Cardiac magnetic resonance-based layer-specific strain in immune checkpoint inhibitor-associated myocarditis
Zheng Li and Rui Zhao contributed equally to this work and shared the first authorship.
Abstract
Aims
To assess the different imaging characteristics between corticosteroid-sensitive (CS) and corticosteroid-refractory (CR) immune checkpoint inhibitor-associated myocarditis (ICIaM) with cardiac magnetic resonance (CMR) and the potential CMR parameters in the early detection of CR ICIaM.
Methods and results
Thirty-five patients diagnosed with ICIaM and 30 age and gender-matched cancer patients without a history of ICI treatment were enrolled. CMR with contrast was performed within 2 days of clinical suspicion. Left ventricular ejection fraction (LVEF) and late gadolinium enhancement (LGE) were assessed by CMR. LV sub-endocardial (GLSendo) and sub-epicardial (GLSepi) global longitudinal strains were quantified by offline feature tracking analysis. CS and CR ICIaM were defined based on the trend of Troponin I and clinical course during corticosteroid treatment. All 35 patients presented with non-fulminant symptoms upon initial assessment. Twenty patients (57.14%) were sensitive, and 15 (42.86%) were refractory to corticosteroids. Compared with controls, 22 patients (62.86%) with ICIaM developed LGE. LVEF decreased in CR ICIaM compared with the CS group and controls. GLSendo (−14.61 ± 2.67 vs. −18.50 ± 2.53, P < 0.001) and GLSepi (−14.75 ± 2.53 vs. −16.68 ± 2.05, P < 0.001) significantly increased in patients with CR ICIaM compared with the CS ICIaM. In patients with CS ICIaM, although GLSepi (−16.68 ± 2.05 vs. −19.31 ± 1.80, P < 0.001) was impaired compared with the controls, GLSendo was preserved. There was no difference in CMR parameters between LGE-positive and negative groups. LVEF, GLSendo, and GLSepi were predictors of CR ICIaM. When LVEF, GLSendo, and GLSepi were included in multivariate analysis, only GLSendo remained an independent predictor of CR ICIaM (OR: 2.170, 95% CI: 1.189–3.962, P = 0.012). A GLSendo of ≥−17.10% (sensitivity, 86.7%; specificity, 80.0%; AUC, 0.860; P < 0.001) could predict CR ICIaM in the ICIaM cohort. Kaplan–Meier analysis showed that in patients with impaired GLSendo of ≥−17.10%, cardiovascular adverse events (CAEs) occurred much earlier than in patients with preserved GLSendo of <−17.10% (Log-rank test P = 0.017).
Conclusions
CR and CS ICIaM demonstrated different functional and morphological characteristics in different myocardial layers. An impaired GLSendo could be a helpful parameter in early identifying corticosteroid-refractory individuals in the ICIaM population.
Introduction
Since the first immune checkpoint inhibitors (ICIs)-Ipilimumab was approved by the US Food and Drug Administration (FDA) in 2011, ICIs have been widely used in treating various malignancies.1-3 However, parallel with the exciting clinical outcomes of cancers brought by ICIs, the resultant immune-related adverse events (IRAEs) frequently complicate this revolutionary cancer therapy and necessitate treatment discontinuation in nearly 40% of patients receiving ICIs.1, 2, 4-6 ICIs ICI-associated myocarditis (ICIaM) is the most severe type of IRAEs, with a mortality rate as high as 25–50%.7-9
The treatment of ICIaM has primarily been based on corticosteroids. In the 2022 European Society of Cardiology (ESC) guideline on cardio-oncology, patients who have worsening myocarditis (clinical worsening or persistent troponin elevation) despite high dose methylprednisolone are labelled as steroid-refractory ICIaM, for whom second-line immunosuppression is recommended.10 Previous studies reported that ICIaM patients with inadequate response to steroids had more cardiovascular events and worse outcomes.11 Therefore, it is essential to detect this particular category of patients early to implement appropriate interventions immediately.
Cardiovascular magnetic resonance (CMR) has the inherent capabilities of advanced tissue characterization, making it the imaging of choice in diagnosing myocarditis.12, 13 Strain parameters derived from CMR feature tracking (FT) analysis have been reported to have additional diagnostic and prognostic value in non-ICI-related myocarditis.14, 15 However, data regarding the usefulness of myocardial deformation function in the ICIaM population are limited. As a result, we incorporated CMR FT-based strain analysis into conventional CMR modalities to look for (1) different imaging characteristics between CS and CR ICIaM and (2) potential CMR parameters in the early detection of CR ICIaM.
Methods
Patient cohort
A total of 38 consecutive patients diagnosed with ICIaM by the Cardio-oncology Multidisciplinary Team of Zhongshan Hospital, Fudan University, from January 2019 to June 2022, were enrolled. Myocarditis was diagnosed with a history of ICI use and the criteria for clinically suspected myocarditis per the European Society of Cardiology (ESC) guidelines.16 The exclusion criteria are as follows: (1) unclear cancer treatment history, (2) incomplete/suboptimal CMR scan and image, (3) loss of follow-up, and (4) history of heart failure, uncontrolled hypertension, or coronary artery disease. Clinical information was documented, including patients' demographics, ICI treatment information, baseline cardiovascular diseases/risk factors, laboratory data, electrocardiograms (ECG), transthoracic echocardiography (TTE), CMR features, medical treatment, and clinical outcomes. Thirty age and gender-matched patients with similar cancer types and stages to patients in the ICIaM group were selected as controls for comparison. This research involved human subjects and was performed under the Declaration of Helsinki. All participants provided informed consent. The ethics committee approval was secured for this study: this study was approved by the Zhongshan Hospital's Institutional Review Board (ID: 1212117-6).
Cardiac magnetic resonance data acquisition protocol
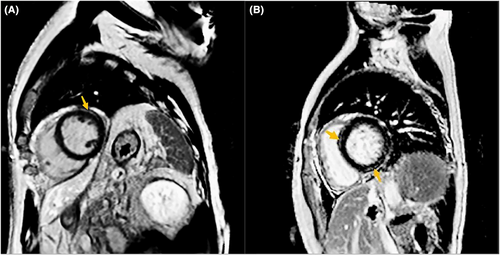
All subjects underwent 1.5 T (Magnetom Aera; Siemens Healthcare, Erlangen, Germany) or 3 T whole-body magnetic resonance scanners (Phillips Medical Systems, Best, Netherlands). With ECG gating and breath holding, standard two-, three- and four-chamber LV long axis views and 8–10 consecutive short axis views covering the whole LV were obtained at the end of the breath. Black blood T2 weighted short tau inversion recovery (STIR) imaging sequences were obtained at three short-axis slices (basal, mid, and apical) and a single long-axis view of LV.17, 18 High signal intensities at the T2-weight STIR sequence were visually assessed by two radiologists blinded to the clinical data. Native T1 mapping was obtained at basal, midventricular, and apical short-axis planes by using a modified Look-Locker inversion-recovery (MOLLI) sequence [5(3)3]. Extracellular volume fraction was quantified using pre- and postcontrast T1 values and patients' blood haematocrit levels. Late gadolinium enhancement (LGE) imaging was performed 10 min after intravenous administration of gadolinium contrast agent with images acquired in matched planes to cine images. Figure 1 shows two examples of LGE in CR and CS ICIaM, respectively.
Cardiac magnetic resonance strain analysis
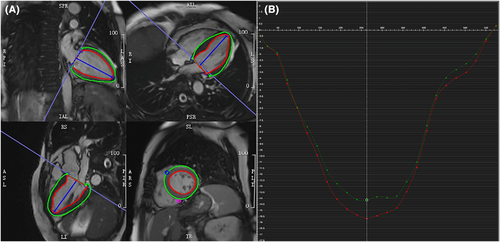
The offline CMR images were analysed by two experienced cardiovascular radiologists blind to clinical information and conventional CMR measurements. Strain parameters were analysed with offline commercial software (CVI 42, release 5.13.2; Circle Cardiovascular Imaging, Calgary, Alberta, Canada). A region of interest covering the LV myocardium was manually drawn by delineating LV's endo- and epicardial borders at the end-diastolic phase of all cine images (Figure 2). Insertions of the right ventricle in LV at the end-diastolic phase of short-axis images were utilized as reference points. Peak longitudinal systolic strains from all long-axis slices were averaged to provide the endocardial GLS (GLSendo) and epicardial GLS (GLSepi) (Figure 2).
Treatment protocol and surveillance
The severity was initially evaluated and categorized at the initial diagnosis of ICIaM according to the classification system suggested by the American Society of Clinical Oncology (ASCO): the mildest grade was referred to as grade 1 (asymptomatic with abnormal cardiac biomarkers or ECG), and the most severe grade was referred to grade 4 (severe symptoms, life-threatening disease with cardiac studies abnormalities in Grade 1–3).19 Standard stratified treatment was started according to the ASCO clinical practice guidelines for IRAE.19 Specifically, patients were initially given high-dose corticosteroids with 1–2 mg/kg/day of prednisone oral or intravenous methylprednisolone with a tapering period lasting 4–6 weeks. In patients without an immediate response to high-dose corticosteroids within 3–5 days, treatment was advanced to higher-dose corticosteroids used in transplant rejection (intravenous methylprednisolone 500–1000 mg daily). In our clinical practice, we monitored patients closely by measuring daily plasma cardiac biomarkers, renal function, and liver function. After reaching the plateau, these indices were checked every 2 weeks. In the final data analytic process, patients were re-classified based on the severity of myocarditis per the European Society of Cardiology (ESC) Guidelines on cardio-oncology10: fulminant type-hemodynamic instability, heart failure requiring non-invasive or invasive ventilation, complete or high-grade heart block, or significant ventricular arrhythmia; non-fulminant type: symptomatic but hemodynamically and electrically stable patients without features of severe disease. Based on the treatment response, patients were also divided into the corticosteroid-refractory (CR) group and corticosteroid-sensitive (CS) group10: corticosteroid-refractory group: non-resolving or worsening myocarditis (clinical worsening or persistent troponin elevation after exclusion of other etiologies) despite high-dose methylprednisolone; corticosteroid-sensitive group: resolving myocarditis and improving troponin level during corticosteroid treatment. In patients with CR ICIaM, tofacitinib, anti-thymocyte globulin, or plasmapheresis were used with high-dose methylprednisolone for additional immunosuppression. Standard myocardial protection agents were also administered, including β-blockers, angiotensin-converting enzyme inhibitors, or angiotensin II receptor blockers.
Outcomes
Cardiovascular adverse events (CAEs) were comprised of cardiovascular death, cardiac arrest, cardiogenic shock, new-onset complete heart block, and heart failure exacerbation requiring hospital admission. A case will be considered cardiovascular death when cardiac arrest, cardiogenic shock, or complete heart block leads to death. When a patient had multiple CAEs, the time of CAEs was determined by the date of the earliest event.
Intraobserver, interobserver, and intervendor variability analysis
Intraobserver and interobserver variability were assessed by randomly selecting 25 patients to be measured by one observer twice and another independent observer. Intraobserver measurements showed intraclass correlation coefficients (ICCs) of 0.976 (95% confidence interval [CI]: 0.945 to 0.989) for GLSendo and 0.972 (95% CI: 0.942 to 0.986) for GLSepi. Interobserver measurement showed ICCs of 0.963 (95% CI: 0.923 to 0.982) for GLSendo and 0.966 (95% CI: 0.930 to 0.983) for GLSepi. To test the intervendor variability, we obtained CMR images with both MRI scanners in 20 volunteers (each volunteer underwent MRI tests twice with both scanners) and used the CVI 42 software to quantify the strain values. The inter-vendor measurement showed ICCs of 0.990 (95% CI: 0.961 to 0.999) for GLSendo and 0.993 (95% CI: 0.979 to 0.999) for GLSepi.
Statistical analyses
Continuous variables were expressed as mean ± standard deviation (SD) or as the median plus interquartile range (IQR) as appropriate. Descriptive statistics were expressed as counts with percentages. The values of variables were compared using either the student-t test or One-way Analysis of Variance (ANOVA) for continuous data. Otherwise, the Chi-square, Fisher exact test, or McNemar's test was used for categorical data whenever appropriate. Possible predictors of CR ICIaM were analysed by logistic regression analysis; odds ratio and 95% confidence intervals (CIs) were calculated. Receiver operating characteristic (ROC) curves were obtained, and the associated cutoff points with the greatest sensitivity and specificity were selected according to Youden's index. The area under the ROC curve (AUC) was calculated to determine the discriminative ability of CMR parameters. The Kaplan–Meier curve was estimated and compared between groups using the Logrank test. A P value of <0.05 was considered statistically significant. All statistical analyses were performed with IBM SPSS Statistics version 26 (IBM Corp., Armonk, NY, USA) and MedCalc software version 15.6.1.0 (MedCalc, New York City, New York).
Results
The final analytic cohort included 35 patients who met the criteria of ICIaM per the ESC guideline on cardio-oncology.10 Baseline clinical characteristics and initial workups are shown in Table 1. No significant difference was found in age and gender between CS and CR ICIaM. ICI agents applied were anti-programmed death 1 (anti-PD-1; including nivolumab, pembrolizumab, toripalimab, and camrelizumab), anti-programmed death-ligand 1 (anti-PD-L1; including durvalumab), and anti-cytotoxic T-lymphocyte-associated protein 4 therapy (anti-CTLA-4; including ipilimumab). Five patients received combination therapy (ipilimumab+ nivolumab). The cancer diagnosis includes gastric (n = 6, 17.14%), hepatic (n = 7, 20.00%), colorectal (n = 5, 14.29%), head–neck (n = 4, 11.43%), lung (n = 3, 8.57%), oesophageal (n = 4, 11.43%), cervical carcinoma (n = 2, 5.71%), breast cancer (n = 3, 8.57%), and renal cell carcinoma (n = 1, 2.86%). All patients were in stages 3–4 of cancer. The median time from starting ICI therapy to the onset of ICIaM was 51 days (IQR: 32–70 days). All of the patients in our study presented with non-fulminant symptoms. There was no difference in ECG or TTE findings between CS and CR ICIaM.
| Total (n = 35) | CS (n = 20) | CR (n = 15) | P value | |
|---|---|---|---|---|
| Male (n, %) | 20 (57.14) | 12 (60.00) | 8 (53.33) | 0.741 |
| Female (n, %) | 15 (42.86) | 8 (40.00) | 7 (46.67) | 0.741 |
| Age (years) | 60.09 ± 8.72 | 60.45 ± 7.21 | 59.60 ± 10.65 | 0.780 |
| SBP (mmHg) | 122.51 ± 7.71 | 121.25 ± 8.38 | 124.20 ± 6.60 | 0.269 |
| DBP (mmHg) | 74.00 ± 6.88 | 74.65 ± 7.23 | 73.13 ± 6.51 | 0.527 |
| HR (b.p.m.) | 76.57 ± 14.21 | 79.80 ± 13.31 | 72.27 ± 14.66 | 0.122 |
| Time from ICI therapy to ICIaM (days) | 51 (32, 70) | 52 (35, 87) | 46 (27, 66) | 0.156 |
| ICIs combination therapy (n, %) | 5 (14.29) | 3 (15.00) | 2 (13.33) | 1.000 |
| Symptoms (n, %) | ||||
| Fatigue, malaise, light-headedness | 26 (74.29) | 14 (70.00) | 12 (80.00) | 0.700 |
| Dyspnoea, palpation | 17 (48.57) | 8 (40.00) | 9 (60.00) | 0.315 |
| Ptosis, diplopia, muscle weakness, myalgia | 6 (17.14) | 4 (20.00) | 2 (13.33) | 0.680 |
| Cardiovascular risk factors/Concomitant diseases | ||||
| COPD (n, %) | 2 (5.71) | 1 (5.00) | 1 (6.67) | 1.000 |
| Diabetes mellitus | 5 (14.29) | 3 (15.00) | 2 (13.33) | 1.000 |
| Smoking history | 11 (31.43) | 7 (35.00) | 4 (26.67) | 0.721 |
| Other IRAEs (n, %) | ||||
| Myositis | 6 (17.14) | 4 (20.00) | 2 (13.33) | 0.680 |
| Hepatitis/transaminitis | 8 (22.86) | 2 (10.00) | 6 (40.00) | 0.051 |
| Myasthenia gravis | 4 (11.43) | 1 (5.00) | 3 (20.00) | 0.292 |
| ECG (n, %) | ||||
| Sinus rhythm | 35 | 20 | 15 | N/A |
| Sinus tachycardia | 7 (20.00) | 5 (25.00) | 2 (13.33) | 0.672 |
| Sinus bradycardia | 4 (11.43) | 1 (5.00) | 3 (20.00) | 0.292 |
| Atrial premature beats | 5 (14.29) | 4 (20.00) | 1 (6.67) | 0.365 |
| Ventricular premature beats | 5 (14.29) | 2 (1.00) | 3 (2.00) | 0.631 |
| ST-T changes | 8 (22.86) | 2 (1.00) | 6 (40.00) | 0.051 |
| TTE | ||||
| PASP, mmHg | 26.05 ± 4.42 | 26.95 ± 4.46 | 24.85 ± 4.21 | 0.836 |
| Pericardial effusion (n, %) | 6 (17.14) | 2 (10.00) | 4 (26.67) | 0.367 |
| TAPSE (mm) | 19.57 ± 1.20 | 19.65 ± 1.23 | 19.47 ± 1.19 | 0.660 |
| Left atrial size (mL/m2) | 27.58 ± 3.80 | 26.97 ± 4.11 | 28.39 ± 3.31 | 0.283 |
- COPD, chronic obstructive pulmonary disease; CR, corticosteroid-refractory; CS, corticosteroid-sensitive; DBP, diastolic blood pressure; ECG, electrocardiogram; HR, heart rate; ICIs, immune checkpoint inhibitors; IRAEs, immune-related adverse events; PASP, pulmonary artery systolic pressure; SBP, systolic blood pressure; TTE, transthoracic echocardiography.
Subgroup analysis of cardiac magnetic resonance parameters
Patterns of LGE are shown in Table 2. CMR parameters in controls and ICIaM patients are shown in Table 3. A comparison of CMR indices between LGE-positive and negative patients is shown in Table 5. Each patient underwent a comprehensive CMR scan with contrast within two days after the clinical suspicion of ICIaM. LGE was observed in 22 patients with patterns including sub-epicardial involvement only (n = 13) and sub-epicardial plus mid-layer involvement (n = 9). No transmural or sub-endocardial LGE pattern was found in the present study. CR ICIaM demonstrated more mid-layer LGE than CS ICIaM (8/12 vs. 1/10, P = 0.011). The positive rate of LGE was comparable between the CS and CR groups (12/15 vs. 10/20, P = 0.089). An elevated T2 weighted signal was observed in 23 patients. There was no difference in the incidence of T2 signal elevation between the CS and CR groups. Native T1 value and extracellular volume significantly increased in CS and CR ICIaM groups compared with controls. However, no difference in native T1 value or extracellular volume was seen between the two subgroups. LVEF decreased in CR ICIaM compared with the CS group and controls. GLSendo and GLSepi significantly increased in patients with CR ICIaM compared with the controls and CS ICIaM. Although GLSepi was impaired in CS ICIaM compared with the controls, GLSendo was preserved. There was no difference in CMR parameters between LGE-positive and negative groups.
| CS | CR | CS | CR | P value | CS | CR | P value | |
|---|---|---|---|---|---|---|---|---|
| LGE pattern | Sub-epicardium only | Sub-epicardium and mid-layer | No enhancement | |||||
| Total | 13 | 9 | 13 | |||||
| Subgroup | 9 | 4 | 1 | 8 | 0.011 | 10 | 3 | 0.089 |
- CR, corticosteroid-refractory; CS, corticosteroid-sensitive; LGE, late gadolinium enhancement.
| Controls (n = 30) | CS (n = 20) | CR (n = 15) | |
|---|---|---|---|
| LVEF (%) | 59.43 ± 3.37 | 58.35 ± 3.86 | 55.80 ± 2.57a,b |
| LGE (n, %) | 0 | 10 (50.0%)a | 12 (80.0%)a |
| Elevated T2 signal (n, %) | 0 | 14 (70.0%)a | 9 (60.0%)a |
| Native T1 value (ms)c | 977.65 ± 26.84 | 1034.58 ± 31.49 a | 1047.50 ± 37.47 a |
| Extracellular volume (%)d | 25.79 ± 2.58 | 31.08 ± 2.94 a | 32.17 ± 2.89 a |
| GLSendo (%) | −19.64 ± 2.26 | −18.50 ± 2.53 | −14.61 ± 2.67a,b |
| GLSepi (%) | −19.31 ± 1.80 | −16.68 ± 2.05 a | −14.75 ± 2.53a,b |
- CS, corticosteroid-sensitive; CR, corticosteroid-refractory; ICIaM, ICIs-associated myocarditis; LVEF, left ventricular ejection fraction; LGE, late gadolinium enhancement; GLS, global longitudinal strain; GLSendo, sub-endocardial GLS; GLSepi, sub-epicardial GLS.
- a P < 0.05, compared with the controls.
- b P < 0.05, compared with the CS group.
- c Native T1 value is available in 19 CS and 14 CR ICIaM patients.
- d Extracellular volume is available in 12 CS and 12 CR ICIaM patients.
Subgroup analysis of inpatient clinical courses
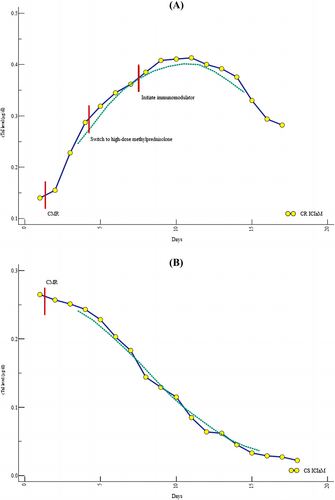
Cardiac biomarkers, clinical courses, and CAEs in CS and CR ICIaM are shown in Table 4. Among all 35 patients, 15 were refractory to corticosteroid-based treatment. Up-trending cTnI was observed in patients with CR ICIaM despite switching to high-dose methylprednisolone after inadequate response to the initial weight-based corticosteroid regimen (Figure 3). Troponin levels decreased gradually during treatment in 20 patients with CS ICIaM. (Figure 3). Compared with patients with CS ICIaM, those with CR ICIaM had more symptom aggravation and disease progression. All the CS ICIaM patients and 10 CR ICIaM patients recovered with the resolution of symptoms and normalization of cTnI, whereas five CR ICIaM patients died despite standard treatment and discontinuation of ICIs. The reasons for death were cardiogenic shock secondary to the progression of myocarditis (n = 4) and severe pneumonia (n = 1). Regarding the treatment, patients in the CR group received more additional immunosuppression therapy. No difference was found in cardiac biomarkers, symptom progression, or all-cause death between LGE-positive and negative patients (Table 5).
| CS (n = 20) | CR (n = 15) | P value | |
|---|---|---|---|
| Baseline cTnI (μg/dL) | 0.16 ± 0.11 | 0.23 ± 0.18 | 0.151 |
| Treatment (n, %) | |||
| Tofacitinib | 0 | 11 (73.33%) | <0.001 |
| Anti-thymocyte globulin | 0 | 5 (33.33%) | 0.009 |
| Plasmapheresis | 0 | 1 (6.67%) | 0.429 |
| Inpatient clinical course (n, %) | |||
| Symptom progression | 4 (20.00%) | 11 (73.33%) | 0.002 |
| All-cause death | 0 | 5 (33.33%) | 0.009 |
| CAEs (n, %) | |||
| Complete heart block | 0 | 1 (6.67%) | 0.429 |
| Cardiogenic shock | 0 | 4 (26.67%) | 0.026 |
| Cardiac arrest | 0 | 2 (13.33%) | 0.176 |
| Heart failure readmission | 2 (10.00%) | 8 (53.33%) | 0.008 |
| Cardiovascular death | 0 | 4 (26.67%) | 0.026 |
- CAEs, cardiovascular adverse events; CR, corticosteroid-refractory; CS, corticosteroid-sensitive; cTnI, cardiac troponin I; ICIaM, ICIs-associated myocarditis.
| LGE-positive (n = 22) | LGE-negative (n = 13) | P value | |
|---|---|---|---|
| Baseline cTnI (μg/dL) | 0.23 ± 0.15 | 0.13 ± 0.12 | 0.077 |
| LVEF (%) | 56.73 ± 3.67 | 58.15 ± 3.31 | 0.259 |
| GLSendo (%) | −16.59 ± 3.63 | −17.24 ± 2.42 | 0.572 |
| GLSepi (%) | −15.68 ± 2.65 | −16.16 ± 2.09 | 0.579 |
| Elevated T2 signal (n, %) | 16 (72.73%) | 7 (53.85%) | 0.292 |
| Native T1 value with (ms) (n = 33) | 1045.29 ± 34.04 | 1030.92 ± 33.97 | 0.252 |
| Extracellular volume (%) (n = 24) | 31.80 ± 3.12 | 31.33 ± 2.65 | 0.712 |
| Inpatient clinical course (n, %) | |||
| Symptom progression | 8 (36.36%) | 7 (53.85%) | 0.481 |
| All-cause death | 5 (22.73%) | 0 | 0.134 |
| CAEs (n, %) | |||
| Complete heart block | 1 (4.55%) | 0 | 1.000 |
| Cardiogenic shock | 4 (18.18%) | 0 | 0.274 |
| Cardiac arrest | 2 (9.09%) | 0 | 0.519 |
| Heart failure readmission | 6 (27.27%) | 4 (30.77%) | 1.000 |
| Cardiovascular death | 4 (18.18%) | 0 | 0.274 |
- CAEs, cardiovascular adverse events; cTnI, cardiac troponin I; GLS, global longitudinal strain; GLSendo, sub-endocardial GLS; GLSepi, sub-epicardial GLS; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction.
Cardiovascular adverse events
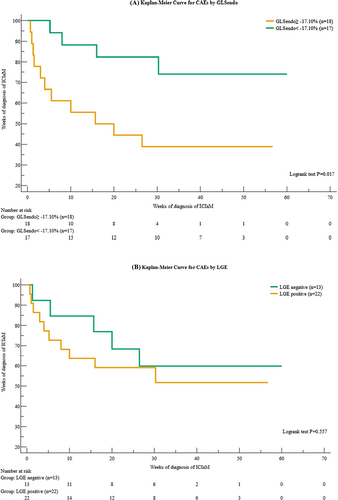
Cardiovascular adverse events in patients with CR and CS ICIaM are shown in Table 4. During a median follow-up time of 28.0 weeks, 15 patients (42.9%) developed CAEs. Two patients with CS ICIaM had acute decompensated heart failure requiring readmission during the post-hospitalization follow-up. No other CAEs or deaths of any cause occurred in patients with CS ICIaM. There was more cardiogenic shock (0/20 vs. 4/15, P = 0.026), cardiovascular death (0/20 vs. 4/15, P = 0.026), and heart failure readmission (2/20 vs. 8/15, P = 0.008) in the CR ICIaM group than in the CS subgroup. Kaplan–Meier curve for the cardiac even-free survival analysis shows that in patients with impaired GLSendo of ≥−17.10%, CAEs occurred much earlier than in patients with preserved GLSendo of <−17.10% (Logrank test P = 0.017, Figure 4). No difference was found in CAEs between LGE-positive and negative patients (Table 5). The Kaplan–Meier curves of LGE-positive and negative groups overlapped with each other (Logrank test P = 0.557, Figure 4).
Logistic regression and receiver operating characteristic curve analysis
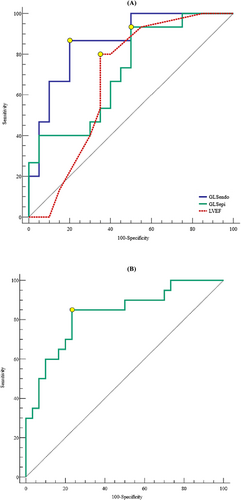
In the ICIaM cohort, univariable analysis of predictors of CR ICIaM is shown in Table 6. Age, gender, baseline cTnI levels, time to the diagnosis of ICIaM, history of ICI combination therapy, or the presence of LGE were not associated with CR ICIaM. Regarding CMR parameters, LVEF, GLSendo, and GLSepi were predictors of CR ICIaM. When LVEF, GLSendo, and GLSepi were included in multivariate analysis, only GLSendo remained an independent predictor of CR ICIaM (OR: 2.170, 95% CI: 1.189–3.962, P = 0.012). In ROC analysis, LVEF could not robustly distinguish CR ICIaM from CS ICIaM (cutoff: <56.00%; sensitivity, 80.0%; specificity, 65.0%; AUC, 0.677; P = 0.059). A GLSepi of ≥−17.23% (sensitivity, 93.3%; specificity, 50.0%; AUC, 0.717; P = 0.013) and a GLSendo of ≥−17.10% (sensitivity, 86.7%; specificity, 80.0%; AUC, 0.860; P < 0.001) could predict the treatment response to corticosteroid in the ICIaM cohort, with the AUC of GLSendo significantly larger than the AUC of GLSepi (0.860 vs. 0.717, P = 0.035) (Table 7 and Figure 5). A GLSepi of ≥−18.74% (sensitivity, 85.0%; specificity, 76.7%; AUC, 0.828; P < 0.001) differentiated CS ICIaM from controls (Table 7 and Figure 5). Ten CS ICIaM patients had positive LGE in the initial CMR test (10/20, sensitivity, 50.0%). The sensitivity of GLSepi was significantly higher than the sensitivity of LGE (85.0% vs. 50.0%, P = 0.039). Among the 10 CS ICIaM patients with negative LGE, eight (8/10, 80.0%) had GLSepi of ≥−18.74%.
| Odds ratio | 95% CI | P value | |
|---|---|---|---|
| Age | 0.989 | 0.915–1.069 | 0.772 |
| Gender | 0.762 | 0.197–2.946 | 0.693 |
| ICIs combination therapy | 1.147 | 0.167–7.898 | 0.889 |
| Time from ICI therapy to ICIaM | 0.978 | 0.949–1.009 | 0.157 |
| Baseline cTnI | 1.036 | 0.986–1.088 | 0.158 |
| GLSendo | 1.688 | 1.208–2.359 | 0.002 |
| GLSepi | 1.464 | 1.038–2.063 | 0.030 |
| LVEF | 0.800 | 0.647–0.991 | 0.041 |
| LGE | 0.250 | 0.054–1.165 | 0.078 |
- CR, corticosteroid-refractory; cTnI, cardiac troponin I; GLS, global longitudinal strain; GLSendo, sub-endocardial GLS; GLSepi, sub-epicardial GLS; ICIaM, ICIs-associated myocarditis; LGE, late gadolinium enhancement; LVEF, left ventricular ejection fraction.
| AUC | P value | 95% CI | Sensitivity | Specificity | Cutoff value | |
|---|---|---|---|---|---|---|
| GLSendo, GLSepi, and LVEF in differentiating CR from CS ICIaM | ||||||
| GLSendo (%) | 0.860 | <0.001 | 0.701–0.954 | 0.867 | 0.800 | −17.10 |
| GLSepi (%) | 0.717 | 0.013 | 0.539–0.855 | 0.933 | 0.500 | −17.23 |
| LVEF (%) | 0.677 | 0.059 | 0.498–0.824 | 0.800 | 0.650 | 56.00 |
| GLSepi in differentiating CS ICIaM from controls | ||||||
| GLSepi (%) | 0.828 | <0.001 | 0.695–0.920 | 0.850 | 0.767 | −18.74 |
- AUC, area under the curve; GLS, global longitudinal strain; GLSendo, sub-endocardial GLS; GLSepi, sub-epicardial GLS; LVEF, left ventricular ejection fraction; ROC, receiver operating characteristic.
Discussions
Summary of findings
To our knowledge, this is the first study to 1) investigate the correlation between morphology and function status of specific myocardial layers in ICIaM with different responses to corticosteroid treatment and 2) assess potential CMR-based parameters in the early detection of CR ICIaM.
Patients with CR ICIaM had more complicated clinical courses, earlier-onset CAEs, and worse prognoses than those with CS ICIaM. A preserved GLSepi, together with a negative LGE, raises confidence in excluding ICIaM. GLSendo was the only independent predictor of CR ICIaM in the ICIaM cohort. GLSendo could early detect CR ICIaM before the full-blown troponin response toward corticosteroid treatment.
The late gadolinium enhancement pattern in immune checkpoint inhibitor-associated myocarditis: A comparison with other characteristic chemotherapy agents
LGE was seen in 22 patients (62.9%), comparable with the positive rate in the other studies.20, 21 Individuals with positive LGE all presented sub-epicardial layer involvement, sparing the sub-endocardium. This LGE pattern was also found in other studies. Faron et al. described sub-epicardium enhancement with associated pericardial enhancement in patients treated with ICIs.22 In contrast, the sub-endocardium is usually the first myocardial layer to demonstrate LGE in patients exposed to anthracycline,23 a conventional antineoplastic agent with a well-recognized cardiotoxic effect responsible for type I cancer therapeutics-related cardiac dysfunction (CTRCD).24 Different mechanisms of cardiotoxicities could cause this phenomenon. The suggested pathophysiology in ICIaM is primarily T-cell-medicated molecular mimicry, which is also the proposed mechanism of lymphocytic viral myocarditis.9, 25 These two types of myocarditis share a similar LGE pattern, in which the epicardial/mid-myocardial layer is almost always involved, relatively sparing the sub-endocardial region.26 Anthracycline can predispose the sub-endocardium to ischemia by causing endothelial cell damage and microcirculation dysfunction. This explains the ischemic injury-type LGE seen in anthracycline-related cardiomyopathy, although without ischemic distribution seen in coronary artery disease.27 It is worth mentioning that in patients receiving cancer therapy, sub-epicardial LGE is not specific to ICIaM. It was also found in patients treated with trastuzumab, a characteristic agent that causes type II CTRCD.24, 28 Thirteen patients did not have LGE in the initial CMR test, of which six patients had no elevated T2-weighted signal. Although data are limited for CMR findings specific to ICIaM, the reported rate of LGE and elevated T2-weighted signal in ICIaM is lower than that in acute non-ICI-associated myocarditis.29, 30 In one multi-centre study of 103 ICIaM patients who underwent CMR, LGE was present in less than 50% of ICIaM, and 42% of cases demonstrated neither LGE nor elevated T2-weighted signal.21 In another study directly comparing the CMR characteristics of ICIaM and viral myocarditis, the positive rate of LGE in viral myocarditis was 100% (85/85), significantly higher than that (82%, 27/33) in ICIaM.31 One possible reason for this discrepancy could be the early timing of the CMR test in the present and previous ICIaM-related studies. Another reason could be the intrinsic characteristic (inflammation process) of ICIaM, which might be different from other myocarditis, even though they share some similar features, especially with viral myocarditis. The subgroup analysis showed no difference in clinical courses and outcomes between LGE-positive and LGE-negative cases, indicating that the initial LGE possessed a limited prognostic value.21
A preserved sub-epicardial strain raises confidence in ruling out immune checkpoint inhibitor-associated myocarditis
Along with the presence of sub-epicardial LGE, the GLSepi was impaired significantly in patients with CS ICIaM compared with the controls. Meanwhile, correlating with the absence of sub-endocardial LGE in CS ICIaM individuals, there was preserved GLSendo. These findings indicated a ‘match’ between morphology and function status in each layer, respectively. However, 10 CS ICIaM patients did not show LGE at the time of the CMR test, while their GLSepi already increased significantly compared with the controls, suggesting that the functional changes occurred earlier than the morphological ones. Therefore, the generation of ‘match’ in the sub-epicardial layer might be an asynchronous process, leading to half of the CS ICIaM cases displaying actual epicardial ‘mismatch’. LGE has been proposed to be a valuable diagnostic tool after initial workups in clinically suspected ICIaM.15 However, due to its relatively low sensitivity, especially in the early phase of the pathology process, LGE cannot reliably rule out ICIaM.21 In the diagnostic test of our study, a GLSepi of ≥−18.74% was able to differentiate CS ICIaM from controls with significantly improved sensitivity. Moreover, GLSepi detected eight LGE-negative CS ICIaM cases in the initial CMR test, which would be misdiagnosed if negative LGE were used as the exclusion criterion. Meanwhile, GLSepi in CR ICIaM increased even further compared with the CS group. In other words, ICIaM can be ruled out more confidently with a preserved GLSepi than a negative LGE. In a previous study, Isaak A et al. investigated the layer-specific strain with CMR-FT analysis in patients with non-ICI-associated myocarditis.14 They reported the excellent diagnostic performance of sub-epicardial longitudinal strain in patients with suspected acute myocarditis. Here, we tested the layer-specific strain in ICIaM individuals, and a similar finding was observed, further expanding the role of CMR-FT-based GLSepi in the diagnosis of this special myocarditis population. Although it is ideal to proceed to endomyocardial biopsy (EMB) in appropriate patients with uncertain diagnoses,21 EMB is an invasive procedure with sampling limitations, especially in the setting of ICIaM, where the myocardium involvement is patchy.9 Repeating CMR has also been suggested if EMB is not an option.9 However, the optimal timing of repeating CMR is still unclear. As a result, we speculate that layer-specific functional analysis could be another fair alternative to facilitate early diagnosis based on our preliminary data.
An impaired sub-endocardial strain detects treatment-refractory patients in immune checkpoint inhibitor-associated myocarditis cohort
In terms of the troponin response in ICIaM patients during treatment, CS ICIaM had a down-trending cTnI. In contrast, CR ICIaM had an up-trending cTnI reaction at both the initial weight-based and high-dose methylprednisolone stages. Regarding the clinical courses and outcomes, we observed more disease progression reflected by symptom aggravation, more CAEs, more hemodynamic instability, and death in patients with CR ICIaM. Patients in our study had non-fulminant symptoms with stable hemodynamic status upon initial clinical encounter. However, even among these non-fulminant ICIaM cases, those with inadequate response toward corticosteroids displayed more complicated clinical courses and worse outcomes. The 2018 ASCO guideline proposed a symptom-based stratified strategy for the management of ICIaM19: systemic prednisone or methylprednisolone at 1 to 2 mg/kg/day for patients with low-grade symptoms and high-dose methylprednisolone at 1000 mg daily for cases with more severe diseases or those who fail to respond to initial corticosteroid dosing within 3 to 5 days. However, in the 2022 ESC guideline on cardio-oncology, initial high-dose intravenous methylprednisolone at 500–1000 mg daily is recommended regardless of the disease severity due to improved prognosis brought by initial higher-dose methylprednisolone.10 Based on the present study's findings, among ICIaM patients with non-fulminant symptoms, there was a subset with the potential for corticosteroid refractory demonstrating rapid disease progression. These individuals may benefit from initial high-dose methylprednisolone, which could partially explain the emerging evidence that an initial higher-dose systemic corticosteroids regimen is associated with improved cardiac outcomes in ICIaM.32
Significant differences existed in strain parameters and LVEF between CR and CS ICIaM at the time of clinical suspicion. Compared with CS ICIaM, CR ICIaM demonstrated a significant drop in LVEF and increases in both GLSepi and GLSendo. Among LVEF and GLS indices, GLSendo was the only independent predictor of inadequate corticosteroid treatment response in the ICIaM cohort. Moreover, patients with impaired GLSendo of ≥−17.10% experienced earlier onset of CAEs than those with preserved GLSendo. It has been reported that strain analysis with CMR-FT may improve the risk stratification in myocarditis.15 Fischer K et al. used feature-tracking CMR to analyse a cohort of non-ICI-associated myocarditis patients.15 They found myocardial strain's independent and incremental prognostic value over clinical features, LVEF, and LGE in patients with myocarditis. In the present study, GLSendo, the only independent predictor of corticosteroid refractory in the ICIaM cohort, correlated with poorer outcomes, further expanding the value of CMR-FT-based GLSendo in the prognosis of this special myocarditis population. From the timing perspective, the initial CMR tests were performed before the troponin response to the treatment was available. In this sense, GLSendo can discern CR ICIaM earlier than the conventional cardiac biomarkers. The advantage of strain analysis in the early clinical stage may bring two benefits. First, GLSendo could predict CR ICIaM, contributing to early risk stratification in the ICIaM population. On the other hand, adjuvant immunomodulators can be administered as soon as the strain is impaired upon initial evaluation instead of waiting for the full-blow trajectory of cTnI.33 Whether this strain-guided earlier intervention with second-line immunosuppression further decreases cardiovascular events and improves the prognosis in CR individuals needs to be verified.
The correlation between sub-endocardial function and morphology in corticosteroid-refractory immune checkpoint inhibitor-associated myocarditis
Although LGE in both ICIaM and viral myocarditis is present as a predominant sub-epicardial enhancement, one previous study observed a considerable proportion of mid-layer penetration of LGE in ICIaM, which was consistent with our findings.31 However, in our study, we further noticed the tendency of mid-layer involvement differed between CS and CR ICIaM. Specifically, LGE in CR ICIaM tended to extend deeper to the mid-layer while relatively confined to the epicardium in CS ICIaM. The discrepancy in clinical outcomes and myocardial layer involvement suggests that CS and CR ICIaM might be distinct disease entities. The CR group also had a ‘match’ of positive LGE and impaired strain in the sub-epicardial layer, similar to the CS counterparts. Although GLSepi increased to a further extent in CR ICIaM, the impaired GLSepi in CS ICIaM may compromise its sensitivity and specificity in differentiating CR ICIaM from CS ICIaM, which explains the smaller AUC of GLSepi in the ROC analysis. Interestingly, unlike the epicardial ‘mismatch’ seen in CS ICIaM, an endocardial ‘mismatch’ was observed in CR ICIaM, where the sub-endocardial layer presented increased strain despite negative LGE. Similar to CS ICIaM, the asynchrony of functional and morphological changes may also be the reason. In CR ICIaM, more mid-layer enhancement was noticed, indicating a pathology propagation from the outer to the inner layer. However, the progression of the ICI toxicity-induced GLS reaction between myocardial layers was more rapid, resulting in the production of LGE lagging behind the damage to sub-endocardial GLS in CR ICIaM. Therefore, the profoundly impaired GLSendo in CR ICIaM and the preserved GLSendo in CS ICIaM accounted for the better diagnostic performance of GLSendo than GLSepi. The ‘mismatches’ seen in both CS and CR ICIaM imply that the pathological change is likely a more time-consuming process, reflecting the cumulative cardiotoxic effects of ICI over time. In contrast, the regional systolic function reacts more instantly toward the insult from ICI toxicity. Although there was a tendency for deeper penetration of pathology in the CR subgroup, sub-endocardial or transmural LGE was absent in the present study, likely due to the early timing of the initial CMR test.
Study limitations
There are several limitations to our study. None of the included cases underwent endomyocardial biopsy, which makes it challenging to correlate the imaging findings to the underlying pathology bases. The number of patients is relatively small. Studies with larger samples and longer follow-ups are required to draw more definitive conclusions. There is a lack of population-based studies on the reference values of CMR-based strain parameters. The reference cutoff points presented in our ROC analysis need to be externally validated and investigated in larger datasets. Additionally, since this is a retrospective study, the CMR protocol was not pre-specified. T2-weight STIR imaging was performed in three short-axis slices and a single long-axis view instead of covering the entire LV length.
Conclusions
Patients with CR ICIaM had more complicated clinical courses, earlier-onset CAEs, and worse prognoses than those with CS ICIaM. CR and CS ICIaM demonstrated different functional and morphological characteristics in different myocardial layers. An impaired GLSendo could early recognize corticosteroid-refractory individuals in the ICIaM population. Initial quantification of layer-specific strain metrics deserves further research in more patients for possible application in the early risk stratification of ICIaM.
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Funding
This study was supported by the National Natural Science Foundation of China (82170359); The Standardized Technology Management and Promotion Project of Shanghai Shenkang Hospital Development Center (SHDC22023207); The Clinical Research Project of Zhongshan Hospital (2020ZSLC21); The Shanghai Municipal Health Commission Fund (202040344); The Youth Fund of Zhongshan Hospital, Fudan University (2020ZSQN74); and The State Key Clinical Specialty Construction Project (YW2021-002).




