Sarcopenia prognosis using dual-energy X-ray absorptiometry and prediction model in older patients with heart failure
Abstract
Aims
This study aimed to determine whether there is a difference in the prognostic value of sarcopenia diagnosed using dual-energy X-ray absorptiometry (DEXA) and that predicted by prediction equations in older patients with heart failure (HF).
Methods and results
We included 269 patients (aged ≥65 years) who were hospitalized for HF. We used two appendicular skeletal muscle mass (ASM) prediction equations: (i) Anthropometric-ASM, including age, sex, height, and weight, and (ii) Predicted-ASM, including sex, weight, calf circumference, and mid-arm circumference. ASM index (ASMI) was calculated by dividing the sum of the ASM in the extremities by the height squared (kg/m2). The cut-off values proposed by the Asian Working Group for Sarcopenia 2019 were used to define low ASMI. The prognostic endpoint was all-cause mortality. The median age of the cohort was 83 years [interquartile range (IQR): 75–87], and 135 patients (50.2%) were men. Sarcopenia diagnosed according to DEXA, Anthropometric measurements, and Predicted-ASM was observed in 134 (49.8%), 171 (63.6%), and 157 (58.4%) patients, respectively. During the median follow-up period of 690 days (IQR: 459–730), 54 patients (19.9%) died. DEXA-sarcopenia [hazard ratio (HR), 2.33; 95% confidence interval (CI), 1.26–4.31; P = 0.007] was associated with all-cause mortality after adjusting for pre-existing risk factors, whereas Predicted-sarcopenia (HR, 1.68; 95% CI, 0.87–3.25; P = 0.123) and Anthropometric-sarcopenia (HR, 1.64; 95% CI, 0.86–3.12; P = 0.132) were not.
Conclusions
Sarcopenia diagnosed using DEXA was associated with poor prognosis in older patients with HF; however, the prediction equations were not.
Introduction
Sarcopenia is a skeletal muscle disorder associated with age-related loss of muscle mass and function, resulting in increased functional decline, mortality, and other adverse events,1-3 and is closely associated with heart failure. In a recent meta-analysis of patients hospitalized with heart failure, the prevalence of sarcopenia was estimated at 35–55%.4, 5 Heart failure is subject to sarcopenia evaluation according to the Asian Working Group for Sarcopenia (AWGS) 2019 algorithm.6 Sarcopenia guidelines currently recommend dual-energy X-ray absorptiometry (DEXA) or bioelectrical impedance analysis (BIA) for diagnosing low appendicular skeletal muscle mass (ASM) in Asians and Europeans.2, 6 Although both DEXA and BIA are useful for risk stratification in patients with heart failure,7-9 we previously reported that DEXA has a better prognostic value than BIA.10
Although DEXA is the gold standard for evaluating ASM, it can only be measured in a limited number of hospitals and is challenging to measure repeatedly owing to its availability.11 In addition, the medical costs of DEXA may be imposed on patients.12 To date, several prediction equations for ASM have been reported, and one that can be easily and repeatedly utilized is the method using age, sex, height, and weight (Anthropometric model).13 Kamiya et al. reported that sarcopenia diagnosed according to the ASM index (ASMI) predicted using the Anthropometric model in patients with cardiovascular disease (not only heart failure) had poor prognosis.14 In contrast, the Predicted model, which was calculated using sex, weight, calf circumference (CC), and mid-arm circumference (MAC), showed excellent correlation with DEXA-measured ASM in patients with heart failure.15 ASM measured using the Predicted model was associated with all-cause mortality in patients with heart failure.16 However, whether sarcopenia is evaluated using Anthropometric or Predicted models has not been directly compared with that diagnosed using the gold standard method, DEXA, in older patients with heart failure.
Therefore, we hypothesized that sarcopenia diagnosed using a Predicted or Anthropometric model (Predicted-sarcopenia or Anthropometric-sarcopenia) may have the same prognostic ability as sarcopenia diagnosed using DEXA (DEXA-sarcopenia) in older patients with heart failure.
Methods
Study design
This study was a sub-analysis of the prevalence and prognostic value of physical and social frailty in geriatric patients hospitalized for heart failure (FRAGILE-HF) and Comparison of Various Methods in Evaluation of Sarcopenia in Patients with Heart Failure (SONIC-HF) at Kameda Medical Centre. We included only patients enrolled in these two prospective registries at the Kameda Medical Centre. The FRAGILE-HF study was a multicentre observational study that investigated the frequency and prognostic impact of frailty in older patients hospitalized for heart failure. The detailed study design and main findings of the FRAGILE-HF have been published elsewhere.17 The SONIC-HF registry evaluates the prognostic impact of muscle mass using ultrasonography. The SONIC-HF study had already completed patient enrolment, and these two studies used identical inclusion/exclusion criteria: hospitalized patients aged ≥65 years with decompensated heart failure who could ambulate at discharge were included. The exclusion criteria for both studies were as follows: previous heart transplantation and implantation of a left ventricular assist device, chronic peritoneal dialysis and haemodialysis, and acute myocarditis. Patients with missing brain natriuretic peptide (BNP) measurements and those with a BNP level of <100 pg/mL on admission were also excluded because the diagnosis of heart failure could be uncertain in this population. Only the first hospitalization was registered for patients who were admitted more than once during the study period. As part of the study protocol, investigators performed ASM assessment using DEXA in both studies before discharge, if the hospital was well equipped to perform the tests during hospitalization. All participants were notified of their participation in the study. The participants were also informed that they could withdraw from the study at any time. The two studies were conducted in accordance with the principles of the Declaration of Helsinki and the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects. As this was an observational study without invasive procedures or interventions, written informed consent was not mandated according to the Ethical Guidelines for Medical and Health Research Involving Human Subjects issued by the Japanese Ministry of Health, Labour and Welfare. The protocols of the two studies and the joint analysis of the results were approved by the ethics committee of our hospital (approval no. 21-013).
Measurement of dual-energy X-ray absorptiometry
ASM was evaluated by a trained radiology technician before discharge when patients were in a compensated, euvolaemic state without intravenous heart failure therapy, including vasodilators, diuretics, or inotropes. DEXA was used to measure body composition with QDR-Horizon A (Hologic, Inc., Commonwealth of Massachusetts, USA). Calibration of DEXA was performed daily to verify the linearity and accuracy of the area measurements, density, and bone mass.
Appendicular skeletal muscle mass of prediction equations
We used two ASM prediction equations (Anthropometric model and Predicted model).13, 15 The formula was as follows: Anthropometric model: ASM (kg) = (0.193 × body weight + 0.107 × height − 4.157 × (men = 1, women = 2) − 0.037 × age − 2.631); and Predicted model: ASM (kg) = 0.214 × weight (kg) + 0.217 × CC (cm) − 0.189 × MAC (cm) + 1.098 × (men = 1, women = −1) + 0.576. MAC and CC were measured to the nearest 1 mm by trained personnel using plastic tape. We measured the MAC midway between the lateral projection of the acromion process and the lateral epicondyle of the humerus with the elbow fully extended.18 The MAC value for the left arm was recorded as the average of measurements from the two trials. To obtain the maximum circumference, a tape measure was placed around the calf, without compressing the measured part. The CC value was recorded as the average of the measurements from two trials for the left leg.19
Evaluation of sarcopenia
This study applied the AWGS 2019 definition of sarcopenia.6 The AWGS 2019 diagnostic criteria for sarcopenia include low muscle strength, poor physical function, and ASM. Low muscle strength was measured using handgrip power. The cut-off values proposed by AWGS 2019 were used to define low muscle strength (<28 kg for men and <18 kg for women). Low physical performance was measured using gait speed < 1.0 m/s, five-time chair-stand test ≥ 12 s, or short physical performance battery ≤ 9. Low ASM was measured using DEXA and prediction equations (Anthropometric model and Predicted model). ASMI was calculated by dividing the sum of the ASM in the extremities by the height squared (kg/m2). The cut-off values proposed by the AWGS 2019 were used to define low ASMI (men, <7 kg/m2; and women, <5.4 kg/m2).
Evaluation of exercise tolerance
Exercise tolerance was assessed via 6 min walking distance (6 MWD) before discharge. The 6 MWD was evaluated according to the guidelines of the American Thoracic Society statement.20
Follow-up and study outcome
Prognostic data for these studies were collected prospectively. The endpoint of this study was all-cause mortality recorded up to May 2021. After discharge, most patients were followed up in outpatient clinics, and prognostic data were retrieved from the medical records of each hospital. For patients without follow-up data in the outpatient clinics of their institution, prognostic data were retrieved through telephone interviews with their families.
Statistical analysis
Continuous variables are described as medians with interquartile ranges (IQRs), and categorical variables are reported as numbers with percentages. Baseline characteristics were compared between groups using the Mann–Whitney U test, χ2 test, or Fisher's exact test. The variables were log-transformed for further analyses. Pearson's product–moment correlation coefficient and the Bland–Altman plots were used to assess the agreement between the ASMI defined by the prediction equations and DEXA.21 In the Bland–Altman plots, systematic bias was calculated as the mean difference between the values obtained using the two methods, and the 95% limits of agreement were calculated as the bias ± 2 SD of the difference between the methods. The association between all-cause mortality and the presence or absence of sarcopenia was examined using Kaplan–Meier estimates and compared using the log-rank test. For prognostic analysis, the Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC) risk score and log-transformed BNP were used in the Cox regression model as adjustment variables to calculate the hazard ratios (HRs) of the sarcopenia and non-sarcopenia groups.22 The MAGGIC risk score comprised 13 independent predictors. Model validation for Japanese patients with heart failure has already been demonstrated.23, 24 In addition, we incorporated 6 MWD as adjustment variables. R Version 3.6.3 was used for all statistical analyses (R Foundation for Statistical Computing, Vienna, Austria; ISBN 3-900051-07-0, URL http://www.R-project.org). Statistical significance was defined as a two-sided P value of <0.05.
Results
Study population and patient characteristics
In total, 269 patients hospitalized for heart failure were included in this study. Table 1 presents the baseline patient characteristics. The median age was 83 years (IQR: 75–87 years), and 135 patients (50.2%) were men. DEXA-sarcopenia, Predicted-sarcopenia, and Anthropometric-sarcopenia were observed in 134 (49.8%), 171 (63.6%), and 157 (58.4%) patients, respectively. With regard to the characteristics of patients with sarcopenia, three methods for defining sarcopenia were consistent, and the sarcopenia groups were associated with older age, male sex, lower body mass index, percentage body fat, and higher BNP levels than the non-sarcopenia group (Table 2).
| Variables | DEXA-sarcopenia | Predicted-sarcopenia | Anthropometric-sarcopenia | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-sarcopenia | Sarcopenia | P value | Non-sarcopenia | Sarcopenia | P value | Non-sarcopenia | Sarcopenia | P value | |
| N = 135 | N = 134 | N = 98 | N = 171 | N = 112 | N = 157 | ||||
| Age (years) | 80 [73–86] | 85 [78–87] | 0.001 | 80 [72–86] | 84 [77–87] | <0.001 | 77 [72–84] | 85 [79–88] | <0.001 |
| Male sex (%) | 47 (34.8) | 88 (65.7) | <0.001 | 32 (32.7) | 103 (60.2) | <0.001 | 69 (61.6) | 66 (42.0) | 0.002 |
| Body mass index (kg/m2) | 23.6 [20.9–25.9] | 19.5 [17.3–21.7] | <0.001 | 24.7 [23.0–27.2] | 19.5 [17.5–21.7] | <0.001 | 24.5 [22.7–26.9] | 19.0 [17.3–21.0] | <0.001 |
| SBP (mmHg) | 116 [106–128] | 113 [102–122] | 0.057 | 117 [108–128] | 113 [102–122] | 0.009 | 116 [108–126] | 113 [102–124] | 0.080 |
| DBP (mmHg) | 61 [58–70] | 61 [55–68] | 0.097 | 62 [58–70] | 61 [56–68] | 0.087 | 62 [58–70] | 60 [55–67] | 0.037 |
| Heart rate (b.p.m.) | 70 [60–77] | 69 [60–78] | 0.814 | 69 [59–75] | 69 [60–78] | 0.509 | 70 [59–78] | 69 [60–77] | 0.732 |
| LVEF (%) | 47 [35–63] | 46 [34–63] | 0.743 | 52 [35–64] | 46 [34–62] | 0.287 | 47 [35–63] | 47 [34–63] | 0.843 |
| NYHA III/IV at admission (%) | 0 (0) | 4 (3) | 0.060 | 0 (0) | 4 (2.3) | 0.300 | 0 (0) | 4 (2.5) | 0.143 |
| Percentage body fat (%) | 25.7 (7.8) | 22.1 (6.7) | <0.001 | 28.5 (7.5) | 21.2 (6.0) | <0.001 | 27.1 (7.3) | 21.6 (6.7) | <0.001 |
| Past medical history (%) | |||||||||
| Heart failure | 44 (32.6) | 46 (34.3) | 0.797 | 30 (30.6) | 60 (35.1) | 0.503 | 41 (36.6) | 49 (31.2) | 0.362 |
| Hypertension | 95 (70.4) | 76 (57.1) | 0.031 | 72 (73.5) | 99 (58.2) | 0.013 | 81 (73.0) | 90 (57.3) | 0.009 |
| Diabetes | 46 (34.1) | 41 (30.6) | 0.602 | 30 (30.6) | 57 (33.3) | 0.686 | 41 (36.6) | 46 (29.3) | 0.235 |
| COPD | 9 (6.7) | 17 (12.7) | 0.103 | 5 (5.1) | 21 (12.3) | 0.084 | 16 (14.3) | 10 (6.4) | 0.037 |
| CAD | 50 (37.0) | 43 (32.1) | 0.442 | 34 (34.7) | 59 (34.5) | >0.999 | 46 (41.1) | 47 (29.9) | 0.069 |
| Prescription at admission (%) | |||||||||
| ACE-I/ARB | 93 (68.9) | 84 (62.7) | 0.306 | 70 (71.4) | 107 (62.6) | 0.145 | 81 (72.3) | 96 (61.1) | 0.068 |
| Beta-blocker | 106 (78.5) | 98 (73.1) | 0.322 | 79 (80.6) | 125 (73.1) | 0.185 | 89 (79.5) | 115 (73.2) | 0.252 |
| MRA | 68 (50.4) | 63 (47.0) | 0.626 | 55 (56.1) | 76 (44.4) | 0.076 | 57 (50.9) | 74 (47.1) | 0.621 |
| Laboratory data at admission | |||||||||
| Haemoglobin (mg/dL) | 12.0 [10.8–14.0] | 11.7 [10.3–13.1] | 0.136 | 12.2 [10.9–14.0] | 11.6 [10.4–13.4] | 0.082 | 12.6 [11.2–14.2] | 11.5 [10.2–13.0] | <0.001 |
| Creatinine (mg/dL) | 1.04 [0.82–1.38] | 1.09 [0.90–1.50] | 0.042 | 1.04 [0.81–1.38] | 1.09 [0.87–1.45] | 0.117 | 1.13 [0.87–1.47] | 1.04 [0.84–1.39] | 0.140 |
| Blood urea nitrogen (mg/dL) | 24 [19–33] | 28 [22–37] | 0.017 | 23 [19–32] | 27 [21–38] | 0.011 | 24 [19–33] | 27 [21–37] | 0.138 |
| Sodium (mEq/L) | 140 [138–142] | 139 [136–142] | 0.030 | 141 [138–142] | 139 [137–142] | 0.003 | 140 [137–142] | 139 [137–142] | 0.468 |
| C-reactive protein (mg/dL) | 0.34 [0.16–0.90] | 0.34 [0.18–0.90] | 0.829 | 0.34 [0.16–0.89] | 0.34 [0.16–0.91] | 0.965 | 0.32 [0.16–0.81] | 0.38 [0.17–0.96] | 0.477 |
| BNP (pg/dL) | 208.7 [133.5–376.8] | 321.2 [158.1–515.7] | 0.011 | 211.1 [136.3–357.7] | 297.7 [152.4–512.1] | 0.015 | 208.6 [129.0–375.2] | 299.9 [158.0–496.4] | 0.024 |
- ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BNP, brain natriuretic peptide; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; DEXA, dual-energy X-ray absorptiometry; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; SBP, systolic blood pressure.
- Values are presented as median [interquartile range] and n (%).
| Unadjusted model | aAdjusted model | |||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P value | Hazard ratio | 95% CI | P value | |
| DEXA-sarcopenia | 2.51 | 1.41–4.45 | 0.002 | 2.33 | 1.26–4.31 | 0.007 |
| Predicted-sarcopenia | 2.06 | 1.10–3.84 | 0.024 | 1.68 | 0.87–3.25 | 0.123 |
| Anthropometric-sarcopenia | 2.30 | 1.26–4.23 | 0.007 | 1.64 | 0.86–3.12 | 0.132 |
- CI, confidence interval; DEXA, dual-energy X-ray absorptiometry.
- a Adjusted for Meta-Analysis Global Group in Chronic Heart Failure risk score, log-transformed brain natriuretic peptide, and 6 min walking distance.
Diagnostic values of Predicted and Anthropometric models
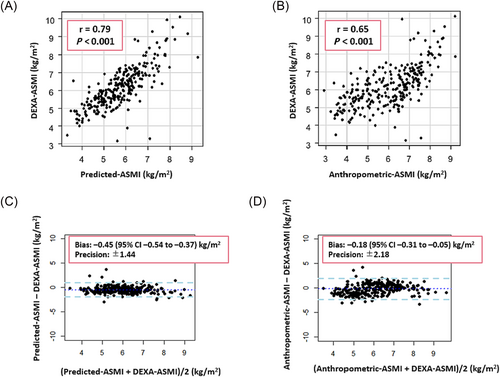
Using the diagnosis of sarcopenia via DEXA-measured ASM as the gold standard, the diagnosis of sarcopenia using the two prediction equations (Predicted-sarcopenia and Anthropometric-sarcopenia) had sensitivities of 94% and 78% and specificities of 67% and 61%, respectively. The ASMIs defined by these two prediction equations (Predicted-ASMI and Anthropometric-ASMI) were significantly correlated with ASMI based on DEXA-measured ASM, with Pearson's correlation coefficients of r = 0.79 and r = 0.65 (P < 0.001 for both). However, the two prediction equations (Predicted-ASMI and Anthropometric-ASMI) underestimated ASMI compared with DEXA, with bias of −0.45 [95% confidence interval (CI), −0.54 to −0.37] and −0.18 (95% CI, −0.31 to −0.05) according to the Bland–Altman plots (Figure 1).
Prognostic comparison of different methods of diagnosing sarcopenia
During a median follow-up of 690 days (IQR: 459–730), all-cause mortality occurred in 54 patients (19.9%). Kaplan–Meier curve analysis and log-rank test revealed that patients with sarcopenia defined by all ASM measures (DEXA-sarcopenia, Predicted-sarcopenia, and Anthropometric-sarcopenia) had significantly higher mortality rates than those without sarcopenia (P = 0.001, P = 0.021, and P = 0.006, respectively) (Figure 2).
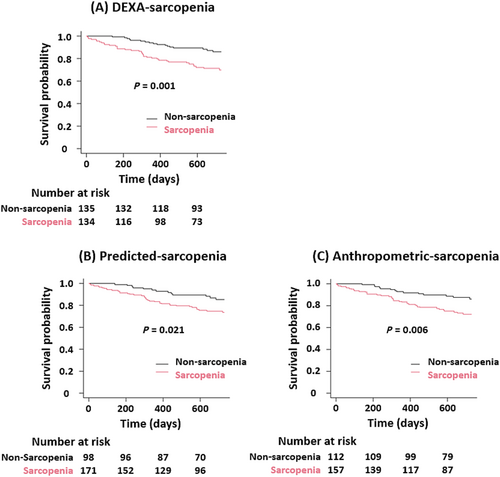
In an unadjusted Cox regression analysis, sarcopenia was significantly associated with a worse prognosis. The association was consistent even after adjustment for MAGGIC risk score, log BNP levels, and 6 MWD in DEXA-sarcopenia (HR, 2.33; 95% CI, 1.26–4.31; P = 0.007). However, sarcopenia predicted by equations did not show a significant association (Predicted-sarcopenia: HR, 1.68; 95% CI, 0.87–3.25; P = 0.123; Anthropometric-sarcopenia: HR, 1.64; 95% CI, 0.86–3.12; P = 0.132) (Table 2).
Discussion
The main findings of this study were as follows: (i) Anthropometric-ASMI and Predicted-ASMI underestimated ASMI using DEXA-measured ASMI as the gold standard, and (ii) DEXA-sarcopenia was associated with mortality independent of pre-existing prognostic models including exercise tolerance, whereas Predicted-sarcopenia and Anthropometric-sarcopenia were not. Thus, DEXA is recommended over the Predicted model for definitive diagnosis of sarcopenia and to obtain additive prognostic information in patients with heart failure.
Appendicular skeletal muscle mass calculated from predictions underestimated dual-energy X-ray absorptiometry-appendicular skeletal muscle mass
Anthropometric and Predicted models are easily available, non-invasive methods for estimating ASM. A previous study of Chinese adults reported no bias in Anthropometric-ASM using DEXA-ASM as a reference.13 Similarly, Katano et al. reported no systemic bias when Predicted-ASM and DEXA-ASM were compared in patients with heart failure; however, the Predicted model underestimated ASM in patients with relatively low percentage body fat mass.15 Considering that the mean percentage of body fat in the present study was 23.9%, which was lower than the 28.5% reported by Katano et al.,15 the difference in the findings of agreement between the Predicted-ASM and the DEXA-ASM between our study and Katano et al.'s study may be attributable to the difference in fat mass between the two cohorts.
Prognostic impact of sarcopenia assessed by guidelines-recommended methods
The prognostic impact of sarcopenia has been reported in patients with heart failure, and previous studies have evaluated sarcopenia using BIA. In a clinical study of 272 hospitalized patients with acute heart failure, sarcopenia diagnosed using BIA was related to cardiac death or heart failure rehospitalization.25 Moreover, we have already reported from FRAGILE-HF that sarcopenia assessed using BIA was independently associated with 1 year mortality, irrespective of left ventricular ejection fraction.7 However, no study has reported an association between sarcopenia defined using DEXA and prognosis, although DEXA may be superior to BIA in terms of its prognostic value in patients with heart failure.10 The current study is the first to show that sarcopenia diagnosed using DEXA is independently associated with all-cause mortality.
Prognostic impact of sarcopenia assessed using prediction equations
Evidence on the prognostic impact of ASMI, defined by Anthropometric and Predicted models, is scarce in patients with heart failure. A retrospective study enrolling 539 patients with heart failure revealed that a low Predicted-ASMI was significantly associated with all-cause mortality and provided additional prognostic value to pre-existing risk factors.16 However, the prognostic impact of sarcopenia assessed using the Predicted-ASMI has not yet been investigated. A clinical study involving 1603 patients with cardiovascular disease (including 702 patients with heart failure) showed that sarcopenia status evaluated using the Anthropometric model was significantly associated with all-cause mortality.14 This finding is inconsistent with our results. The reasons for this discrepancy may be due to different baseline characteristics [Kamiya et al.: mean age, 74.4 ± 6.2 years; 65.4% men; 29.8% sarcopenia; present study: median age, 83 (IQR, 75–87) years; 50.2% men; 58.3% sarcopenia] or the heterogeneity of aetiology of the study participants.14 In the present study, Predicted-sarcopenia and Anthropometric-sarcopenia were not found to be independently associated with all-cause mortality. One possible explanation is the low specificity for diagnosing sarcopenia, indicating that these two methods tend to overestimate the sarcopenia status. Further studies are needed to clarify the association between these two non-invasive methods and the prognosis of patients with heart failure.
Clinical implications
To the best of our knowledge, our study is the first to demonstrate a significant association between sarcopenia, as defined using DEXA, and mortality in patients with heart failure. Given that sarcopenia defined by Anthropometric and Predicted models did not exhibit such an association, DEXA should be employed for a definitive diagnosis of sarcopenia.
The predictive model showed an acceptable sensitivity for diagnosing sarcopenia; however, its specificity was insufficient. Therefore, the Predicted model, an inexpensive and repeatedly available method, is likely to be useful as a screening tool for sarcopenia diagnosis and could be prioritized over the Anthropometric model. If patients are suspected of having sarcopenia based on the Predicted model in patients with heart failure, they should be evaluated using DEXA.
When sarcopenia is identified, specific interventions should be considered, even in older patients with heart failure. Increased physical activity and exercise therapy are recommended for both community-dwelling older adults26 and those with concurrent sarcopenia.27 Recently, progressive rehabilitation interventions28 and electrical muscle stimulation,29 in addition to usual care, have demonstrated improvements in physical function for older patients with heart failure.
Limitations
This study has several limitations. First, the present study was not prespecified and was performed retrospectively, although two multicentre registries were prospectively enrolled. Furthermore, only tertiary hospitals participated in the present study, which may limit the generalizability of the findings. Second, the present study used DEXA at discharge to diagnose sarcopenia; however, it is unclear whether repeated diagnosis of sarcopenia after discharge is associated with a worse prognosis. Finally, because our study was conducted only in Asians, the results may differ by race, and caution should be exercised when adapting the results of this study.
Conclusions
Among older patients hospitalized with heart failure, sarcopenia diagnosed using DEXA was significantly associated with all-cause mortality independent of other prognostic factors; however, sarcopenia evaluated by prediction equations was not. Our study results imply that although the Predicted model can be used for screening, DEXA is recommended to obtain a definitive diagnosis of sarcopenia and additive prognostic information in patients with heart failure.
Conflict of interest
Y.M. received an honorarium from Otsuka Pharmaceutical Co., Novartis Pharma K.K., Bayer Inc., and AstraZeneca and research grants from Pfizer Japan Inc., Otsuka Pharmaceutical Co., EN Otsuka Pharmaceutical Co., Ltd., and Nippon Boehringer Ingelheim Co., Ltd. N.K. is affiliated with a department funded by Philips Japan, Inter Reha Co., Ltd., KYOCERA Corporation, AMI Inc., and Fukuda Denshi Co. Ltd. based on collaborative research agreements and received honorarium from Novartis Japan, Otsuka Pharmaceutical Co., Ltd., and Eli Lilly Japan K.K. The other authors declare no conflicts of interest.
Funding
This research was partially funded by EN Otsuka Pharmaceutical Co., Ltd.




