previously RV paced patients in one observational study27 responded better to CRT regarding LV function than those who had not experienced RV pacing. In addition, they had an adjusted 33% lower risk of death or HF while tending to have an adjusted lower risk of death than those not RV paced. The authors concluded that patients upgraded to CRT with prior RV pacing respond to CRT at least as well as de novo. However, the true cut-off for which extent and duration of RV pacing may be harmful has not been fully elucidated. These findings indicate that HF due to RV pacing may be ‘easier’ to successfully treat with CRT than HF of other causes. In our survey we do not have information on the extent or duration of RV pacing in our patients upgraded to CRT, and hence have no information as to whether RV pacing had contributed to HF symptoms in our patients. But, in general, patients upgraded in this survey were in worse disease state regarding HF symptoms and had more AF, ischaemic HF, renal function impairment and were older, all of which contributed to HF development. In a recent meta-analysis, there was no significant difference in clinical response or mortality after CRT upgrade compared to de novo implantations.29
These results and the wide use of upgrading in the current and previous survey call for the need for a clinical trial. A randomized study evaluating upgrading from a PM or an ICD to CRT vs. no upgrading could be a valid way to scientifically either support or refute the present extensive choice of upgrading demonstrated in this survey, despite lack of scientific evidence. The ongoing BUDAPEST-CRT Upgrade study30 may provide such evidence. In BUDAPEST-CRT, 360 patients with LVEF <35%, NYHA class II–IV with paced QRS duration ≥150 ms and RV pacing of ≥20% will be randomized to be upgraded to a CRT-D or an ICD or remain in ICD therapy. The primary endpoint is a composite of all-cause mortality, time to first HF hospitalization and ≤ 15% reduction in LV end-systolic volume at 12 months.
It has been reported that there are more complications in upgrades from a previous device compared to new implantations from the Danish Pacemaker Registry31 and the US REPLACE Registry.32 However, in our first CRT survey, peri-procedural and in-hospital complications were similar in upgrades compared to de novo.14 Similarly, in the current survey, we did not find either peri-procedural or in-hospital device-related complications to be different in upgrades compared to de novo patients despite worse HF disease state and more co-morbidities in upgraded patients. However, the upgraded patient cohort had more bleeding complications during surgery, probably reflecting that they more often were on oral anticoagulants. De novo patients more often experienced lead-related complications, reflecting that they had more than one lead implanted instead of LV lead only. Taken together our results suggest no reason to stop physicians from considering upgrade to CRT and/or ICD in indicated patients due to fear of complications.
Limitations
The strength and ability of a survey to address questions are related to the strength of its methodology, its representativeness and size. Overall, 11% of patients implanted with CRT in participating countries were enrolled in the survey. We cannot assess the degree of selection bias in the choice of enrolled patients or in the reporting of unsuccessful implants, complications and mortality rates. Moreover, the majority of participating centres were university and teaching hospitals. This is a limitation, since CRT implantations in the real word are also performed at smaller hospitals.
Furthermore, the interpretation of questions in the eCRFs was up to the investigator. The IHF conducted ‘front-end’ data check and post database lock quality control analyses designed to prevent incorrect data being analysed. It is difficult to quantify the effect of the most recent ESC HFA guidelines which were published during this survey. The adoption of new guidelines take time to be applied in clinical practice.33
Conclusion
Despite lack of evidence-based data, close to one fourth of all CRT implantations across ESC countries in the CRT Survey II of more than 11 000 patients are upgrades from a previous PM or ICD. Upgraded patients had more co-morbidities and older age but similar implantation success and complication rates compared to de novo implantations. These results call for more studies.
Acknowledgements
We thank Tessa Baak, Operation for her dedication and excellent organiZational skills. The work was supported by the European Heart Rhythm Association, the Heart Failure Association, Biotronik, Boston Scientific, Medtronic, Sorin, St. Jude, Abbott, Bayer, Bristol-Myers Squibb, and Servier. Thanks to the industry representatives Joel Courville, Thomas Herrmann, Dave Hollants, Marijke Laarakker, Art Pilmeyer, Nico Uwents and Alphons Vincent. Thanks to the National Coordinators and the implanters for providing us with such a wealth of data.
Conflict of interest: C.M.L. has received research grants from Astra Zeneca, Stockholm County Council and Heart–Lung Foundation and speaker honoraria from Abbott, Medtronic, Vifor and Novartis. A.A. has served as a consultant for Medtronic, Boston Scientific, LivaNova, Merit, and St. Jude Medical; has received speaker fees from Medtronic, Boston Scientific, and LivaNova. C.S. serves as consultant for Abbott, Biosense Webster, Biotronik, Boston Scientific, Medtronic, LivaNova; has received speaker honoraria from Biosense Webster, Biotronik, Boston Scientific, Medtronic, LivaNova. A.M. has received speaker honoraria from LivaNova and Medtronic. The other authors report no conflicts of interest.




