Exercise training and high-sensitivity cardiac troponin-I in patients with heart failure with reduced ejection fraction
ClinicalTrials.org: NCT000917046
Abstract
Aims
The aims of this sub-study of the SMARTEX trial were (1) to evaluate the effects of a 12-week exercise training programme on serum levels of high sensitivity cardiac troponin I (hs-cTnI) in patients with moderate chronic heart failure (CHF), in New York Heart Association class II-III with reduced ejection fraction (HFrEF) and (2) to explore the associations with left ventricular remodelling, functional capacity and filling pressures measured with N-terminal pro brain natriuretic peptide (NT-proBNP).
Methods and results
In this sub-study, 196 patients were randomly assigned to high intensity interval training (HIIT, n = 70), moderate continuous training (MCT, n = 59) or recommendation of regular exercise (RRE), (n = 67) for 12 weeks. To reveal potential difference between structured intervention and control, HIIT and MCT groups were merged and named supervised exercise training (SET) group. The RRE group constituted the control group (CG). To avoid contributing factors to myocardial injury, we also evaluated changes in patients without additional co-morbidities (atrial fibrillation, hypertension, diabetes mellitus, and chronic obstructive pulmonary disease). The relationship between hs-cTnI and left ventricular end-diastolic diameter (LVEDD), VO2peak, and NT-proBNP was analysed by linear mixed models. At 12 weeks, Hs-cTnI levels were modestly but significantly reduced in the SET group from median 11.9 ng/L (interquartile ratio, IQR 7.1–21.8) to 11.5 ng/L (IQR 7.0–20.7), P = 0.030. There was no between-group difference (SET vs. CG, P = 0.116). There was a numerical but not significant reduction in hs-cTnI for the whole population (P = 0.067) after 12 weeks. For the sub-group of patients without additional co-morbidities, there was a significant between-group difference: SET group (delta −1.2 ng/L, IQR −2.7 to 0.1) versus CG (delta −0.1 ng/L, IQR −0.4 to 0.7), P = 0.007. In the SET group, hs-cTnI changed from 10.9 ng/L (IQR 6.0–22.7) to 9.2 ng/L (IQR 5.2–20.5) (P = 0.002), whereas there was no change in the CG (6.4 to 5.8 ng/L, P = 0.64). Changes in hs-cTnI (all patients) were significantly associated with changes in; LVEDD, VO2peak, and NT-proBNP, respectively.
Conclusions
In patients with stable HFrEF, 12 weeks of structured exercise intervention was associated with a modest, but significant reduction of hs-cTnI. There was no significant difference between intervention group and control group. In the sub-group of patients without additional co-morbidities, this difference was highly significant. The alterations in hs-cTnI were associated with reduction of LVEDD and natriuretic peptide concentrations as well as improved functional capacity.
Introduction
Low-level, chronic elevation of cardiac troponins, even within the normal range, provides independent information concerning risk in chronic heart failure (CHF).1, 2 Longitudinal observational data from epidemiological studies suggest that higher physical activity and cardiorespiratory fitness are associated with lower concentrations of cardiac troponins3. Additionally, exercise training (ET) is associated with improved functional capacity and endothelial function and reduced levels of N-terminal B-type natriuretic peptide (NT pro-BNP)4-7 in patients with heart failure with reduced ejection fraction (HFrEF). According to the European Society of Cardiology 2021 HF guidelines, regular aerobic exercise training has therefore the highest recommendation in this population with level of evidence class IA.8
We have previously reported that serum high sensitivity cardiac Troponin T (hs-cTnT) concentrations were reduced in patients with stable HFrEF after 12 weeks of exercise training—for the whole SMARTEX population. The group of patients participating in a structured training programme and the group of patients advised to exercise were merged. Additionally, higher peak oxygen uptake (VO2peak) correlated with lower hs-cTnT, suggesting a positive long-term effect of increasing VO2peak on subclinical myocardial injury in HFrEF. However, the effect of a structured training programme compared with recommendations of exercise training was not evaluated.
Recent studies have revealed interesting biological and epidemiological differences between cardiac troponin T and I.9 Some studies have shown that cardiac troponin I has been more closely associated with heart failure and atrial fibrillation than troponin T (cTnT)10 and might be a better surrogate marker. Currently, it is unclear if a structured ET programme with high adherence and adequate intensity also affects the cardiac troponin I (cTnI) levels in patients with CHF. Furthermore, we do not know if an improvement in left ventricular remodelling after a structured exercise training programme is associated with a decrease in hs-cTnI levels.
The aims of this sub-study of the SMARTEX trial were (1) to evaluate the effects of a 12-week structured exercise training programme on serum levels of high sensitivity cardiac troponin I (hs-cTnI) in patients with moderate CHF in New York Heart Association class II-III with reduced ejection fraction (HFrEF) compared with the alterations in serum levels in patients with recommendations to exercise (control group) and (2) to explore the associations of changes in hs-cTnI with left ventricular remodelling and filling pressures measured with brain natriuretic peptide (BNP).
Methods
The multicentre randomized SMARTEX Heart Failure Study randomized 231 patients with HFrEF to different training modalities, and 215 completed the 12-week assessment.
Eligible patients with symptomatic (New York Heart Association class II–III), stable, on optimal tolerated, guideline recommended therapy were randomized 1:1:1 to a 12-week exercise programme of high-intensity interval training (HIIT, n = 82), moderate continuous training (MCT, n = 73), or recommendation of regular exercise (RRE, n = 76), stratified by trial centre and aetiology of HF (ischaemic vs. non-ischaemic). Inclusion criteria and interventions of the study have been described previously.11, 12 At the time of inclusion, sacubitril–valsartan and sodium glucose cotransport 2 transport inhibitors were not commercially available and consequently not a part of heart failure therapy.
In this sub-study, blood samples for analysis of hs-cTnI were available in 196 patients after 12 weeks (Figure 1).
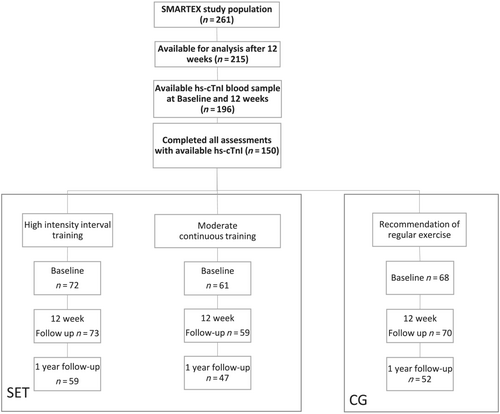
Blood samples were drawn after an overnight fast at baseline (BL) and at completion of the exercise programme (12 weeks).
Cardiopulmonary exercise testing (CPET) was performed at baseline and more than 48 h after the last exercise training session and following blood samples. CPET was performed on either a bicycle or a treadmill, using an incremental protocol with 10 W or 20 W increase in workload per minute until exhaustion, starting at 20 W or 40 W, respectively. Standard equipment for indirect calorimetry was used to measure the VO2peak as described elsewhere.12
BL and 12-week tests were performed using the same protocol and exercise method.
Left ventricular end-diastolic diameter (LVEDD) was assessed by the echocardiographic core laboratory blinded to group assignment according to standard procedure, which has been described previously.11
Intervention
Participants in the MCT and HIIT groups had three supervised exercise training sessions per week, on a treadmill or bicycle. MCT sessions lasted approximately 47 min, and HIIT sessions approximately 38 min including warm-up and cool down periods. Attempts were made to match the sessions with similar energy burden. The exercise protocols have been published previously.11-13 In brief, the HIIT group was targeted to exercise at 90–95% of maximal heart rate (HR max). The MCT group was targeted to exercise at 60–70% of HRmax. Testing was performed on the same modality as the training (treadmill or bicycle). hs-cTnI was measured in serum using a commercially available assay hs-cTnI from Abbott Diagnostics, Illinois, USA (STAT Troponin-I analysed on an Abbott Architect SR2000i). The analytical range was 2.0–500 000 ng/L. All frozen samples were thawed and analysed on the same analyser on the same day with CVa (coefficient of variation analytical) 6% at hs-cTnI level 14 ng/L.
As highlighted in the original publication,12 adherence to the training sessions was generally excellent; however, the adherence to the targeted exercise deviated from the protocol. Of the patients doing HIIT, 51% worked out with lower intensity than prescribed, whereas 80% in the MCT group exercised with higher intensity. Because of this overlap, we combined the patients randomized to HIIT and MCT into the supervised exercise training group (SET). Patients randomized to RRE constituted the control group (CG).
To additionally evaluate effect of exercise training on hs-cTnI, in a group of patients adjusted for confounding co-morbidities, we divided the patients into groups with or without additional co-morbidities including atrial fibrillation, hypertension, diabetes mellitus, and chronic obstructive pulmonary disease.
Statistical analysis
Continuous data are presented as medians and interquartile ranges. The Mann–Whitney U test was used to compare continuous variables between two groups. The one-sample Wilcoxon signed rank test was used to test whether median of the sample was different from zero. All the tests studying the differences of hs-cTnI, LVEDD, NT-proBNP, and VO2peak between baseline and follow-up visits in the unadjusted analyses were paired; data only for patients who had both measurements were used for calculation of the medians.
Unadjusted analyses of hs-cTnI between baseline and 12 weeks were performed using nonparametric tests because even after log-transformation, changes of hs-cTnI did not follow the normal distribution, mainly due to the presence of outliers, and the t-test was not applicable. For adjusted analyses at baseline and 12 weeks, linear mixed models were used with log-transformed hs-cTnI as the outcome variable. Applying the models to larger data sets and adjustment for covariates reduced the presence of outliers, and the distribution of the model's residuals was close to normal. Residuals were studied using Cook's distance.
To assess the relationship between hs-cTnI and LVEDD, NT-proBNP, and VO2peak, we used linear mixed models. For each model, the outcome variable was log-transformed measurement of hs-cTnI, while the predictor variables were time (as a categorical variable, with categories being the study time points: baseline and 12-weeks follow-up), and the characteristic of interest measured at this time point, the training group (intervention or control), age, and sex. Values of NT-proBNP were log-transformed to attain normality of residuals. Random effects were the patient ID and training centre. The covariance structure was assumed as compound symmetry. The proportion of explained variance was assessed using Nakagawa's pseudo R2.
To evaluate difference in hs-cTnI dynamics between training groups, the interaction term [Time × Training group] were evaluated for these models. However, it showed not to be significant for any model and was removed.
All tests were two-sided; P-values <0.05 were assumed statistically significant for regression coefficients and baseline comparisons.
For comparison of changes from baseline to 12 weeks, we applied Bonferroni correction for multiple testing, and the difference was regarded as significant if P < 0.016, because three tests were performed: whether the change in SET was different from zero, whether the change in CG was different from zero, and whether changes in SET and CG differed.
All statistical analyses were performed using R Project for statistical computing, version 4.1.2. Linear mixed models were implemented using lme4 package version 1.1–27.1.
Results
Baseline characteristics are presented in Table 1. Nineteen per cent of the patients were female. The majority of patients had ischaemic heart failure, were in New York Heart Association class II, and with an LVEF of 30%. Patients were on optimal tolerated medical treatment with a combination of angiotensin-converting enzyme inhibitors or angiotensin II receptor blocker, diuretics and beta-blockers in the majority of patients. The two groups were comparable and well matched with the exception of presence of hypertension, which was more common in the control group, 49% versus 33% (P = 0.03) (Table 1). Median age in the control group was 60 versus 61 years in the intervention group (P = 0.28). At baseline, there were no differences in cardiac troponin I levels between the study cohorts, SET and CG (P = 0.49). Baseline characteristics for the patients without additional co-morbidities are presented in Table 2.
| Characteristic | Control (n = 67) | Intervention (SET) (n = 129) | P-value |
|---|---|---|---|
| Age, years | 57.0 (51.0–69.0) | 61.0 (54.0–70.0) | 0.21 |
| Female sex, n (%) | 12 (18) | 24 (19) | 1 |
| Heart failure <12 months, n (%) | 13 (20) | 17 (13) | 0.33 |
| NYHA class 2, n (%) | 51 (76) | 86 (67) | 0.23 |
| NYHA class 3, n (%) | 16 (24) | 43 (33) | 0.23 |
| Left ventricular ejection fraction, % | 30.0 (24.0–33.0) | 29.0 (24.0–34.0) | 0.75 |
| Aetiology, ischaemic, n (%) | 37 (55) | 76 (59) | 0.73 |
| Previous myocardial infarction, n (%) | 28 (42) | 71 (55) | 0.10 |
| Previous CABG, n (%) | 16 (24) | 29 (22) | 0.52 |
| Previous PCI, n (%) | 30 (45) | 48 (37) | 0.38 |
| Atrial fibrillation chronic, n (%) | 6 (9) | 18 (14) | 0.43 |
| Atrial fibrillation paroxysmal, n (%) | 12 (18) | 15 (12) | 0.32 |
| History of hypertension, n (%) | 35 (52) | 42 (33) | 0.01 |
| History of diabetes mellitus, n (%) | 13 (19) | 34 (26) | 0.37 |
| History of COPD, n (%) | 2 (3) | 11 (9) | 0.23 |
| Current smoking, n (%) | 31 (46) | 60 (47) | 1 |
| Alcohol per week, median (IQR) | 1 (0–3.5) | 1 (0–7) | 0.56 |
| Medications, n (%) | |||
| ACE inhibitor/ARB | 65 (97) | 119 (92) | 0.23 |
| Beta-blocker | 65 (97) | 122 (95) | 0.72 |
| Aldosterone receptor antagonist | 35 (52) | 77 (60) | 0.36 |
| Diuretic | 46 (69) | 97 (75) | 0.42 |
| Digoxin or digitoxin | 6 (9) | 23 (18) | 0.15 |
| Statin | 41 (61) | 88 (68) | 0.41 |
| Body mass index, kg/m2 | 27.7 (24.6–30.6) | 27.5 (25.1–31.8) | 0.64 |
| Systolic blood pressure, mmHg | 120 (110–130) | 118 (110–130) | 0.24 |
| Diastolic blood pressure, mmHg | 76 (70–82) | 71 (65–80) | 0.06 |
| NT-proBNP, ng/L | 895 (408–1618) | 976 (454–1813) | 0.51 |
| hs-cTnI, ng/L | 11.4 (6.4–15.1) | 11.9 (7.1–21.8) | 0.45 |
| CRP, mg/L | 2.5 (1.5–4.5) | 2.1 (1.1–5) | 0.45 |
| Triglyceride level, mmol/L | 1.6 (1.0–2.1) | 1.5 (1.0–2.3) | 0.77 |
| Left ventricular end diastolic diameter, mm | 68 (64–72) | 68 (63–74) | 0.76 |
- Baseline characteristics for all patients.
- ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; hs-cTnI, high sensitivity cardiac troponin I; IQR, interquartile ratio; NT-proBNP, N-terminal pro brain natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SET, structured exercise training group.
| Characteristic | Control (n = 20) | Intervention (SET) (n = 50) | P-value |
|---|---|---|---|
| Age, years | 55.0 (48–65) | 57.0 (48–69) | 0.68 |
| Female sex, n (%) | 4 (20) | 16 (32) | 0.48 |
| Heart failure <12 months, n (%) | 5 (26) | 9 (18) | 0.67 |
| NYHA class 2, n (%) | 17 (85) | 39 (78) | 0.74 |
| NYHA class 3, n (%) | 3 (15) | 11 (22) | 0.74 |
| Left ventricular ejection fraction, % | 30.5 (25–33) | 29.0 (24–35) | 0.92 |
| Aetiology, ischaemic n (%) | 11 (55) | 28 (56) | 1 |
| Previous myocardial infarction, n (%) | 10 (50) | 30 (60) | 0.59 |
| Previous CABG, n (%) | 4 (20) | 8 (16) | 0.30 |
| Previous PCI, n (%) | 9 (45) | 18 (36) | 0.67 |
| Atrial fibrillation chronic, n (%) | 0 (0) | 0 (0) | 0 |
| Atrial fibrillation paroxysmal, n (%) | 0 (0) | 0 (0) | 0 |
| History of hypertension, n (%) | 0 (0) | 0 (0) | 0 |
| History of diabetes mellitus, n (%) | 0 (0) | 0 (0) | 0 |
| Current smoking, n (%) | 10 (50) | 21 (42) | 0.73 |
| Alcohol per week, median (IQR) | 2 (1–5.5) | 1 (0–5) | 0.27 |
| Medications, n (%) | |||
| ACE inhibitor/ARB | 19 (95) | 49 (98) | 0.49 |
| Beta-blocker | 19 (95) | 46 (92) | 1 |
| Aldosterone receptor antagonist | 11 (55) | 25 (50) | 0.79 |
| Diuretic | 11 (55) | 33 (66) | 0.56 |
| Digoxin or digitoxin | 1 (5) | 7 (14) | 0.51 |
| Statin | 11 (55) | 30 (60) | 0.91 |
| Body mass index, kg/m2 | 26.3 (24.5–31.8) | 26.7 (24.7–28.9) | 0.86 |
| Systolic blood pressure, mmHg | 120 (108–124) | 114 (110–120) | 0.23 |
| Diastolic blood pressure, mmHg | 72 (69–80) | 70 (64–80) | 0.40 |
| NT-proBNP, ng/L | 510 (319–746) | 860 (402–1496) | 0.04 |
| TnI, ng/L | 6.4 (4.9–10) | 10.9 (6–22.7) | 0.04 |
| CRP, mg/L | 2.1 (1.3–4) | 1.7 (0.8–3) | 0.48 |
| Triglyceride level, mmol/L | 1.2 (1.0–1.7) | 1.2 (0.9–2.2) | 0.98 |
| Left ventricular end diastolic diameter, mm | 69 (65.8–74.2) | 68 (63–74.8) | 0.46 |
- Baseline characteristics for patients without additional co-morbidities (atrial fibrillation, hypertension, diabetes mellitus, and chronic obstructive pulmonary disease).
- ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin II receptor blocker; CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease; CRP, C-reactive protein; IQR, interquartile ratio; NT-proBNP, N-terminal pro brain natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; SET, structured exercise training group; TnI, troponin I.
High sensitivity cardiac troponin I at 12 weeks
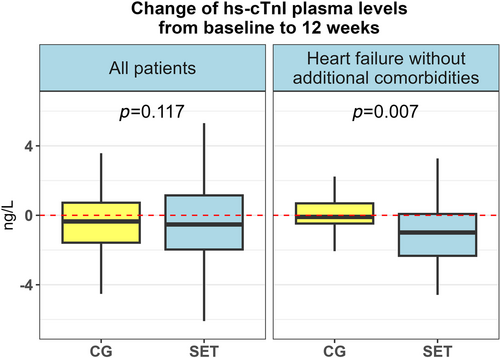
For the whole population (SET and control groups pooled), there was a non-significant, small numerical reduction of hs-cTnI median 11.8 ng/L (interquartile ratio, IQR 7.1–21.1) at baseline to median 11.3 (IQR 6.7–19.8) at 12 weeks, P = 0.067. hs-cTnI levels were modestly, but significantly reduced in the SET from median of 11.9 ng/L (IQR 7.1–21.8) to 11.5 ng/L (IQR 7.0–20.7), P = 0.030. The reduction was not statistically significant in the CG [11.4 ng/L (IQR 6.4–15.1) vs. 10.7 ng/L (IQR 6.2–17.6), P = 0.95]. There was no difference in changes in serum levels of hs-cTnI, from baseline to 12 weeks, between SET and CG (P = 0.117, Figure 2, Table 3).
| Characteristic | Baseline | 12 weeks | Change | Baseline | 12 weeks | Change | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| All patients (N = 196) | SET | Rel. Change (%) | CG | Rel. Change (%) | P-value | P-value (Compares Rel change) | ||||
| hs-cTnI (ng/L) | 11.9 (7.1; 21.8) | 11.5 (7.0; 20.7) | −0.7 (−2.6; 1.7) | −6.7 (−21.3; 14.9) | 11.4 (6.4; 15.1) | 10.7 (6.2; 17.6) | −0.2 (−1.5; 1.6) | −2.7 (−13.6; 16.8) | 0.117 | 0.184 |
| LVEDD (mm) | 68.0 (63.0; 74.0) | 66.0 (60.0; 72.0) | −2.0 (−5.0; 2.0) | 68.0 (64.0; 72.0) | 69.0 (63.2; 74.0) | 0.0 (−3.0; 4.0) | 0.015 | |||
| NT-proBNP (ng/L) | 975.5 (453.8; 1813.2) | 848.0 (438.0; 1771.5) | 15.0 (−168.2; 208.2) | 2.0 (−24.8; 31.3) | 895.0 (408.0; 1617.5) | 835.0 (397.5; 1896.5) | 8.0 (−147.5; 268.5) | 1.0 (−20.1; 27.9) | 0.795 | 0.891 |
| VO2peak (mL/kg/min) | 16.8 (14.1; 19.8) | 17.3 (14.6; 22.0) | 1.0 (−0.6; 2.9) | 18.4 (14.8; 20.7) | 17.5 (14.6; 22.2) | −0.1 (−1.6; 1.2) | 0.001 | |||
| Patients without co-morbidities (N = 70) | ||||||||||
| hs-cTnI (ng/L) | 10.9 (6.0; 22.7) | 9.2 (5.2; 20.5) | −1.2 (−2.7; 0.1) | −11.1 (−22.9; 1.4) | 6.4 (4.9; 10.0) | 5.8 (4.7; 9.4) | −0.1 (−0.4; 0.7) | −2.1 (−7.8; 11.9) | 0.007 | 0.050 |
| LVEDD (mm) | 68.0 (63.0; 74.8) | 63.0 (60.0; 70.0) | −2.0 (−7.0; 0.0) | 69.0 (65.8; 74.2) | 65.0 (62.0; 72.5) | −2.0 (−8.0; 2.5) | 0.920 | |||
| NT-proBNP (ng/L) | 860.5 (402.5; 1496.5) | 748.5 (451.0; 1431.0) | −27.5 (−197.0; 178.8) | −7.6 (−26.3; 28.2) | 509.5 (319.2; 746.2) | 470.0 (288.5; 793.8) | −39.0 (−153.8; 100.2) | −12.2 (−34.7; 27.1) | 0.612 | 0.780 |
| VO2peak (mL/kg/min) | 18.8 (15.8; 20.9) | 20.9 (16.7; 24.4) | 1.3 (0.0; 3.3) | 20.4 (16.4; 23.3) | 20.6 (15.9; 23.9) | −1.1 (−1.7; 1.0) | 0.006 | |||
- Changes between SET and CG, for all patients and for patients without additional co-morbidities. The distributions were described using medians together with the 1st and 3rd percentiles.
- CG, control group; LVEDD, left ventricular end-diastolic diameter; hs-cTnI, high sensitivity cardiac troponin I; NT-proBNP, N-terminal pro brain natriuretic peptide; SET, structured exercise training group.
Population restricted to patients without additional co-morbidities (atrial fibrillation, hypertension, diabetes mellitus, and chronic obstructive pulmonary disease)
The difference in changes of serum levels of hs-cTnI between SET (n = 50) and CG (n = 20) was statistically significant [delta −1.2 ng/L (IQR −2.7 to 0.1) vs. delta −0.1 ng/L (IQR −0.4 to 0.7), P = 0.007], (Figure 2, Table 3). In the SET, hs-cTnI changed from 10.9 ng/L (IQR 6.0–22.7) to 9.2 ng/L (IQR 5.2–20.5) (P = 0.002), whereas there was no change in the CG (6.4 to 5.8 ng/L, P = 0.64).
Left ventricular end-diastolic diameter at 12 weeks (all patients)
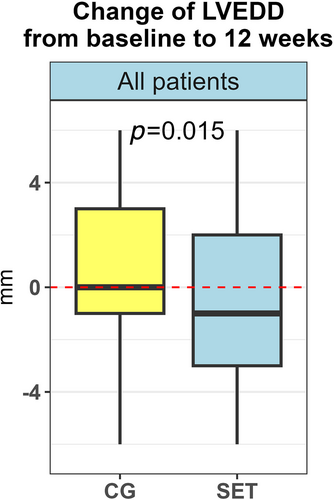
There was a modest reduction in LVEDD from median 68.0 mm (IQR 63.0–73.8) to median 67.0 mm (IQR 61.0–72.0), (P = 0.009) at 12 weeks.
Furthermore, there was a difference in delta values (defined as absolute difference between the 12-week measurement and baseline) of LVEDD between SET and CG at 12 weeks (P = 0.015, Figure 3, Table 3). For the SET, the values of LVEDD changed from median of 68.0 mm (IQR 63.0–74.0) at baseline to 66.0 mm (IQR 60–72) at 12 weeks, while for the CG, the change at 12 weeks was not statistically significant (P = 0.57).
Cardiac troponin I and left ventricular end-diastolic diameter
Levels of hs-cTnI and LVEDD were related: the model predicted that each mm decrease in LVEDD was associated with 1.2% (95% CI 0.6–1.9%, P < 0.001) decrease in the hs-cTnI level. The model explained 86.8% of variation of the data (R2 conditional); however, only 6.9% of the variation was attributed to the LVEDD, age, and sex (R2 marginal).
The relationship was similar in the subgroup of patients without additional co-morbidities with a predicted decrease of 1.7% in hs-cTnI (95% CI 0.7–2.8, P = 0.001).
N-terminal pro brain natriuretic peptide at 12 weeks
For the whole group (SET and CG), NT-proBNP did not change from baseline to 12 weeks (P = 0.26). There was no difference in delta values of NT-proBNP between SET and CG at 12 weeks (P = 0.80, Table 3).
Cardiac troponin I and N-terminal pro brain natriuretic peptide
There was a significant relationship between hs-cTnI and NT-proBNP at all time points; the model predicted that at any study time point, we expected 3.0% (95% CI 2.4–3.5%, P < 0.0001) decrease in hs-cTnI if NT-proBNP decreased by 10%. This relation was again valid for all study time points (baseline and 12 weeks) as well as the changes of scores between these two time points. The model explained 87.6% of variation of the data (R2 conditional); 18.5% of the variation was explained by NT-proBNP, age, and sex (R2 marginal).
Functional capacity
VO2peak at 12 weeks
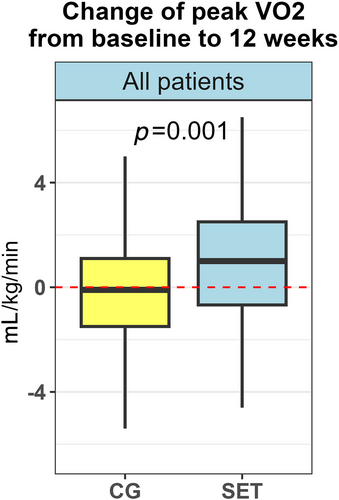
For the whole group, VO2peak changed from median 17.1 mL/kg/min (IQR 14.3–20.3) to median 17.3 mL/kg/min (IQR 14.6–22.0) (P = 0.003). There was a difference in delta values of VO2peak between SET and CG at 12 weeks (P = 0.001) (Figure 4, Table 3). In the SET, the values changed from median 16.8 mL/kg/min (IQR 14.1–19.8) at baseline to 17.3 mL/kg/min (IQR 14.6–22.0) at 12 weeks (P < 0.0001). In the CG, VO2peak did not change from baseline to 12 weeks (P = 0.44).
Cardiac troponin I and VO2peak
The model evaluating the relationship between VO2peak and hs-cTnI predicted that 1 mL/kg/min increase in VO2peak was associated with 2.2% (95% CI 3.6–0.9%, P = 0.001) decrease of hs-cTnI. The model explained 87.3% of variation of the hs-cTnI data (R2 conditional); 7.6% of the variation was explained by VO2peak, age and sex (R2 marginal).
Discussion
In the present study, we found only a modest and non-significant reduction in hs-cTnI for the whole group after 12 weeks of exercise training. When evaluating the effect of a structured training programme (SET group) with controls who were advised to exercise, there was a significant but modest reduction of hs-cTnI in the SET group only, whereas the reduction in the CG was not statistically significant. There was no statistical significant difference between SET and CG.
In a subgroup of patients, there was a statistically significant, clinical relevant difference in changes in levels of hs-cTnI from baseline to 12 weeks between SET and CG when adjusting for additional co-morbidities.
The reduction of hs-cTnI was associated with improved left ventricular remodelling and increased functional capacity.
High sensitivity cardiac troponin I alterations after 12 weeks
Although not statistically significant, there was a trend towards a small numerical reduction of hs-cTnI for the whole population (SET and CG merged). This is in accordance with our previous findings for hs-cTnT which was modestly, but statistically significant reduced in the whole SMARTEX population after 12 weeks (all groups; MCT, HIIT, and RRE).14
The results are thus partly in contrast to the results of the HF Action trial in which there was no effects of the intervention on cardiac troponin T (cTnT). However, in the HF-ACTION trial, high-sensitivity assay was not available, and only 14% of patients had measurable cTnT. Additionally, only 30% exercised at or above target exercise minutes per week and the training effect on functional capacity was modest.15
When it comes to the effect of structured training compared with controls who received recommendations to exercise (RRE), there was a numerical small, but statistical significant reduction in hs-cTnI in the intervention group (SET) only, but there was no statistical significant difference between SET and CG.
However, when adjusting for co-morbidities, excluding additional factors that might put strain on the myocardium, there was a statistically significant, clinical relevant difference also between intervention groups, indicating effect of a structured training programme in this sub-set of patients.
Thus, the findings of a positive trend on the effect of a structured exercise training programme on hs-cTnI is partly in accordance with the results found for hs-cTnT in the SMARTEX trial. However, the small and borderline significant reduction in hs-cTnI in the whole group makes the clinical significance questionable for the crude population.
On the other hand, when we excluded patients with co-morbidities the hs-cTnI changed from 10.9 to 9.2 ng/L, a reduction of 1.7 ng/L and became statistically significant. This group consisted of 70 patients out of the original 196 patients included. The significant training effect on chronic myocardial injury was then found in only otherwise more healthy heart failure patients (Table 2).
The importance of levels of troponins in stable CHF is documented in the large multicentre Val-HeFT trial, in which baseline concentration of troponin predicted adverse outcome (all-cause mortality or hospital admission for HF) in patients with stable HF with reduced left ventricular systolic function. A progressive and significant increase occurred in the unadjusted risk of death with increasing deciles of hs-cTnT [from 6.2% in decile 1 to 46.3% in decile 10; decile 10 vs. 1, (P < 0.0001)]. Similar trends were observed for the end point of hospitalization for HF.16, 17
However, elevations in hs-cTnI are more strongly associated with some cardiovascular disease (CVD) outcomes in long term follow up in the general population than elevated levels of TnT, which are more associated with non-CVD death.18
The progressive importance of increased hs-cTnI levels is documented in a study of 520 patients with HFrEF and HFmrEF after 2-year where hs-cTnI levels ≥17 ng/L represented an independent increased risk of an adverse prognosis.19
In another study, in 95 stable outpatients with non-ischaemic CHF, not only the serum concentration of hs-cTnI at baseline but also an increase in hs-cTnI were independent and useful prognostic predictors in patients with non-ischaemic CHF. The hazard ratio for mortality of patients with high hs-cTnI (≥0.03 ng/mL) and an increase in hs-cTnI (Δhs-cTnI ≥0 ng/mL) was 3.59 (95% CI 1.3–9.9, P = 0.014) compared with that of those with high hs-cTnI (≥0.03 ng/mL) and a decrease in hs-cTnI (Δhs-cTnI <0 ng/mL).20
These findings support the hypothesis that even a small reduction in hs-cTnI may have prognostic clinical significance. These findings are consistent with the results in a recent study in black adults with subclinical myocardial injury, in which higher levels of physical activity were associated with reduced risk of heart failure with preserved ejection fraction (HFpEF).21 Subclinical myocardial injury were defined as hs-cTnI >6 ng/L, a level considerably lower than in our population.
A reduction in hs-cTnI of 1.7 ng/L after 12 weeks of exercise training might therefore indicate a prognostic significance in this population.
Cardiac troponin I and ventricular remodelling
In patients with CHF, increased cardiac troponin levels correlate with myocardial remodelling including increased LVEDD and elevated levels of natriuretic peptides indicating cardiac dysfunction due to low grade chronic myocardial injury.22
The findings of a relationship between reduction of LVEDD and hs-cTnI in the current study are in line with the findings of Kusumoto and co-workers who revealed a relationship between cTnT and LVEDD.22 Our findings of an association between a reduction in LVEDD and a decrease in hs-cTnI after a structured ET programme further extends the beneficial effects of ET to indicate that an exercise-induced reduction of myocardial injury might be associated with improved remodelling in CHF.
Cardiac troponin I and N-terminal pro brain natriuretic peptide
Despite a modest positive result on left ventricular remodelling after a structured ET programme, there was no difference in changes of NT-proBNP between SET and CG after 12 weeks. This is in accordance with the findings of Nilsson et al. who found no significant changes in NT-proBNP levels after aerobic interval training, despite significant improvement of functional capacity.23
On the other hand, recent meta-analyses and systematic reviews have indicated that aerobic exercise does not solely improve NT-proBNP and aerobic capacity but also left ventricular function in patients with HF.6, 24, 25
The findings of a moderate correlation between NT-proBNP and hs-cTnI at any time point in the present study are in line with these previous studies and may elucidate the mechanisms at play with improved haemodynamics and reduced chronic low grade myocardial injury.
Cardiac troponin I and functional capacity
There was an inverse association of troponin I with VO2peak. The correlation of troponins with prognosis and risks in HF is well known and has been well documented in recent years. The same holds true for functional capacity and prognosis in HF patients. However, there are limited data on the association of hs-cTnI and functional capacity in heart failure.
Our results are in contrast to earlier findings in HF-ACTION where improvements in cTnT were not associated with increases in VO2peak. This must however be seen in light with our refined hs-cTnI measurements as noted above.
Limitations
SMARTEX HF was a large international multicentre randomized controlled trial and in our sub-study the two intervention groups were merged into one group, due to significant overlap in training intensity as described earlier. Thus, we got one larger intervention group with better power to detect differences between intervention and controls. However, pooling created more heterogeneity of the data, making it harder to give concrete recommendations regarding which intensity levels to exercise at.
As noted in other SMARTEX publications, there were limited physical activity data from the control group, and it is difficult to assess at what intensity level they exercised. This could be a potential confounder in the analysis.
In the subset of patients without additional co-morbidities, patients in the SET group have significantly higher baseline levels of NT-proBNP and hs-cTnI. This skewed values at baseline could also represent a possible limitation for evaluating effect of the intervention in this population. Changes in levels of hs-cTnI might also have been influenced by haemoconcentration after a period of exercise training. On the other hand, there was no significant changes in haemoglobin levels or haematocrite values across the groups during the study period.
Conclusions
In patients with stable HFrEF, 12 weeks of structured exercise intervention was associated with a very modest, but significant reduction of hs-cTnI. However, there was no significant difference between intervention group and control group. The alterations in hs-cTnI were associated with reduction of LVEDD and natriuretic peptide concentrations as well as improved functional capacity. In patients without additional co-morbidities, there was a modest but significant difference in delta hs-cTnI between groups.
Acknowledgements
All contributors to the Smartex-HF study are given in the original publication by Ellingsen et al.12
Conflict of interest
E.R. has received a grant from the Western Norway health trust. T.O. has received speaker and/or consultancy honoraria from Abbott Diagnostics, Bayer, CardiNor, Roche Diagnostics, and Siemens Healthineers, and has received research support from Abbott Diagnostics, Novartis, Roche Diagnostics, via Akershus University Hospital. The rest of the authors declare no conflict of interest.
Funding
The Smartex trial was supported by St. Olavs Hospital; Faculty of Medicine and Health Sciences, NTNU – Norwegian University of Science and Technology; Norwegian Health Association; Danish Research Council; Central Norwegian Health Authorities/NTNU; Western Norway Health Authorities; Simon Fougner Hartmanns Familiefond; Else-Kröner-Fresenius-Stiftung; and Société Luxembourgeoise pour la recherche sur les maladies cardio-vasculaires.




