Increased interleukin-6 is associated with higher risk of heart failure in people with type 2 diabetes
Abstract
Aims
We aimed to determine the association between serum interleukin-6 (IL-6) concentrations and new-onset heart failure (HF) in persons with type 2 diabetes (T2D).
Methods and results
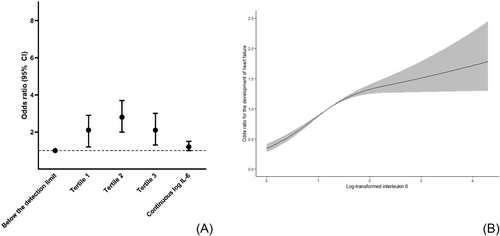
We performed a case–control study nested in the Diabetes Care System Cohort, a prospective cohort of persons with T2D in primary care. We included 724 participants, of whom 141 developed HF during 5 years of follow-up and 583 were age- and sex-matched controls. IL-6 was measured at baseline and categorized into four groups: Group 1 was composed of participants with IL-6 below the detection limit of 1.5 pg/mL, and the remainder were divided into tertiles. We performed logistic regression analyses with categorized IL-6 or continuous IL-6 as the determinant and new-onset HF as the outcome adjusted for follow-up time, age, sex, glycated haemoglobin, estimated glomerular filtration rate, albumin/creatinine ratio, and cardiovascular disease at baseline. Effect modification by sex was tested. Participants were 70.7 ± 9.0 years, and 38% were women. In comparison with Group 1, all tertiles were associated with an increased risk of HF with odds ratios of 2.1 [95% confidence interval (CI): 1.2–2.9], 2.8 (95% CI: 2.0–3.7), and 2.1 (95% CI: 1.3–3.0), respectively, for Tertiles 1–3. Continuous IL-6 was associated with the development of HF with an odds ratio of 1.2 (95% CI: 1.0–1.5). No effect modification by sex was observed.
Conclusions
Higher IL-6 levels are associated with the development of HF in persons with T2D. Further research should determine whether IL-6-lowering interventions could prevent the development of HF.
Introduction
The prevalence of heart failure (HF) among people with type 2 diabetes (T2D) is high, ranging from 5% to 28%.1, 2 Indeed, people with T2D are at a two- to four-fold increased risk of HF in comparison with individuals without T2D, in particular for HF with preserved ejection fraction (HFpEF).3 Inflammation could play a key role in the pathophysiology of HFpEF.4 In general population cohorts, higher interleukin-6 (IL-6) levels have been associated with increased incidence of HF.5, 6 To date, the association between IL-6 and risk of HF has not been studied in persons with T2D.
Methods
We performed a case–control study nested in the Diabetes Care System Cohort, a prospective cohort that consists of persons with T2D in primary care of the West-Friesland region in the Netherlands.7 In 2008–14, as part of a biobank, we have drawn blood in a random subsample of 5629 participants.8 From this subsample, we selected all participants who developed HF during follow-up (N = 142) and were 1:4 matched on age and sex with 592 controls who did not develop HF, which resulted in a total study population of 724 participants. The study has been approved by the Ethical Review Committee of the Vrije Universiteit University Medical Center, Amsterdam. Each participant in this biobank subset gave informed consent for the use and storage of their material.
Interleukin-6 measurement
Fasting blood samples were drawn from the participants between 2008 and 2014, which is considered the baseline in this study, and stored at −80°C until analysis in 2021. The IL-6 assay was performed on serum samples using the e601 module of the cobas 6000 (Roche Diagnostics, Mannheim, Germany). The intra-assay and interassay coefficients for variation were 3.5% and 4.3%, respectively.5
Incident heart failure
HF was self-reported, and the events were verified against the medical records from the regional hospital and general practitioner. In this registration, HF was coded as ICD-9 Codes 428 and 429. Only the verified events were included as events in this current study.
Covariates
Glycated haemoglobin (HbA1c) was measured by the turbidimetric inhibition immunoassay (cobas c501, Roche Diagnostics, Mannheim, Germany) and is expressed in percentages.7 Kidney function was estimated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) estimated glomerular filtration rate (eGFR) equation.9 Albumin/creatinine ratio (mg/mmol) was measured from an overnight first-voided urine sample. Weight and height were measured when participants were barefoot and wore light clothes. Body mass index (BMI) was calculated by dividing weight by the square of height. Systolic and diastolic blood pressure were measured twice while the participant was sitting on their right arm using a random-zero sphygmomanometer. Total cardiovascular disease (CVD; ICD-9 Codes 390–459 and 8099.60), acute myocardial infarction (ICD-9 Codes 410 and 411), coronary heart disease (ICD-9 Codes 410–414), peripheral arterial disease (ICD-9 Codes 440–443.9 and 444.2), cerebrovascular accident and transient ischaemic attack (ICD-9 Codes 430–438), and cancer (ICD-9 Codes 140–239) at baseline were self-reported by the participants during their visit.
Statistical analyses
Continuous variables were depicted as mean ± SD or median [inter-quartile ranges], and numbers (percentages) for categorical variables. IL-6 was categorized into four groups: Group 1 was composed of participants with levels below the detection limit of 1.5 pg/mL, and the remainder of the levels were divided into tertiles. Continuous IL-6 was log-transformed as it was not normally distributed. Logistic regression analyses were used to determine the association between both categorized IL-6 with Group 1 as the reference group, as well as continuous log-transformed IL-6, and new-onset HF. The analyses were adjusted for a priori selected confounders: follow-up time, age, sex, HbA1c, eGFR, albumin/creatinine ratio, and CVD status at baseline. Additionally, sex was tested as an effect modifier by adding an interaction term (IL-6 × sex) to the model. We used ANOVA to determine improved model fit where we would stratify the results when the P value was lower than 0.05. To visualize the association between log-transformed IL-6 levels and HF, we used restricted cubic splines, with three knots placed at the 10th, 50th, and 90th percentiles. Missing values of the determinant (2% missing) and covariates (~10% missing) were imputed with multiple imputation.10 Analyses were performed in RStudio Version 4.2.1, and Figure 1 was drawn in GraphPad Prism 9.
Results
Participants with IL-6 levels below the detection limit were younger (67 ± 9 vs. 71.5 ± 9.0 years) and had less often CVD at baseline (20% vs. 40%) than participants in Tertiles 1–3 (Table 1). Both cases and controls had a mean age of 71 ± 9 years, and the female-to-male distribution was similar (Supporting Information, Table S1). Both cases and controls had a similar BMI of ~30 kg/m2, but cases had more often CVD at baseline than controls (66% vs. 31%). Indeed, 75.8% used antihypertensive medication, 73.5% used lipid-lowering medication, and 62.6% and 31.8% used metformin and sulfonylurea (SU) derivatives, respectively. Because the guidelines for diabetes management of Dutch general practitioners did not allow sodium–glucose cotransporter-2 (SGLT2) inhibitors until November 2021, no patients used SGLT2 inhibitors during the period of the study.
| Total population (N = 724) | Categorized IL-6 (pg/mL) | ||||
|---|---|---|---|---|---|
| Below the detection limit (N = 127) |
Tertile 1 (N = 199) |
Tertile 2 (N = 199) |
Tertile 3 (N = 199) |
||
| ≤1.5 | 1.52–2.94 | 2.96–5.38 | ≥5.4 | ||
| Age (years) | 70.7 ± 9.0 | 67.0 ± 8.6 | 70.5 ± 8.6 | 72.3 ± 8.6 | 71.7 ± 9.5 |
| Women | 272 (37.6%) | 43 (33.9%) | 83 (41.7%) | 71 (35.7%) | 75 (37.7%) |
| BMI (kg/m2) | 29.6 ± 5.0 | 27.7 ± 3.5 | 29.3 ± 4.3 | 30.1 ± 5.3 | 30.6 ± 5.8 |
| Diabetes duration (years) | 6.2 [3.0–10.7] | 6.1 [3.2–10.4] | 6.1 [2.9–11.0] | 6.3 [3.1–10.4] | 6.4 [2.7–10.8] |
| HbA1c (%) | 6.5 [6.1–7.1] | 6.4 [6.0–6.9] | 6.6 [6.2–7.0] | 6.6 [6.1–7.1] | 6.5 [6.1–7.2] |
| eGFR (mL/min/1.73 m2) | 73.8 ± 18.4 | 79.4 ± 14.5 | 73.3 ± 18.3 | 72.8 ± 18.9 | 71.8 ± 19.5 |
| Albumin/creatinine ratio | 0.7 [0.2–1.8] | 0.5 [0–1.07] | 0.5 [0–1.4] | 1.0 [0.3–2.3] | 0.9 [0.3–2.8] |
| HDL cholesterol (mmol/L) | 1.25 ± 0.36 | 1.32 ± 0.38 | 1.27 ± 0.36 | 1.21 ± 0.32 | 1.21 ± 0.36 |
| Systolic blood pressure (mmHg) | 146.6 ± 21.4 | 140.6 ± 20.4 | 146.5 ± 21.9 | 150.1 ± 20.4 | 147.0 ± 21.9 |
| Diastolic blood pressure (mmHg) | 77.2 ± 9.0 | 77.8 ± 8.5 | 78.0 ± 9.1 | 77.2 ± 9.0 | 75.8 ± 9.1 |
| Current smoker | 112 (15.5%) | 19 (15.0%) | 19 (9.5%) | 34 (17.1%) | 40 (20.1%) |
| Total CVD at baseline | 276 (38.1%) | 25 (19.7%) | 78 (39.2%) | 85 (42.7%) | 88 (44.2%) |
| AMI at baseline | 84 (11.6%) | 7 (5.5%) | 24 (12.1%) | 27 (13.6%) | 26 (13.1%) |
| CHD at baseline | 171 (23.6%) | 17 (13.4%) | 47 (23.6%) | 57 (28.6%) | 50 (25.1%) |
| PAD at baseline | 53 (7.3%) | 4 (3.1%) | 9 (4.0%) | 22 (11.1%) | 18 (9.0%) |
| CVA and TIA at baseline | 53 (7.3%) | 4 (3.1%) | 16 (8.0%) | 15 (7.5%) | 18 (9.0%) |
| Use of antihypertensive medication | 549 (75.8%) | 81 (63.8%) | 154 (77.4%) | 154 (77.4%) | 160 (80.4%) |
| Use of lipid-lowering medication | 532 (73.5%) | 92 (72.4%) | 160 (80.4%) | 150 (75.4%) | 130 (65.3%) |
| Use of cardiac medication | 102 (14.1%) | 8 (6.3%) | 25 (12.6%) | 41 (20.6%) | 28 (14.1%) |
| Metformin use | 453 (62.6%) | 76 (59.8%) | 127 (63.8%) | 130 (65.3%) | 120 (60.3%) |
| Insulin use | 170 (23.5%) | 23 (18.1%) | 35 (17.6%) | 49 (24.6%) | 63 (31.7%) |
| SU derivative use | 230 (31.8%) | 38 (29.9%) | 61 (30.7%) | 61 (30.7%) | 70 (35.2%) |
| Follow-up time (years) | 5.0 [4.4–9.1] | 5.3 [4.7–9.3] | 5.2 [4.5–9.2] | 4.9 [4.2–9.2] | 4.8 [3.1–8.5] |
| IL-6 (pg/mL) | 4.0 [2.6–6.8] | 1.5 | 2.3 [1.9–2.6] | 4.0 [3.4–4.7] | 8.8 [6.8–13.5] |
- AMI, acute myocardial infarction; BMI, body mass index; CHD, coronary heart disease; CVA, cerebrovascular accident; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; IL-6, interleukin-6; PAD, peripheral arterial disease; SU, sulfonylurea; TIA, transient ischaemic attack.
In comparison with Group 1, all tertiles were associated with an increased risk of HF with odds ratios of 2.1 [95% confidence interval (CI): 1.2–2.9], 2.8 (95% CI: 2.0–3.7), and 2.1 (95% CI: 1.3–3.0), respectively, for Tertiles 1–3 (Figure 1). Sex did not modify this association (P value for improved model fit = 0.24). Continuous IL-6 was borderline associated with the development of HF with an odds ratio of 1.2 (95% CI: 1.0–1.5) (Figure 1), and the spline regression showed a non-linear association (Figure 1).
Aim
We aimed to determine the association between IL-6 concentrations and new-onset HF in persons with T2D.
Conclusions
This study showed that higher serum IL-6 concentrations are associated with an increased risk of HF in persons with T2D. To our knowledge, our study is the first to determine the association between IL-6 and new-onset HF in persons with T2D. Our findings are partially in line with earlier findings in a Dutch general population and US community-dwelling population.5, 6 For our continuous results, we found borderline significant associations, as opposed to the previous studies that found significant associations for continuous log-transformed IL-6 and the development of HF. However, as we found a non-linear association between IL-6 and HF, the continuous results are not a good reflection of the actual relationship. For the association of IL-6 in tertiles with HF, we found similar effect estimates as the study in the Dutch general population.5
A limitation is that we could not differentiate between HFpEF and HF with reduced ejection fraction (HFrEF). Results from another study have shown that the association of IL-6 with HFpEF is stronger than the association with HFrEF.5 As our cohort consists only of persons with T2D, who predominantly suffer from HFpEF, our findings could be driven by HFpEF.2 Another limitation is that we use self-reported HF with verification from medical records, which is prone to underestimate the number of cases. Last, as we used routine care data, we did not have additional information on other inflammatory biomarkers.
It remains unclear whether IL-6 could be a target to prevent the development of HF in persons with T2D and in the general population. Recent Mendelian randomization studies suggested that IL-6 is not causally associated with HF.11-13 These studies were performed in a European general population; thus, it is unclear whether these results could be extrapolated to a T2D population. Moreover, results with the treatment of monoclonal antibodies for the IL-6 receptor have been contradictory.14 Therefore, further research in specific patient populations, such as persons with T2D, is needed to confirm the causal role of IL-6 in the development of HF.
In conclusion, IL-6 is associated with new-onset HF in persons with T2D. Further research is warranted to further confirm whether IL-6 might play a causal role in the development of HF, and especially HFpEF, in T2D populations.
Acknowledgements
We would like to thank Coba Smit for her contribution in the measurement of the IL-6 levels.
Conflict of interest
None declared.
Funding
This work was supported by RECONNECT and by the Netherlands Cardiovascular Research Initiative: an initiative with support of the Dutch Heart Foundation (CVON2016-Early HFpEF). J.W.J.B. is supported by a ZonMw VIDI grant (91718304).




