Mesenchymal stromal cells to treat patients with non-ischaemic heart failure: Results from SCIENCE II pilot study
Clinical Trial Registration Information: NCT03797092.
Abstract
Aims
Allogeneic stem cell therapy is more logistically suitable compared with autologous cell therapy for large-scale patient treatment. We aim to investigate the clinical safety and efficacy profile of the allogeneic adipose tissue derived mesenchymal stromal cell product (CSCC_ASC) as an add-on therapy in patients with chronic non-ischaemic heart failure with reduced left ventricular ejection fraction (HFrEF) < 40%.
Methods and results
This is a single-centre investigator-initiated randomized phase I/II study with direct intra-myocardial injections of 100 million allogeneic CSCC_ASC. A total of 30 HFrEF patients with New York Heart Association (NYHA) class ≥II despite optimal anticongestive heart failure medication and plasma NT-proBNP > 300 pg/mL (>35 pmol/L) were included and randomized 2:1 to CSCC_ASC or standard care. The primary endpoint left ventricular end systolic volume (LVESV) and other echo related parameters were analysed by an investigator blinded for treatment allocation. No difference in serious adverse events was observed between groups. LVESV decreased significantly from baseline to 6 months follow-up in the ASC group (153.7 ± 53.2 mL and 128.7 ± 45.6 mL, P < 0.001) and remained unchanged in the standard care group (180.4 ± 39.4 mL and 186.7 ± 48.9 mL, P = 0.652). There was a significant difference between the groups in LVESV change (31.3 ± 11.0 mL, P = 0.009). The difference from baseline to follow-up between the two groups in left ventricular end diastolic volume (LVEDV) was 18.7 ± 12.4 mL, P = 0.146 and in left ventricular ejection fraction (LVEF) −7.8 ± 2.1%, P = 0.001. Considering the baseline values of LVESV, LVEDV and LVEF as covariates, the difference between groups for change from baseline to follow-up resulted in a P-value of 0.056, 0.076, and 0.738, respectively. NYHA class and self-reported health did also improve significantly in the ASC group compared with the standard care group (0.7 ± 0.2, P = 0.001 and −12.8 ± 5.3, P = 0.025; respectively). There was no difference in NT-proBNP (−371 ± 455 pmol/L, P = 0.422) or in 6 min walk test (12 ± 31 m, P = 0.695) between groups.
Conclusions
Intramyocardial injections of allogeneic CSCC_ASC in patients with chronic non-ischaemic HFrEF was safe and improved LVESV, LVEF, NYHA class, and self-reported health compared with standard care group.
Introduction
The prevalence of heart failure in the western world is around 1-2% and 30-40% of these patients have a non-ischaemic aetiology.1, 2 The overall 5-years mortality rate for patients diagnosed with heart failure is around 50%.3 These patients suffer from severe morbidity and mortality despite optimal medical therapy. The prevalence of patients with non-ischaemic heart failure with reduced left ventricular ejection fraction (HFrEF) is increasing with age. Thus, with an aging population in the western world, the total number of patients with non-ischaemic HFrEF will increase. This will challenge the health care systems as the patients with non-ischaemic HFrEF have reduced quality of life, increased rate of hospital admissions, reduced life expectancy, and additionally are hit by premature exit from the labour market. More than half of the adult patients undergoing heart transplantation are non-ischaemic HFrEF patients.4 Consequently, there is an unmet need for an effective treatment capable of reversing non-ischaemic HFrEF to improve patient's survival, quality of life, and reduce health care costs.
In the United States, the number of adults living with HFrEF increased from about 5.7 million (2009–2012) to 6.5 million (2011–2014).5 It is estimated that there will be a continuous increase in prevalence in future and similar tendencies are seen worldwide.
Until now, cell therapy has mainly focused on regeneration of damaged tissue in, for example, ischaemic heart disease, neurological diseases, and bone- and joint diseases especially in chronic stages. However, interest is now expanding to diseases in which a substantial part of the pathology involves inflammatory and immune mechanisms.
Mesenchymal stromal cell is one of the most promising cell types, which can be obtained from bone marrow and adipose tissue, having a potential to facilitate tissue regeneration and modulate the immune system.6
The impact of this potential beneficial immunomodulatory effect of adipose tissue derived mesenchymal stromal cell (ASC) has only been modestly investigated clinically in heart failure patients.7 Latest evidence suggests that the immunomodulatory potential may be clinically important.8 By selecting patients with an inflammatory aetiology, the cell therapy may be more likely to be effective such as in patients with non-ischaemic HFrEF.9
The obstacles experienced with clinical use of autologous stem cell therapies are numerous, and thus, an allogeneic cell product (CSCC_ASC) has been developed. This allogeneic product is a clinically approved cryopreserved off-the-shelf ASC product and has been used in three clinical trials in patients with long-term chronic ischaemic heart failure.10-12 These trials have shown that treatment with CSCC_ASC was safe.
In this study, we wanted to explore the effect of CSCC_ASCs to restore the cardiac function in patients with non-ischaemic HFrEF.
Methods
Study overview
The SCIENCE II Pilot study is a single-centre phase I/II, open randomized clinical trial with a 2:1 parallel assignment to either cell treatment on top of standard care or standard care alone (no sham procedure performed) to investigate the safety and efficacy of intra-myocardial injections of 100 million allogeneic CSCC_ASCs in patients with non-ischaemic HFrEF.
The study protocol is approved by the Slovenian Ethical Committee (0120–354/1018/4) and the Slovenian Medicines Agency (1050-93/1018-4), and it complies with the Declaration of Helsinki. The study is registered in ClinicalTrials.gov (NCT03797092) and EudraCT (2018-002538-19). All patients received oral and written information about the study and signed a written informed consent prior to inclusion.
Patient population
Thirty patients were consecutive enrolled aged 30–80 years with non-ischaemic HFrEF with left ventricular ejection fraction (LVEF) < 40% and New York Heart Association (NYHA) class ≥II despite optimal heart failure treatment without further treatment options. Moreover, the patients had to have plasma NT-proBNP > 300 pg/mL (>35 pmol/L). Inclusion and exclusion criteria are described in Table 1.
| Inclusion criteria. |
|
• Age between 30 to 80 years • Signed informed consent • Known non-ischaemic dilated cardiomyopathy • NYHA ≥II despite optimal heart failure treatment and no other treatment options • Heart failure medication unchanged 2 months prior to inclusion • LVEF ≤ 40% • Plasma NT-pro-BNP > 300 pg/mL (>35 pmol/L) • Patients can only be included 3 months after implantation of a CRT and at least 1 month after an implantation of ICD unit |
| Exclusion criteria |
|
• Heart failure NYHA I • Moderate to severe aortic stenosis (valve area < 1.3 cm2) or valvular disease with option for surgery or interventional therapy • Heart failure caused by cardiac valve disease or untreated hypertension • If the patient is expected to be candidate for MitraClip therapy of mitral regurgitation in the 12 months follow-up period • Cardiomyopathy with a reversible cause that has not been treated, for example, thyroid disease, alcohol abuse, hypophosphataemia, hypocalcaemia, cocaine abuse, selenium toxicity, and chronic uncontrolled tachycardia • Cardiomyopathy in association with a neuromuscular disorder, for example, Duchenne's progressive muscular dystrophy • Previous cardiac surgery • Diminished functional capacity for other reasons such as COPD with (FEV1) < 1 L/min, moderate to severe claudication or morbid obesity • Clinical significant anaemia (haemoglobin < 6 mmol/L), leukopenia (leucocytes < 2 × 109/L), leucocytosis (leucocytes > 14 × 109/L), or thrombocytopenia (thrombocytes < 50 × 109/L) • Reduced kidney function (eGFR < 30 mL/min) • Left ventricular thrombus • Anticoagulation treatment that cannot be paused during cell injections • Patients with reduced immune response • History with malignant disease within 5 years of inclusion or suspected malignancy – except treated skin cancer other than melanoma • Pregnant women • Woman of childbearing potential unless β-HCG is negative and contraception is used during the trial • Other experimental treatment within 4 weeks of baseline tests • Participation in another interventional trial • Life expectancy <1 year |
- β-HCG, beta human chorionic gonadotropin; COPD, chronic obstructive pulmonary disease; CRT, cardiac resynchronisation therapy device; eGFR, estimated glomerular filtration rate; FEV1, forced expiratory volume in 1 s; LVEF, left ventricle ejection fraction; ICD, implantable cardioverter device; NYHA, New York Heart Association.
Cell manufacturing
The investigational cell product, CSCC_ASC, was produced in an approved Good Manufacturing Practice facility in Cardiology Stem Cell Centre (CSCC) at Rigshospitalet, University Hospital, Copenhagen, Denmark. CSCC holds a manufacturing authorization (no. 23909) and a tissue establishment authorization (no. 32298) issued and inspected every second year by Danish Medicines Agency and Danish Patient Safety Authority, respectively. The manufacturing procedure follows the EU Guidelines for Good Manufacturing Practice of Medicinal Products for Human Use (certificate of GMP compliance no. DK IMP 92217).
The donors were tested for HIV, hepatitis B and C, syphilis and HTLV I/II serology by serum analysis within 30 days prior to liposuction and on the day of donation. Donor tissue typing (low HLA I and II genotyping) was performed.
Liposuction was performed in local anaesthesia from abdominal adipose tissue in a healthy donor by an experienced plastic surgeon, and in full compliance with surgical procedures for sterile cosmetic surgery. Approximately 100–150 mL lipoaspirate was obtained from each donor, followed by an animal-free expansion in growth medium supplemented with human platelet lysate (Sexton Biotechnologies) in automated closed bioreactor systems (Quantum Cell Expansion System, Terumo BCT) as previously described.13-15 The final cell product was used after two passages in the bioreactor system. Harvested CSCC_ASCs were cryopreserved in Cell Seal vials (COOK Regentec/Sexton Biotechnologies) (110 million cells in 5 mL) in CryoStor CS10 (BioLife Solutions) and stored below −180°C in nitrogen dry storage until clinical use. Release criteria were sterility, endotoxin <70 IU/mL, viability (>80%), and purity (stable positive markers CD90, CD105, CD73 > 80%, and negative markers CD45 and HLA-DR) (Appendix A). The presence of mycoplasma was tested from all bioreactor expansions immediately prior to cell harvest and the presence of bacteria, fungi, and endotoxins was tested on the final product, immediately prior to cryopreservation.
Two years stability documenting sterility, viability, recovery, CSCC_ASC purity, and proliferation potential, after thawing of the final product, has been documented and approved by competent authorities.
A total of three donors were used to produce the vials for this clinical trial. A patient in the active therapy arm was treated with one vial of CSCC_ASC, which consisted of ASCs obtained from only one donor.
World Courier shipped the CSCC_ASC vials to the treatment site in a qualified portable nitrogen dry shipper in accordance with European rules for Good Distribution Practices, to the on-site vapour phase liquid nitrogen storage freezer.
Electromechanical mapping and intramyocardial injections of CSCC_ASC
A 3D electromechanical map of left ventricle was created using the NOGA® system (Biologics Delivery Systems, CA, USA) prior to the injections. For each patient, colour-coded unipolar voltage and their corresponding ‘bull's-eye’ maps, consisting of at least 150 sampling points were generated. Points were acquired when the catheter tip was stable on the endocardium (defined as simultaneous stability of local activation time, location stability, loop stability, and cycle length stability).
Functional myocardium was defined as unipolar voltage (UV) ≥ 8.27 mV and non-functional myocardium was defined as areas with UV < 8.27 mV.
Trans-endocardial delivery of cell suspension was then performed with MyoStar® (Biosense Webster) injection catheter targeting the areas with preserved myocardial function. After acquiring a stable mapping point with the tip of the catheter perpendicular to the endocardial surface, the endocardial surface was punctured and the needle was advanced into the myocardium, and trans-endocardial injections were performed. Segments with electromechanical mismatch were defined as areas with average UV ≥ 8.27 mV and average linear shortening (LS) < 6%, scarred myocardium was defined as areas with UV < 8.27 mV and LS < 6%, and normal myocardium was defined as areas with UV ≥ 8.27 mV and LS ≥ 6%. The cells were injected in the target zones with UV ≥ 8.27 mV and LS < 6% using Myostar catheter (Biosense-Webster, Diamond Bar, CA, USA). Each patient received between 10 and 15 injections of CSCC_ASC suspension (0.3 mL each, total 100 million ASCs).
Endpoints
The primary endpoint was change in left ventricle end-systolic volume (LVESV) at 6 months follow-up between ASC and standard care group measured by echocardiography. The secondary echocardiographic endpoints were changes in LVEF and end-diastolic volume (LVEDV) at 6 months follow-up. Additional secondary endpoints were changes in NYHA, EQ-5D-5L questionnaire, 6 min walking test, and NT-proBNP. Safety data for both groups were collected, and difference in serious adverse events at 6 months follow-up was analysed.
Echocardiography
The transthoracic echocardiography data were recorded at baseline and 6 months follow-up for both the ASC and standard care group according to American Society of Echocardiography (ASE) and European Association of Cardiovascular Imaging (EACVI) recommendations. For each patient, at least five end-expiratory full cardiac cycles were recorded for each protocol specified view. All acquired images were de-identified and transferred to an independent imaging core-lab (Stanford Cardiovascular Institute Clinical Biomarker & Phenotyping Core Lab). The recordings were analysed at the end of the study by an independent echo-cardiographer, who was blinded to the patient's treatment status and the timing of the recordings. LVESV, LVEDV, and LVEF were measured using the Simpson's biplane method.
Olink analysis
Plasma EDTA samples were collected at baseline, and 1, 3, 6 and 12 months after randomization. The samples were analysed using Olink Target 96 Inflammation at BioXpedia (Aarhus, Denmark). Normalized protein expression values were processed in R16 and RStudio17 using the Olink Analyse package (version 3.4.1), and variables with more than 75% missing values were excluded (all below lower limit of detection). To highlight temporal changes and minimize baseline variability, we normalized the data by dividing each time point's values by the corresponding baseline readings.
Statistical analysis
All statistical analysis is performed using SPSS version 28.0 (SPSS Inc., Chicago, Illinois). Continuous variables are presented as mean ± standard deviation (SD), or 95% confidence interval and categorical variables are presented as numbers and percentages. Paired t-test is used for comparison of continuous data within groups, while unpaired t-test is used for comparison between groups. Comparison between groups for change in echo data from baseline to follow-up was also analysed using analysis of covariance (ANCOVA). Categorical data are compared using Fisher's exact or chi-square test as appropriate. A two-sided P-value of <0.05 is considered statistically significant.
The Olink Analyse package performs a Welch two-sample t-tests at a confidence level of 0.95, and the results were plotted using EnhancedVolcano18 with unadjusted P-value cut-off at 0.05. A linear mixed effect model of treatment with time point as random effect was created using olink_lmer at a significance level of Benjamini & Hochberg adjusted P-value < 0.05.
Results
A total of 30 patients were included into this study from December 2018 to January 2020. Demographic data are presented in Table 2. Overall, 24 men and 6 women were included and randomized 2:1 either to treatment with CSCC_ASC (n = 20) or standard care (n = 10) (Figure 1). No significant differences in baseline data were observed between the two groups.
| Parameter | ASC (n = 20) | Standard care (n = 10) | P-value |
|---|---|---|---|
| Baseline profile | |||
| Age (years) | 62.7 ± 7.9 | 63.8 ± 7.3 | 0.725 |
| Gender (male) | 15 (75.0) | 9 (90.0) | 0.333 |
| NYHA class | 2.3 ± 0.5 | 2.4 ± 0.5 | 0.356 |
| LVESV (mL) | 153.7 ± 53.2 | 180.4 ± 39.4 | 0.215 |
| LVEDV (mL) | 212.1 ± 60.8 | 243.3 ± 45.5 | 0.205 |
| LVEF (%) | 28.7 ± 5.6 | 25.9 ± 4.2 | 0.206 |
| 6-min walking test (m) | 470 ± 93 | 435 ± 103 | 0.349 |
| EQ-5D-5L score | 60.8 ± 15.7 | 58.3 ± 18.5 | 0.718 |
| Medication | |||
| ACEi/ARB/ARNI | 20 (100.0) | 9 (90.0) | 0.161 |
| Beta-blocker | 20 (100.0) | 10 (100.0) | 1.00 |
| MRA | 20 (100.0) | 10 (100.0) | 1.00 |
| SGLT2i | 1 (5.0) | 0 (0.0) | 0.489 |
| Loop diuretic | 10 (50.0) | 5 (50.0) | 1.000 |
| Digoxin | 5 (25.0) | 2 (20.0) | 0.770 |
| Biochemical profile | |||
| NT-proBNP (pmol/L) | 1403 ± 1344 | 1643 ± 1626 | 0.669 |
| Total cholesterol (mmol/L) | 4.8 ± 1.2 | 5.0 ± 1.6 | 0.624 |
| LDL-C (mmol/L) | 2.9 ± 1.0 | 3.1 ± 1.2 | 0.539 |
| HDL-C (mmol/L) | 1.3 ± 0.3 | 1.2 ± 0.2 | 0.286 |
| Triglycerides (mmol/L) | 1.8 ± 1.1 | 2.2 ± 1.6 | 0.486 |
| Creatinine (μmol/L) | 91.8 ± 29.3 | 103.4 ± 33.2 | 0.334 |
- Values are mean ± SD or n (%).
- ACE, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blockers; ARNI, angiotensin receptor Neprilysin inhibitor; ASC, adipose tissue derived mesenchymal stromal cells; HDL, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LVEDV, left ventricular end diastolic volume; LVEF, left ventricular ejection fraction; LVESV, left ventricular end systolic volume; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; SGLT2i, sodium glucose co-transporter 2 inhibitor.
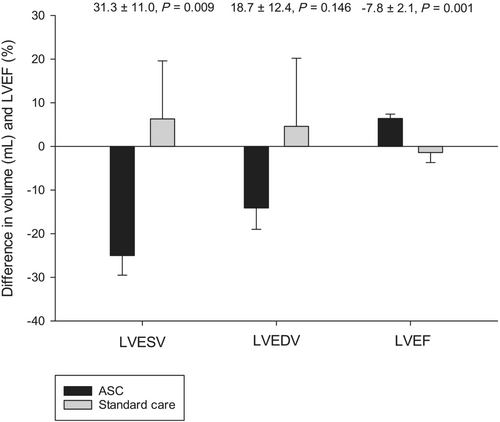
After randomization, one patient from the standard care group was heart transplanted 3 months after randomization (Table 3). One patient from the standard care group died due to multi-organ failure because of sepsis after hysterectomy due to acute haemorrhage. One patient from the ASC group was lost to follow-up as he migrated to another country and was not able to attend after 3 months follow-up due to COVID-related travel restrictions. No complications were observed related to the cell injection procedure.
| ASC (n = 19) | Standard care (n = 10) | P-value | |
|---|---|---|---|
| Death | 0 (0.0) | 1 (10.0) | 0.161 |
| Heart transplantation | 0 (0.0) | 1 (10.0) | 0.161 |
| Hospitalization for heart failure | 3 (15.8) | 3 (30.0) | 0.369 |
- Values are n (%).
Cardiac function
In the ASC group, the LVESV was 153.7 ± 53.2 mL, the LVEDV was 212.1 ± 60.8 mL, and the LVEF was 28.7 ± 5.6% at baseline (Table 4). In the standard care group, the LVESV was 180.4 ± 39.4 mL, the LVEDV was 243.3 ± 45.5 mL, and the LVEF 25.9 ± 4.2%. There were no significant differences between the two groups at baseline in LVESV (26.7 ± 21.0 mL, P = 0.215), LVEDV (31.2 ± 24.0 mL, P = 0.205), or LVEF (−2.8 ± 2.2%, P = 0.206).
| ASC group | Standard care group | ASC group | Standard care group | Between groups | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 months FU | Baseline | 6 months FU | Change from baseline to 6 months follow-up | P-value | Change from baseline to 6 months follow-up | P-value | Mean difference | 95% CI | P-value | ||
| Lower | Upper | |||||||||||
| LVESV (mL) | 153.7 ± 53.2 | 128.7 ± 45.6 | 180.4 ± 39.4 | 186.7 ± 48.9 | −25.0 ± 4.5 | <0.001 | 6.3 ± 13.3 | 0.652 | 31.3 ± 11.0 | 8.7 | 54.0 | 0.009/0.056* |
| LVEDV (mL) | 212.1 ± 60.8 | 198.0 ± 53.6 | 243.3 ± 45.5 | 247.9 ± 55.3 | −14.1 ± 4.9 | 0.010 | 4.6 ± 15.6 | 0.779 | 18.7 ± 12.4 | −7.0 | 44.3 | 0.146/0.076* |
| LVEF (%) | 28.7 ± 5.6 | 35.2 ± 8.0 | 25.9 ± 4.2 | 24.5 ± 5.8 | 6.4 ± 1.0 | <0.001 | −1.4 ± 2.3 | 0.562 | −7.8 ± 2.1 | −12.0 | −3.5 | 0.001/0.738* |
- Values are mean ± SD.
- CI, confidence interval; FU, follow-up; LV, left ventricular; LVEDV, LV end-diastolic volume; LVEF, LV ejection fraction; LVESV, LV end-systolic volume.
- * ANCOVA.
The primary endpoint LVESV decreased significantly from baseline to 6 months follow-up in the ASC group (153.7 ± 53.2 mL and 128.7 ± 45.6 mL, P < 0.001) but remained unchanged in the standard care group (180.4 ± 39.4 and 186.7 ± 48.9 mL, P = 0.652) (Table 4). The difference between the two groups for change in LVESV from baseline to follow-up was 31.3 ± 11.0 mL, P = 0.009 (Figure 1).
There was also a significant decrease in LVEDV (−14.1 ± 4.9 mL, P = 0.010) and a significant increase in LVEF (6.4 ± 1.0%, P < 0.001) in the ASC group from baseline to 6 months follow-up, which was not seen in the standard care group (LVEDV: 4.6 ± 15.6 mL, P = 0.779 and LVEF: −1.4 ± 2.3%, P = 0.562).
Between the two groups, a significant difference in LVEF from baseline to 6 months follow-up was observed on −7.8 ± 2.1%, P = 0.001. Between the two groups, no significant difference was observed in change for LVEDV 18.7 ± 12.4 mL, P = 0.146.
Comparing the change from baseline to follow-up in LVESV, LVEDV, and LVEF between ASC and standard care groups using ANCOVA resulted in a P-value of 0.056, 0.076, and 0.738, respectively (Table 4).
NYHA class and self-reported quality of life
NYHA class improved from baseline to 6 months follow-up by 0.8 ± 0.1, P < 0.001 in the ASC group, while no significant change was observed in the standard care group (0.1 ± 0.1, P = 0.351). A significant difference of 0.7 ± 0.2, P = 0.001 between groups were seen (Table 5).
| ASC group | Standard care group | Between groups | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | 6 months FU | P-value | Baseline | 6 months FU | P-value | Mean difference in change | 95% CI | P-value | ||
| Lower | Upper | |||||||||
| NT-proBNP (pmol/L) | 1403 ± 1344 | 1384 ± 1598 | 0.811 | 1643 ± 1626 | 1394 ± 893 | 0.365 | −371 ± 455 | −1310 | 566 | 0.422 |
| NYHA class | 2.3 ± 0.5 | 1.4 ± 0.1 | <0.001 | 2.4 ± 0.5 | 2.3 ± 0.3 | 0.351 | 0.7 ± 0.2 | 0.3 | 1.1 | 0.001 |
| EQ-5D-5L score | 60.8 ± 15.7 | 74.2 ± 3.7 | <0.001 | 58.3 ± 18.5 | 57.5 ± 8.0 | 0.895 | −12.8 ± 5.3 | −23.8 | −1.8 | 0.025 |
| 6-min walking test (m) | 470 ± 93 | 506 ± 32 | 0.051 | 435 ± 103 | 524 ± 56 | 0.013 | 12 ± 31 | −52 | 76 | 0.695 |
- Values are mean ± SD.
- CI, confidence interval; FU, follow-up; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association.
Self-reported quality of life measured as EQ-5D-5L score did also increase significantly in the ASC group from 60.8 ± 15.7 to 74.2 ± 3.7, P < 0.001, while it remained unchanged from baseline to 6 months follow-up in patients allocated to standard care (58.3 ± 18.5 and 57.5 ± 8.0, P = 0.895). There was a significant difference in EQ-5D-5L score change between the two groups of −12.8 ± 5.3, P = 0.025.
NT-proBNP and 6-min walk test
There were no changes in NT-proBNP in the groups or between the two groups (Table 5). The 6-min walk test, improved in both groups although only significant in the standard care group. No significant difference in the change was observed from baseline to 6 months follow-up between the two groups (12 ± 31 m, P = 0.695).
Olink data
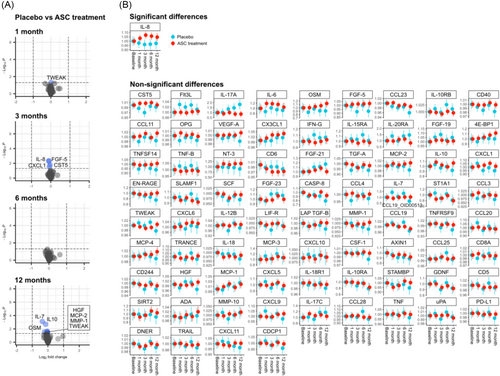
The Olink analysis revealed subtle variations in plasma protein expression levels at different time points, each showing less than a two-fold change (Figure 2A). Evaluating the time course by mixed effect modelling revealed a statistically significant, albeit small, relative change in interleukin-8 (IL-8) (Figure 2B). The difference in IL-8 levels did not surpass 10.77% at 3 months follow up, while changes in the remaining 76 variables remained statistically insignificant.
Discussion
This is the first clinical trial using the CSCC_ASC product to investigate the safety and efficacy as an add-on therapy in patients with non-ischaemic HFrEF. Previously, two large studies using CSCC_ASC have shown neutral results in patients with long-term chronic ischaemic HFrEF. However, there is some evidence of an inflammatory component in patients with non-ischaemic HFrEF and clinical cell therapy trials have indicated that mesenchymal stromal cell therapy may be beneficial in patients with an inflammatory component.19, 20
In this trial, mesenchymal stromal cells were obtained from healthy donors who were not known with any diseases or receiving medical treatment. Cells were culture expanded in automated closed bioreactor system. Afterwards, they were delivered to the treatment site where they were stored until treatment. The entire process went smoothly without any obstacles.
Data from this pilot study indicate that the treatment with CSCC_ASC is safe and improve cardiac functional parameters, which were analysed by an independent observer from another core lab. The echo parameters were also re-measured by a second blinded independent observer. An intraclass correlation coefficient estimate and its 95% confidence interval were calculated based on a single-rating, consistency, two-way random-effects model. This yielded a value of 0.91 (0.88–0.95), suggesting good reliability of the performed echocardiographic measurements.
As this is a small study with 30 patients included, the change in LVESV, LVEDV, and LVEF from baseline to follow-up were also compared between the two groups using ANCOVA which takes the baseline values into account. This resulted in a P-value of 0.056, 0.076, and 0.738, between the two groups for LVESV, LVEDV, and LVEF, respectively. Thus, the LVESV and LVEDV were only borderline statistically in favour of the CSCC_ASC treated group compared with the standard care group.
NYHA class and self-reported health status did also improve significantly between the two groups in the favour of cell therapy. However, there was an increase in 6-min walk test in both groups.
Every cell batch production is tested for viability and functionality, and the mechanisms of actions for ASC capacity are suggested to be through modulation of ongoing immunological and inflammatory processes. Thus, patients with non-ischaemic HFrEF with an on-going inflammatory disease may benefit from this therapy compared with patients with a disease being in a chronic non-inflammatory state, in which the myocardial micro milieu is not able to benefit from the cells delivered.
Interestingly, there may be a higher level of inflammation in early stages of non-ischaemic HFrEF disease and it can be speculated whether the cell treatment should be given early in the disease process.
Interestingly, the DREAM-HF Phase III trial with allogeneic bone marrow-derived mesenchymal stromal cells (rexlemestrocel-L) or placebo given to 537 patients with HFrEF showed in a subgroup analysis that early treatment after the HFrEF diagnosis prevented progression to NYHA class III for patients with NYHA class II and also reduced cardiac death by 60%.8 Another clinical cell trial, treated patients 4–12 months after a myocardial infarction with allogeneic cardiosphere derived cells demonstrating improvement in cardiac magnetic resonance measured functional cardiac parameters suggesting an early intervention.21
It remains challenging to attribute systemic inflammation markers to the therapeutic impact of CSCC_ASC treatment or disease progression. Our findings indicate a minor relative increase in IL-8 plasma level, peaking transiently at 3 months in ASC group before converging with changes seen in the placebo group. Although typically associated with immune activity, especially neutrophil mobilization, IL-8 has also been implicated in cardiac disease.22 However, this cytokine serves a multifaceted role, acting as an effective promoter of angiogenesis23 and potentially enhancing mesenchymal stromal cell mediated angiogenesis.24 In any case, the observed difference between groups is minimal and cannot surely be linked to clinical outcome. It is possible that the circulating inflammatory mediators may not adequately reflect the local cardiac environment and IL-8 finding may be a result of multiple testing. Our primary focus was on the Olink inflammation panel driven by the hypothesis that the immune modulatory properties of the cell product would manifest through changes in inflammatory markers. However, other markers such as galectin-3, ST-2, and GDF-15 could be of importance. The predefined nature of the Olink panels restricted our ability to include these specific markers in the current study, which is a part of the Cardiovascular III panel.
The present trial is a single centre small study but with the primary endpoint data analysed by an independent core centre blinded to the randomization. However, more investigations are warranted to fully explore the potential clinical effect of CSCC_ASC treatment in patients with non-ischaemic HFrEF.
Conclusions
Allogeneic cell therapy is growing as a treatment option and now logistically feasible. In this clinical pilot study of patients with non-ischaemic HFrEF, intramyocardial injections of CSCC_ASC on top of standard anti-congestive heart failure treatment showed improved cardiac function, NYHA class, and self-reported health. Further larger randomized clinical trials are needed to confirm these findings.
Acknowledgements
This trial was supported by the Innovation Fund Denmark grant (CSCC grant no. 6153-00002A) and by Aase and Ejnar Danielsens Foundation.
Conflict of interest
Jens Kastrup, Annette Ekblond, and Mandana Haack-Sørensen are inventors of a granted patent (‘Stem Cell Therapy Based On Adipose-Derived Stem Cells’) (WO2017068140A1 EP3365432A1) owned by the Capital Region of Denmark and Rigshospitalet, Copenhagen University Hospital, Denmark. The patent is granted in Europe and Australia. Applications are submitted in Canada, China, Hong Kong, Japan, Korea, and USA. Jens Kastrup, Annette Ekblond, and Mandana Haack-Sørensen are the founder of Cell2Cure ApS, which has a licence to commercialize the patent. The authors declare no conflict of interest except above mentioned.
Appendix A
| Attribute | Acceptance criteria |
|---|---|
| Number of cells | 100–120 million cells |
| Viability | >80% |
| Donor serology | Negative for anti-HIV1, 2 (antibody + Ag) |
|
Negative for anti-HCV |
|
|
Negative for HBsAg |
|
| Negative for anti-HBc | |
| Negative for syphilis | |
| Negative for HTLV I/II | |
| Sterility | |
| Bacteria/fungi | Negative/negative |
| Endotoxin level | <70 EU/mL |
| Mycoplasma | Negative |
| Purity | CD 90 > 80% |
| (immunophenotype) | CD 105 > 80% |
| CD 73 > 80% | |
| CD 45 < 3% | |
| HLA-DR < 5% |




