Prognostic effect of systematic geriatric assessment on patients with acute heart failure
Abstract
Aims
Frailty and dependence are frequent in patients admitted for acute heart failure (AHF), but their prognostic significance is unknown, especially in young adults. We aimed to study in adults admitted for AHF, regardless of age, the effect of frailty and dependence on the incidence of mortality and a combined event of mortality, readmissions for AHF, and visits to the emergency room (ER) for AHF at 1 and 6 months.
Methods and results
We designed a prospective cohort study by including all the patients with AHF admitted in our Cardiology Department from July 2020 through May 2021. A multidimensional geriatric assessment was performed during the admission. We clinically followed up the patients 6 months after discharge. We enrolled 202 patients. The mean age was 73 ± 12.32 years, and 100 (49.5%) of the patients were elderly (>75 years). Just 78 patients (38.6%) were women, and 100 (49.5%) had previous HF. Frailty (FRAIL ≥ 3) was observed in 68 (33.7%) patients (mean FRAIL score: 1.88 ± 1.48). Dependence (Barthel < 100) was observed in 65 (32.2%) patients (mean Barthel index: 94.38 ± 11.21). Frailty and dependence showed a significant association with both prognostic events at 1 and 6 months. In the multivariable analysis, frailty was associated with higher mortality at 1 month [hazard ratio (HR) 12.61, 95% confidence interval (CI) 1.57–101.47, P = 0.017] but not at 6 months (HR 2.25, 95% CI 0.61–8.26, P = 0.224) or with the combined endpoint at neither 1 month (HR 1.64, 95% CI 0.54–5.03, P = 0.384) nor 6 months (HR 1.35, 95% CI 0.75–2.46, P = 0.320). Dependence was related to higher mortality at 1 month (HR 13.04, 95% CI 1.62–104.75, P = 0.016) and 6 months (HR 7.18, 95% CI 1.99–25.86, P = 0.003) and to higher incidence of the combined event at 1 month (HR 5.93, 95% CI 1.63–21.50, P = 0.007) and 6 months (HR 2.62, 95% CI 1.49–4.61, P = 0.001).
Conclusions
In AHF patients, frailty and dependence implied a worse prognosis, rising mortality, readmissions, and ER visits for AHF.
Introduction
Frailty and heart failure (HF) are two highly prevalent conditions that are correlated. The worldwide prevalence of HF is estimated to be 64 million,1 and this prevalence increases with age,2 observing more than 10% of HF patients in adults over 70 years.3 The prevalence in the United States is estimated at ~6 million.4 The frail condition usually appears in HF patients, occurring up to six times more frequently in those diagnosed with HF compared with those without HF. Frailty is a condition that increases a patient's vulnerability to the negative effect of stressors, independent of age,5 and has been associated with a higher risk of death and rehospitalization for HF.6, 7 At the cellular and molecular levels, some forms of damage occur that reduce the physiological reserves, often resulting in physical inactivity and malnutrition. Frailty is usually focused on physical function and on clinical variables, which define frailty as the result of health deficits. Several scales have been developed to define frailty, including the FRAIL8 and Short Physical Performance Battery (SPPB) scales,9 based on clinical and functional variables.
In a comprehensive geriatric assessment, other factors such as dependence determination10 and quality of life11 assessment should also be considered. Dependence is a frequent occurrence in patients with cardiovascular disease12 and is linked to a higher incidence of adverse outcomes in patients with acute heart failure (AHF) attending the emergency room (ER).13
Conditions such as frailty and geriatric syndromes are usually measured in older patients14 with chronic heart failure (CHF),15 who are often admitted to internal medicine or geriatric units. However, limited research has been conducted on this condition among all age groups and AHF patients admitted to cardiology units. It appears to be more extensively studied among elderly patients with CHF. Some studies, including FRAILTY-AHF16 and Sze et al.,17 analyse AHF in elderly patients and find that frailty is associated with a worse prognosis.
Our study aimed to assess the prognostic impact of frailty and dependence on all-cause mortality and a combination of all-cause mortality, readmissions, and emergency visits because of HF among patients admitted with AHF to the Cardiology Department.
Methods
Study design and patients
We performed a single-centre, observational, and prospective cohort study. We enrolled all patients with AHF who were admitted to the cardiology unit of our hospital from July 2020 through May 2021, 18 years of age or older. AHF was defined according to the European Society of Cardiology guidelines criteria.18 Therefore, we included de novo AHF and patients with exacerbation of CHF. Patients diagnosed with acute coronary syndrome, advanced atrioventricular block, or stroke within the last 7 days were excluded. With this decision, we tried to avoid the inclusion of congestive patients due to other aetiologies than primary HF itself. We also excluded patients with systemic disease and a life expectancy of <1 year, as this has a greater impact on the prognosis than does AHF.
The study protocol observed the principles of the Declaration of Helsinki and was evaluated and approved by the ethics committee of our hospital (reference CEIC-2364). Prior to inclusion, all the patients signed written informed consent. Inclusion in the study did not influence clinical decisions on the diagnosis and treatment of the patients, which were made by the patient's regular physician. Personal data were processed confidentially and in accordance with the provisions of Regulation (EU) 2016/679 of the European Parliament.
Data collection
Local investigators collected data during the admission. Baseline clinical features (age, sex, history of HF, and comorbidities such as hypertension and atrial fibrillation) and echocardiographic [left ventricular ejection fraction (LVEF)] data and electrocardiogram (type of rhythm) were studied. We selected 75 years as a cut-off point to dive the sample in young and old patients according to previous research.12 Blood samples were collected at admission and before discharge to determine the estimated glomerular filtration rate, N-terminal pro-brain natriuretic peptide (NT-proBNP), and troponin T ultrasensible. Comorbidities were expressed by the Charlson Comorbidity Index (CCI).19 We collected medication data prescribed at discharge as angiotensin-converting enzyme inhibitors (ACE-Is), angiotensin receptor neprilysin inhibitors (ARNIs), beta-blockers (BBs), and mineralocorticoid receptor antagonists.
We conducted a clinical follow-up of the patients for 6 months from the time of discharge by reviewing electronic medical records and on-site and telephone clinical interviews to collect adverse cardiovascular events: admissions for AHF, visits to the ER for AHF, or death.
Geriatric assessment
A geriatric assessment was performed always for the same trained cardiologist involved in the research on the day of the discharge including the evaluation of the quality of life (measured by the Minnesota scale),11 frailty (defined by a FRAIL score ≥ 3),8 and dependence (defined by a Barthel index < 100).10
We have decided to include pre-frail patients in the non-frail group because these patients have not yet developed the characteristics of the vulnerability of a frail patient, although they are at higher risk.20 All grades of dependence are included in the dependent group of patients (light, moderate, and severe dependence).
The FRAIL is a validated five-item questionnaire that includes questions about fatigue, resistance, ambulation, comorbidities, and weight loss. It is an easy and fast scale to determine frailty.8 It predicts functional decline, mortality, and healthcare utilization,21 and it is validated in HF patients.22
The Barthel index is recommended by the British Geriatrics Society to measure physical independence. It evaluates 10 basic activities of daily living and their performance or not independently: eating, washing, dressing, grooming, continence in bowel movements and urination, using the bathroom, moving around, walking, and climbing stairs. The dependence or independence in each activity is rated with 0, 5, 10, or 15 points. The resulting range is from 0 to 100; the more dependent, the lower the value.10 Only the result of 100 is considered independent, with the lower values having different grades of dependence.
The Minnesota scale (Minnesota Living with Heart Failure Questionnaire) is the most widely used scale to determine the quality of life in patients with HF; it contains 21 items and 2 dimensions: physical and emotional. It presents excellent reliability and validity estimators.11 The CCI is a system for evaluating life expectancy at 10 years, depending on the age at which it is evaluated and the subject's comorbidities. In addition to age, it consists of 19 items, which, if present, have been found to have a specific influence on the subject's life expectancy.19
The Meta-Analysis Global Group in Chronic (MAGGIC) scale was also measured. This is a validated risk score for predicting the prognosis of HF patients. It evaluates age, ejection fraction, systolic blood pressure, body mass index, creatinine, New York Heart Association class, gender, current smoker, presence of diabetes, chronic obstructive pulmonary disease, HF diagnosed more than 18 months ago, and treatment with BB and ACE-I.23 The MAGGIC risk score has been validated by several studies as a good risk tool for predicting the prognosis of HF patients, and also, it is related to left ventricular remodelling.24
Statistical analysis
A 2.5-fold increase in the risk of admission due to AHF or mortality at 6 months was assumed in frail subjects compared with non-frail subjects and an incidence of events of 25% in non-frail subjects. With these data, it was estimated to include 172 patients with a power of 80% and a bilateral significance level of 0.05.
The results were presented as a number (percentage) for the discrete variables and a mean and standard deviation for the continuous variables. We used the χ2 test for differences in frequency. The variables were classified into normal and non-normal with the Kolmogorov–Smirnov test. First, we analysed the differences in baseline conditions between frail and non-frail patients and between dependent and independent patients by using a Student's t-test (for the normal variables) and a Mann–Whitney U test (for the non-normal variables). Significant results were deemed statistically significant if the P value was <0.05.
Second, we analysed the prognostic effect of frailty (FRAIL ≥ 3) and dependence (Barthel < 100) with the Kaplan–Meier method and log-rank test for univariate survival analysis. Then, a backward Wald–Cox proportional odd regression model was performed for each scale by including those variables with P < 0.005 in univariate analysis to avoid excessive comparisons. For this reason, we also did not include in the multivariate analysis those variables that showed collinearity analysed by the contingency coefficient. Nevertheless, a post hoc Bonferroni adjustment was developed to control the overall probability of a Type I error for multiple hypothesis tests by dividing the critical P value (α) by the number of comparisons. Statistical analysis was performed using SPSS Statistics for Windows software, Version 20.0 (IBM, Chicago, IL, USA).
Results
We enrolled 202 patients. The mean age was 73 years. Only 78 patients (38.6%) were women, and 50.5% were younger than 75 years old. Frailty (FRAIL ≥ 3) was observed in 68 patients (33.7%). The mean FRAIL score was 1.88 ± 1.48. Most of the patients (137 patients, 67.8%) were completely independent (Barthel index = 100). According to the CCI, 114 (56.4%) had high comorbidity (≥3 points). We summarized the distribution of the main geriatric scales in Supporting Information, Figure S1. In Table 1, we show the differences in main basal variables between frail and non-frail patients and between dependent and independent patients. In the frail group, the patients were older and had more comorbidities. Similar results were observed in the dependent group; nevertheless, the higher presence of women is remarkable among the dependent patients. The dependent group was prescribed fewer ARNIs and more diuretics at discharge but there was not any significant difference in prescription at discharge between frail and non-frail patients. Dependent and frail patients presented higher values of HF-related biomarkers like NT-proBNP and troponin at discharge.
| Total population (N = 202) | Independent (N = 137) | Dependent (N = 65) | P | Frail (N = 68) | Non-frail (N = 134) | P | |
|---|---|---|---|---|---|---|---|
| Elderly (>75 years) | 100 (49.5%) | 46 (33.6%) | 54 (83.1%) | <0.001 | 51 (75%) | 48 (35.8%) | <0.001 |
| Age (years) | 73 ± 12.32 | 68 ± 11.57 | 81 ± 8.81 | <0.001 | 80 ± 8.68 | 69 ± 12.37 | <0.001 |
| Women | 78 (38.6%) | 45 (32.8%) | 33 (50.8%) | 0.015 | 28 (41.2%) | 50 (37.3%) | 0.622 |
| Diabetes mellitus | 64 (31.7%) | 42 (30.7%) | 22 (33.8%) | 0.649 | 28 (41.2%) | 36 (27.1%) | 0.042 |
| Hypertension | 140 (69.3%) | 86 (62.8%) | 54 (83.1%) | 0.003 | 58 (85.3%) | 82 (61.2%) | 0.001 |
| Chronic kidney disease | 30 (14.8%) | 14 (10.2%) | 16 (24.6%) | 0.007 | 16 (23.5%) | 14 (10.4%) | 0.014 |
| COPD | 22 (10.9%) | 13 (9.5%) | 9 (13.8%) | 0.353 | 14 (20.6%) | 8 (6.0%) | 0.002 |
| Stroke | 18 (8.9%) | 12 (8.8%) | 6 (9.2%) | 0.912 | 7 (10.3%) | 11 (8.3%) | 0.635 |
| Ischaemic dilated cardiomyopathy | 27 (13.4%) | 18 (13.1%) | 9 (13.8%) | 0.890 | 14 (20.6%) | 13 (9.8%) | 0.033 |
| Coronary artery disease | 49 (24.2%) | 28 (20.4%) | 21 (32.3%) | 0.066 | 26 (38.2%) | 23 (17.2%) | 0.001 |
| Atrial fibrillation | 90 (44.5%) | 52 (38.0%) | 38 (58.5%) | 0.006 | 39 (57.4%) | 51 (38.1%) | 0.010 |
| Previous HF | 100 (49.5%) | 57 (41.6%) | 43 (66.2%) | 0.001 | 42 (61.8%) | 58 (43.3%) | 0.015 |
| LVEF ≤ 40% | 86 (42.5%) | 65 (47.4%) | 21 (32.3%) | 0.042 | 27 (39.7%) | 58 (43.3%) | 0.596 |
| Haemoglobin at discharge (g/dL) | 13.31 ± 2.28 | 13.53 ± 2.26 | 12.86 ± 2.29 | 0.050 | 13.04 ± 2.40 | 13.45 ± 2.22 | 0.231 |
| Creatinine at discharge (mg/dL) | 1.44 ± 1.24 | 1.37 ± 1.05 | 1.57 ± 1.56 | 0.166 | 1.39 ± 0.68 | 1.45 ± 1.44 | 0.106 |
| C-reactive protein at discharge (mg/L) | 17.38 ± 30.51 | 13.98 ± 19.24 | 24.76 ± 45.76 | 0.026 | 21.31 ± 42.71 | 15.36 ± 21.92 | 0.333 |
| NT-proBNP at discharge (pg/mL) | 4813.81 ± 6497.20 | 3610.27 ± 5163.36 | 7335.52 ± 8137.05 | <0.001 | 6473.95 ± 7779.56 | 3772.75 ± 5154.22 | 0.003 |
| Troponin at discharge (ng/L) | 85.87 ± 168.46 | 77.69 ± 169.17 | 103.02 ± 167.01 | 0.002 | 92.32 ± 154.13 | 82.80 ± 176.52 | 0.043 |
| ST-2 at discharge (ng/mL) | 52.24 ± 49.35 | 45.05 ± 30.80 | 67.82 ± 73.30 | 0.004 | 63.13 ± 71.54 | 46.63 ± 32.32 | 0.177 |
| ACE-I at discharge | 76 (37.6%) | 54 (39.7%) | 22 (34.9%) | 0.518 | 23 (34.8%) | 53 (39.6%) | 0.494 |
| ARNI at discharge | 60 (30.0%) | 48 (35.3%) | 12 (19.0%) | 0.020 | 18 (27.3%) | 42 (31.3%) | 0.533 |
| BB at discharge | 156 (77.2%) | 111 (81.6%) | 45 (71.4%) | 0.104 | 53 (80.3%) | 103 (76.9%) | 0.644 |
| MRA at discharge | 85 (42.1%) | 63 (46.3%) | 22 (34.9%) | 0.130 | 26 (39.4%) | 59 (44.0%) | 0.505 |
| SGLT2i at discharge | 56 (27.7%) | 38 (27.9%) | 18 (28.6%) | 0.927 | 21 (31.8%) | 35 (26.1%) | 0.416 |
| Diuretic at discharge | 166 (82.2%) | 108 (79.4%) | 58 (92.1%) | 0.026 | 57 (86.4%) | 109 (81.3%) | 0.431 |
| High comorbidity (Charlson score ≥ 3) | 114 (56.4%) | 71 (51.8%) | 43 (66.2%) | 0.055 | 47 (69.1%) | 67 (50. %) | 0.011 |
| Minnesota scale | 45.70 ± 15.98 | 41.50 ± 15.11 | 54.49 ± 14.17 | <0.001 | 58.31 ± 12.19 | 39.26 ± 13.71 | <0.001 |
| MAGGIC scale | 20.61 ± 6.36 | 18.82 ± 6.05 | 24.48 ± 5.23 | <0.001 | 24.08 ± 5.04 | 18.89 ± 6.26 | <0.001 |
| Dependence (Barthel < 100) | 65 (32.2%) | — | — | — | 39 (57.4%) | 26 (19.4%) | <0.001 |
| Frailty (FRAIL ≥ 3) | 68 (33.7%) | 29 (21.2%) | 40 (61.5%) | <0.001 | — | — | — |
| Days of admission | 8 ± 5.06 | 8 ± 5.00 | 9 ± 5.04 | 0.007 | 8 ± 4.45 | 8 ± 5.30 | 0.240 |
- ACE-I, angiotensin-converting enzyme inhibitor; ARNI, angiotensin receptor neprilysin inhibitor; BB, beta-blocker; COPD, chronic obstructive pulmonary disease; HF, heart failure; LVEF, left ventricular ejection fraction; MAGGIC, Meta-Analysis Global Group in Chronic; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-brain natriuretic peptide; SGLT2i, sodium-glucose cotransporter type 2 inhibitor; ST-2, suppression tumorigenicity 2.
- High comorbidity was considered as a Charlson score ≥ 3 points. Dependence was considered as a Barthel index < 100, and frailty was considered as a FRAIL score ≥ 3 points. Significant associations are in bold.
During the 6 month follow-up, 11 patients (5.4%) died at 1 month and 20 (9.9%) at 6 months, and the combined event was detected in 20 (9.9%) at 1 month and 58 (28.7%) at 6 months.
Frail patients had higher mortality at 1 month [9 (13.2%) vs. 2 (1.5%), P < 0.001] and at 6 months [14 (20.6%) vs. 6 (4.5%), P < 0.001] and higher incidence of combined event at 1 month [14 (20.6%) vs. 6 (4.5%), P < 0.001] and at 6 months [29 (42.6%) vs. 29 (21.6%), P = 0.001]. Dependent patients had higher mortality at 1 month [10 (15.4%) vs. 1 (0.7%), P < 0.001] and at 6 months [17 (26.2%) vs. 3 (2.2%), P < 0.001] and higher incidence of combined event at 1 month [17 (26.2%) vs. 3 (2.2%), P < 0.001] and at 6 months [34 (52.3%) vs. 24 (17.5%), P < 0.001].
In Tables 2 and 3, we summarize the variables that showed significant associations with mortality and the combined event, respectively, at 1 or 6 months. We observed that frailty and dependence were related to mortality and the combined event at any time. In Figure 1, we show Kaplan–Meier curves for frailty and dependence (we included mortality tables in the supporting information).
| Mortality at 1 month | Mortality at 6 months | |||||
|---|---|---|---|---|---|---|
| Dead | Alive | P | Dead | Alive | P | |
| Elderly (>75 years) | 10 (10%) | 90 (90%) | 0.005 | 16 (16%) | 84 (84%) | 0.004 |
| Revascularization | 6 (14%) | 36 (86%) | 0.004 | 9 (21%) | 33 (79%) | 0.004 |
| Ischaemic cardiomyopathy | 4 (15%) | 23 (85%) | 0.018 | 6 (22%) | 21 (78%) | 0.016 |
| Previous LVEF ≤ 40% | 6 (16%) | 32 (84%) | 0.001 | 10 (26%) | 28 (74%) | <0.001 |
| Valvular heart disease | 7 (10%) | 63 (90%) | 0.037 | 11 (16%) | 59 (84%) | 0.041 |
| Moderate to severe mitral regurgitation | 6 (10%) | 53 (90%) | 0.056 | 11 (19%) | 48 (81%) | 0.007 |
| Coronary artery disease | 6 (12%) | 43 (88%) | 0.015 | 11 (22%) | 38 (78%) | 0.001 |
| Previous ARNI | 5 (22%) | 18 (78%) | <0.001 | 7 (30%) | 16 (70%) | <0.001 |
| Previous MRA | 3 (11%) | 24 (89%) | 0.170 | 6 (22%) | 21 (78%) | 0.022 |
| Previous HF | 9 (9%) | 91 (91%) | 0.029 | 18 (18%) | 82 (82%) | <0.001 |
| Previous diuretic | 9 (8%) | 101 (92%) | 0.061 | 18 (16%) | 92 (84%) | 0.001 |
| Inotropic | 4 (16%) | 21 (84%) | 0.009 | 6 (24%) | 19 (76%) | 0.007 |
| LVEF ≤ 40% | 7 (8%) | 79 (92%) | 0.141 | 13 (15%) | 71 (85%) | 0.031 |
| Frailty | 9 (13%) | 59 (87%) | <0.001 | 14 (21%) | 54 (79%) | <0.001 |
| Dependence | 10 (15%) | 55 (85%) | <0.001 | 17 (26%) | 48 (74%) | <0.001 |
| NT-proBNP at discharge (pg/mL) | 18 228 ± 11 081.05 | 4813.81 ± 6497.19 | 0.001 | 1969.94 ± 6708.98 | 4813.81 ± 6497.19 | <0.001 |
| Troponin at discharge (ng/L) | 136.32 ± 219.98 | 86.87 ± 168.45 | 0.053 | 85.55 ± 173.94 | 85.87 ± 168.45 | 0.022 |
- ARNI, angiotensin receptor neprilysin inhibitor; HF, heart failure; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal pro-brain natriuretic peptide.
- Dependence was considered as a Barthel index < 100, and frailty was considered as a FRAIL score ≥ 3 points.
| Combined event at 1 month | Combined event at 6 months | |||||
|---|---|---|---|---|---|---|
| Presence of event | Lack of event | P | Presence of event | Lack of event | P | |
| Elderly (>75 years) | 18 (18%) | 82 (82%) | <0.001 | 40 (40%) | 60 (60%) | <0.001 |
| Hypertension | 37 (26%) | 103 (74%) | 0.121 | 48 (34%) | 92 (66%) | 0.004 |
| Chronic kidney disease | 3 (10%) | 27 (90%) | 0.033 | 16 (53%) | 14 (47%) | <0.001 |
| Revascularization | 10 (24%) | 32 (76%) | <0.001 | 17 (40%) | 25 (60%) | 0.054 |
| Coronary artery disease | 11 (22%) | 38 (78%) | 0.001 | 22 (45%) | 27 (55%) | 0.004 |
| Valvular heart disease | 11 (16%) | 59 (84%) | 0.046 | 24 (34%) | 46 (66%) | 0.177 |
| Moderate to severe mitral regurgitation | 10 (17%) | 49 (83%) | 0.032 | 24 (41%) | 35 (59%) | 0.013 |
| Previous HF | 17 (17%) | 83 (83%) | 0.001 | 42 (42%) | 58 (58%) | <0.001 |
| Previous ARNI | 5 (22%) | 18 (78%) | 0.041 | 11 (48%) | 12 (52%) | 0.010 |
| Previous diuretic | 15 (14%) | 95 (86%) | 0.056 | 47 (43%) | 63 (57%) | <0.001 |
| Previous LVEF ≤ 40% | 9 (24%) | 29 (76%) | 0.001 | 21 (55%) | 17 (45%) | <0.001 |
| Inotropic | 6 (24%) | 19 (76%) | 0.006 | 12 (48%) | 13 (52%) | 0.004 |
| LVEF ≤ 40% | 9 (10%) | 77 (90%) | 0.801 | 31 (36%) | 55 (64%) | 0.020 |
| Frailty | 14 (21%) | 54 (79%) | <0.001 | 29 (43%) | 39 (57%) | 0.001 |
| Dependence | 17 (26%) | 48 (74%) | <0.001 | 34 (52%) | 31 (48%) | <0.001 |
| NT-proBNP at discharge (pg/mL) | 5020.67 ± 6805.37 | 4813.81 ± 6497.20 | 0.002 | 4928.09 ± 6597.46 | 4813.81 ± 6497.20 | <0.001 |
| Troponin at discharge (ng/L) | 91.72 ± 180.34 | 85.87 ± 168.46 | 0.089 | 87.67 ± 171.74 | 85.87 ± 168.45 | 0.029 |
- ARNI, angiotensin receptor neprilysin inhibitor; HF, heart failure; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide.
- Dependence was considered as a Barthel index < 100, and frailty was considered as a FRAIL score ≥ 3 points.
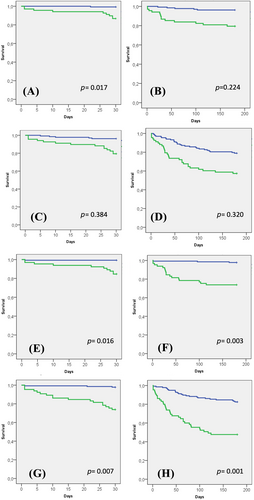
In Tables 4 and 5, we show the multivariable survival analysis. Frail patients had higher mortality at 1 month [hazard ratio (HR) 12.61, 95% confidence interval (CI) 1.57–101.47, P = 0.017], but there was no difference at 6 months (HR 2.25, 95% CI 0.61–8.26, P = 0.224). Also, they did not present significantly worse results in the combined endpoint for 1 month (HR 1.64, 95% CI 0.54–5.03, P = 0.384) and 6 months (HR 1.35, 95% CI 0.75–2.46, P = 0.320). Besides, dependent patients also had higher mortality at 1 month (HR 13.04, 95% CI 1.62–104.75, P = 0.016) and 6 months (HR 7.18, 95% CI 1.99–25.86, P = 0.003) and an increase in the incidence of the combined event at 1 month (HR 5.93, 95% CI 1.63–21.50, P = 0.007) and 6 months (HR 2.62, 95% CI 1.49–4.61, P = 0.001). According to the Bonferroni correction, frailty and dependence lost the association with 1 month mortality, but dependence maintained the association with the other three endpoints. As NT-proBNP showed a consistent prognostic effect in our sample, we developed a receiver operating characteristic curve to analyse the diagnostic performance of this biomarker and the two geriatric scales studied (Barthel and FRAIL) for 6 month mortality and the combined event (Supporting Information, Figures S2 and S3). Dependence showed the best performance (area under the curve of 0.81 for mortality and 0.68 for the combined event), although the net reclassification improvement did not reach statistical significance.
| Mortality at 1 month | ||
|---|---|---|
| Variable | HR (95% CI) | P value (significance threshold after Bonferroni adjustment: P < 0.012) |
| Revascularization | 1.25 (0.26–6.11) | 0.784 |
| Previous LVEF ≤ 40%a | 3.06 (0.79–11.82) | 0.105 |
| Frail | 12.61 (1.57–101.47) | 0.017 |
| NT-proBNP at discharge | 8.66 (1.76–41.92) | 0.007 |
| Mortality at 6 months | ||
|---|---|---|
| Variable | HR (95% CI) | P value (significance threshold after Bonferroni adjustment: P < 0.010) |
| Elderly (>75 years) | 4.30 (0.95–19.41) | 0.058 |
| Coronary artery diseaseb | 2.32 (0.83–6.49) | 0.107 |
| Previous HFc | 10.87 (1.41–83.70) | 0.022 |
| Frailty | 2.25 (0.61–8.26) | 0.224 |
| NT-proBNP at discharge | 4.39 (1.47–13.06) | 0.008 |
| Combined event at 1 month | ||
|---|---|---|
| Variable | HR (95% CI) | P value (significance threshold after Bonferroni adjustment: P < 0.010) |
| Elderly (>75 years) | 13.45 (1.78–101.73) | 0.012 |
| Coronary artery diseaseb | 2.74 (1.06–7.09) | 0.037 |
| Previous HFc | 4.74 (1.06–21.23) | 0.042 |
| Frailty | 1.64 (0.54–5.03) | 0.384 |
| NT-proBNP at discharge | 2.48 (0.97–6.36) | 0.059 |
| Combined event at 6 months | ||
|---|---|---|
| Variable | HR (95% CI) | P value (significance threshold after Bonferroni adjustment: P < 0.006) |
| Elderly (>75 years) | 2.33 (1.28–4.24) | 0.005 |
| Hypertension | 1.18 (0.54–2.57) | 0.681 |
| Chronic kidney disease | 1.41 (0.73–2.71) | 0.305 |
| Coronary artery diseaseb | 1.59 (0.90–2.81) | 0.111 |
| Previous HFc | 2.99 (1.64–5.48) | <0.001 |
| Inotropic | 1.59 (0.76–3.32) | 0.221 |
| Frailty | 1.35 (0.75–2.46) | 0.320 |
| NT-proBNP at discharge | 2.39 (1.37–4.17) | 0.002 |
- CI, confidence interval; HF, heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide.
- Frailty was considered as a FRAIL score ≥ 3 points. Significant associations after Bonferroni adjustment are in bold.
- a Previous LVEF < 40% showed collinearity with previous diuretics and previous angiotensin receptor neprilysin inhibitors (P < 0.001), so we only included previous LVEF < 40% when more than one of the three variables showed P < 0.005 in the univariate analysis.
- b Revascularization and coronary artery disease showed collinearity (P < 0.001), so we only included coronary artery disease when both showed P < 0.005 in the univariate analysis.
- c Previous HF showed collinearity with previous LVEF < 40%, previous diuretics, and previous angiotensin receptor neprilysin inhibitors (P < 0.001), so we only included previous HF when more than one of the three variables showed P < 0.005 in the univariate analysis.
| Mortality at 1 month | ||
|---|---|---|
| Variable | HR (95% CI) | P value (significance threshold after Bonferroni adjustment: P < 0.012) |
| Revascularization | 1.80 (0.42–7.66) | 0.428 |
| Previous LVEF ≤ 40%a | 2.51 (0.71–8.13) | 0.153 |
| Dependence | 13.04 (1.62–104.75) | 0.016 |
| NT-proBNP at discharge | 8.07 (1.69–38.53) | 0.009 |
| Mortality at 6 months | ||
|---|---|---|
| Variable | HR (95% CI) | P value (significance threshold after Bonferroni adjustment: P < 0.010) |
| Elderly (>75 years) | 2.18 (0.34–13.97) | 0.410 |
| Coronary artery diseaseb | 2.96 (1.07–8.21) | 0.037 |
| Previous HFc | 2.91 (0.60–14.15) | 0.185 |
| Dependence | 7.18 (1.99–25.86) | 0.003 |
| NT-proBNP at discharge | 6.75 (2.23–20.45) | 0.001 |
| Combined event at 1 month | ||
|---|---|---|
| Variable | HR (95% CI) | P value (significance threshold after Bonferroni adjustment: P < 0.010) |
| Elderly (>75 years) | 7.70 (0.94–62.90) | 0.057 |
| Coronary artery diseaseb | 3.01 (1.22–7.45) | 0.017 |
| Previous HFc | 2.29 (0.62–8.48) | 0.214 |
| Dependence | 5.93 (1.63–21.50) | 0.007 |
| NT-proBNP at discharge | 2.11 (0.82–5.44) | 0.121 |
| Combined event at 6 months | ||
|---|---|---|
| Variable | HR (95% CI) | P value (significance threshold after Bonferroni adjustment: P < 0.006) |
| Elderly (>75 years) | 1.77 (0.88–3.57) | 0.111 |
| Hypertension | 1.08 (0.51–2.27) | 0.842 |
| Chronic kidney disease | 1.24 (0.65–2.39) | 0.516 |
| Coronary artery diseaseb | 1.69 (0.95–2.98) | 0.072 |
| Previous HFc | 2.31 (1.26–4.24) | 0.007 |
| Inotropic | 1.69 (0.84–3.42) | 0.142 |
| Dependence | 2.62 (1.49–4.61) | 0.001 |
| NT-proBNP at discharge | 2.36 (1.36–4.11) | 0.002 |
- CI, confidence interval; HF, heart failure; HR, hazard ratio; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal pro-brain natriuretic peptide.
- Dependence was considered as a Barthel index < 100. Significant associations after Bonferroni adjustment are in bold.
- a Previous LVEF < 40% showed collinearity with previous diuretics and previous angiotensin receptor neprilysin inhibitors (P < 0.001), so we only included previous LVEF < 40% when more than one of the three variables showed P < 0.005 in the univariate analysis.
- b Revascularization and coronary artery disease showed collinearity (P < 0.001), so we only included coronary artery disease when both showed P < 0.005 in the univariate analysis.
- c Previous HF showed collinearity with previous LVEF < 40%, previous diuretics, and previous angiotensin receptor neprilysin inhibitors (P < 0.001), so we only included previous HF when more than one of the three variables showed P < 0.005 in the univariate analysis.
Discussion
The main results of the study are as follows: (i) geriatric syndromes are very common in patients admitted for HF; (ii) dependent and frail patients with AHF presented more comorbidity and higher values of NT-proBNP and troponin; (iii) HF treatment at discharge was similar in frail and non-frail patients, but dependent patients received less treatment with ARNIs and more with diuretics; and (iv) frailty and dependence have an impact on the prognosis during the follow-up.
The mean age of our patients was 73 years, but more than half of the patients (50.5%) were younger than 75 years old, which makes it different from other studies, which only include older patients.16, 25 Few studies analyse frailty in highly selected groups of young patients with AHF, specifically in the transplant waiting list26 focused on frailty prevalence, but data about prognosis effects are lacking.
Just 38% of our participants were women. This result is concordant with other studies, in which the population is mostly male and elderly.17, 27 According to geriatric syndromes, one-third of participants were frail and one-third were dependent. This is a little different from other studies12 and meta-analyses28 in which a higher prevalence of frailty in HF patients (up to 44.5%) was observed. These differences could be explained by the proportion of young patients enrolled in our study and the great heterogeneity of the geriatric scales used in previous research. For example, in a meta-analysis,28 26 published studies were analysed, including a total of 6896 patients. The studies were classified as measured ‘Physical Frailty’ with scales as Fried phenotype criteria, SPPB test, handgrip strength, or gait speed and as a ‘Multidimensional Frailty’ including scales as a frailty index or the Tilburg Frailty Indicator. This meta-analysis found significant and substantive variability in the prevalence of frailty in HF across studies.
We used the FRAIL scale to measure frailty in our sample because it predicts functional impairment, mortality, and healthcare utilization.21 It is usually applied to the elderly with HF,29 although it is also suitable for use in a younger population.21 For dependence, we used the Barthel index, recommended by the British Geriatrics Society, to measure physical independence.30 It is the most common scale to measure independence, and it is used in other studies similar to ours.31
We observed that frail and dependent patients were older and had higher comorbidities such as hypertension, chronic kidney disease, atrial fibrillation, and previous diagnosis of HF.14, 32, 33 These results are frequently described in CHF, but now, our study shows that it also occurs in the AHF population.34 They also have higher values of biomarkers such as NT-proBNP and troponin at admission and discharge, similar to previous studies.31 It is known that high levels of certain biomarkers, such as NT-proBNP, involve a worse prognosis in frail elderly populations with HF.34-36 In this sense, in young patients, frailty seems to be more determined by age and comorbidities; however, in the elderly, the HF severity represented by myocardial and remodelling stress marked by NT-proBNP has an impact on frailty.37
In certain studies, it has been observed that frail patients have less often guideline-recommended medical treatment at discharge and follow-up,38 although they obtained the most significant benefit.39 Our data show similar neurohormonal treatment prescriptions at discharge between frail and non-frail patients but lower prescriptions in dependent patients than in independent ones.
We consider the most important result of our study to be the impact of frail and dependence on prognosis. We observed that frail patients had higher mortality at 1 month and that dependent patients had worse results for mortality and the combined event at 1 and 6 months. In the multivariable analysis, we did not find any influence of age in this prognostic value of dependence and frailty. It reflects the huge impact of geriatric syndromes on the prognosis of our patients in the short and middle terms. Dependence is a status with less variability over time, and that is probably why we can see the worst results at 1 and 6 months. However, the frailty status is more changeable and theoretically reversible over time, so, maybe, patients after discharge might improve in their frailty status. We performed a frailty assessment during admission, and the results might be influenced by the severity of the acute illness.26
This result is concordant with other studies that showed frailty as a predictor of all-cause mortality and hospital readmission in HF.25, 40, 41 One possible explanation is the relation between geriatric syndromes and cardiac damage reflected by an increase in cardiac biomarkers in frail and dependent patients.37 Another possible underlying mechanism is the worse prognosis usually associated with comorbidities that are also more frequent in frail and dependent patients.20
Similar to us, Sze et al. studied the prognostic effect of frailty in AHF patients.17 They followed up 256 patients for 9 months and observed that frailty condition and malnutrition implied higher mortality. In this study, all the patients had LVEF ≤ 40%; however, in our study, we also included patients with mildly reduced and preserved LVEF, so we showed the best reflection of daily clinical practice where an increasing prevalence of HF with preserved LVEF is detected. Furthermore, this study used the Clinical Frailty Scale, which is based on the subjective evaluation of the frailty status of the patient by the researcher. We used the FRAIL index, which includes a multidimensional assessment of frailty based on patients' responses. Similar results were observed in other studies in a short term,12 more likely to our study.
Some studies relate dependence and worse prognosis in AHF. Chivite et al.42 included 2195 patients older than 75 years (mean age of 83 years). The median preadmission Barthel index was 90, and almost 22% had Barthel index < 60. They observed that the presence of dependence conferred a significant risk of 1 year mortality. Now, we show similar results in an unselected population by age, and we confirm that the prognostic impact of dependence occurred very soon after the discharge, in the first month.
Our article presents several limitations. The design was observational, and the sample was relatively small. We only included patients admitted to the cardiology unit, not in other units such as internal medicine or geriatric, in which the incidence of geriatric syndromes and elderly people is higher. These facts probably led to the loss of a significant association between dependence and frailty with 1 month mortality after Bonferroni adjustment. Although the use of combined events is frequent in this type of research, combining the results would lead to less power to detect significant associations. Another limitation is the time of follow-up, just 6 months. The geriatric evaluation was performed during HF admission, so the results might be slightly different from the baseline evaluation of these patients, and they might be influenced by the severity of the acute episode. However, several studies show the impact of prognosis with the geriatric assessment during the admission.17, 27 It would be interesting, for future studies, to re-evaluate the geriatric scales in the follow-up of the patients.
Nevertheless, one of the strengths of our study is the inclusion of the adult participants regardless of age; more than half of our patients were younger than 75 years old, an uncommon feature in other studies. Also, we included patients with AHF, unlike other studies that include CHF43, 44 or older patients with AHF16 but not AHF in adults of all ages. Finally, the geriatric assessment was performed by cardiologists, which implies that this type of evaluation is fast and feasible to do by trained cardiologists.
We believe that the importance of identifying and measuring frailty lies in its potential reversibility. Close monitoring of frailty can potentially guide better management,5, 41 including a close follow-up of these patients45 for inclusion in a multidimensional rehabilitation programme.46
In conclusion, in non-selected AHF patients, frailty and dependence implied a worse prognosis, rising mortality, readmissions, and ER visits for AHF. A geriatric assessment should be useful during the admission of AHF patients to detect high-risk patients.
Conflict of interest
The authors report no conflict of interest.
Funding
This work was supported by the GRS 2525/A/22 and INT/M/04/22 grants from the Junta de Castilla y León.




