Association between haemoglobin, albumin, lymphocytes, and platelets and mortality in patients with heart failure
Abstract
Aims
The combination of haemoglobin, albumin, lymphocytes, and platelets (HALP) is a new metric used to assess patient prognosis in many diseases. This study aimed to assess the relationship between HALP and short- and long-term mortality in patients with heart failure.
Methods and results
This retrospective cohort study included adult patients with heart failure who were hospitalized between 2019 and 2021. The primary outcomes were 1-month mortality and 1-year mortality. The multivariable logistic regression analysis was used to evaluate the association between HALP and the risk of mortality. Stratified analyses were conducted based on New York Heart Association functional classification (NYHA) stage (II/III, IV) and left ventricular ejection fraction (LVEF, <50%, ≥50%). The area under the receiver operating characteristic curve (AUC) was used to evaluate the ability of HALP, prognostic nutritional index (PNI), C-reactive protein (CRP), and the Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC-HF) risk score in predicting mortality in patients with heart failure. A total of 730 patients with heart failure were included, of whom 61 (8.36%) died within 1 month and 77 (10.55%) died within 1 year. High HALP scores were associated with a reduced risk of 1-month mortality (odds ratio (OR) = 0.978, 95% confidence interval (CI): 0.963–0.992, P = 0.003) and 1-year mortality (OR = 0.987, 95% CI: 0.977–0.997, P = 0.009) in patients with heart failure. In patients with different NYHA stages or LVEF levels, high HALP scores were correlated with a reduced risk of 1-year mortality in patients with NYHA stage II/III (OR = 0.978, 95% CI: 0.957–1.000, P = 0.045) or LVEF ≥50% (OR = 0.970, 95% CI: 0.945–0.996, P = 0.024). The AUC for HALP, PNI, CRP, and MAGGIC-HF to predict 1-year mortality in patients with heart failure were 0.677 (95% CI: 0.619–0.735), 0.666 (95% CI: 0.608–0.723), 0.638 (95% CI: 0.572–0.704), and 0.654 (95% CI: 0.591–0.717), respectively.
Conclusions
HALP may be a potential marker for predicting mortality in patients with heart failure. Further exploration based on HALP may yield better clinical predictors of prognosis in patients with heart failure.
Introduction
Heart failure is a chronic progressive clinical syndrome caused by structural or functional cardiac abnormalities.1 The clinical syndrome of heart failure is poor oxygen delivery, fatigue with slight physical exertion, fluid retention, shortness of breath, and impaired function of the heart itself, manifesting as diastolic or systolic hypofunction.2 Traditional cardiovascular risk factors such as hypertension, diabetes, obesity, sedentary lifestyle, hyperlipidaemia, and smoking also play an important role in the development of heart failure.3 Heart failure affects more than 64.0 million people worldwide, with an incidence of 1–20 cases per 1000 population and 1-year mortality of 15–30%, while the 5-year mortality rises to 50–75%.4 Therefore, prognostic evaluation is essential for the management of patients with heart failure.
Recently, a new metric, the combination of haemoglobin, albumin, lymphocytes, and platelets (HALP), has been proposed for assessing patient prognosis in many diseases.5, 6 Previous studies supported that a high HALP score was related to a lower risk of mortality in patients with solid cancers or acute ischaemic stroke.5, 6 The components of HALP (haemoglobin, albumin, lymphocytes, and platelets) have also been reported to be associated with prognosis in patients with heart failure. Haemoglobin is an important indicator of prognosis in patients with heart failure.7 Anaemia (lower than normal haemoglobin levels) is a common co-morbidity in patients with heart failure and is associated with an increased risk of hospitalization and death.8 Low lymphocyte counts are associated with a high risk of mortality in patients with heart failure.9 In patients with heart failure, lymphopenia may be associated with inflammation, malnutrition, peripheral congestion, and beta-adrenergic activation.10-15 Mechanisms of peripheral congestion may lead to lymphocyte loss and endotoxin transfer, and endotoxin may induce apoptosis of specific subtypes of lymphocytes, resulting in lymphocytopenia.10-12 Sustained sympathetic-adrenergic activation may result in a decrease in circulating lymphocytes.14, 15 The prevalence of malnutrition in heart failure patients is estimated to be between 15% and 90%, and nutritional status has become an important indicator for assessing prognosis.16 Cheng et al. demonstrated that a high prognostic nutritional index (PNI, calculated by albumin level and lymphocyte count) was independently associated with a lower risk of mortality in patients with heart failure.13 Serum albumin is a nutritionally influenced liver protein. Liu et al. found that low serum albumin (<3.4 g/dL) was an independent predictor of all-cause mortality in patients with heart failure.17 In addition, platelet activation and hypercoagulability are evident in patients with heart failure.18 Platelets play a central role in the development of thrombosis at sites of arterial wall injury. Ruptured plaques or denuded arterial endothelium leads to the exposure of various prothrombotic factors, which promote platelet adhesion, activation, and ultimately the generation of platelet-rich thrombi.18 This may indicate that HALP scores are equally applicable to the assessment of prognosis in patients with heart failure. However, studies on the relationship between HALP and the prognosis of patients with heart failure have been less reported. Only Kocaoglu et al. analysed the effectiveness of the HALP score in predicting 1-week and 3-month mortality in patients with heart failure.19 However, their study may have been limited by a small sample size, unadjusted for confounding by disease history and treatment, and no comparisons with heart failure risk scores used in clinical practice. Therefore, more evidence is needed on the association between HALP and the short- and long-term risk of mortality in heart failure patients.
Thus, the purpose of this study was to assess the relationship between HALP and the short- and long-term risk of mortality in patients with heart failure. The predictive value of HALP for mortality in patients with heart failure was analysed.
Methods
Study design and patients
The admitted patients with heart failure included in this retrospective cohort study were collected from The Affiliated Taizhou People's Hospital of Nanjing Medical University between 2019 and 2021. The diagnostic criteria for heart failure were based on the Chinese guidelines for the diagnosis and treatment of heart failure 2018.20 Specifically, the presence of heart failure is clarified according to the following steps: (1) symptoms (dyspnoea at rest, dyspnoea with mild activity, or peripheral oedema) or signs (tachypnoea, pulmonary congestion with rales, or elevated jugular pressure) of suspected heart failure; (2) an abnormal electrocardiogram or chest X-ray with pulmonary congestion; and (3) natriuretic peptide testing [B-type natriuretic peptide (BNP) ≥ 35 ng/L or N-terminal pro-BNP (NT-proBNP) ≥ 125 ng/L] and ultrasoundcardiogram (structural or functional abnormalities of the heart). Inclusion criteria were as follows: (1) age ≥18 years old; (2) meeting the diagnostic criteria for heart failure; and (3) with complete clinical data on haemoglobin, albumin, lymphocyte, and platelet. Exclusion criteria were as follows: (1) patients with end-stage malignancies; (2) patients receiving immunosuppressive therapy in the 6 months before admission; (3) patients who gave up treatment; and (4) patients with neurological disease and communication disorders. This study was approved by the Ethics Committee of The Affiliated Taizhou People's Hospital of Nanjing Medical University. The need for written informed consent was waived by the Ethics Committee of The Affiliated Taizhou People's Hospital of Nanjing Medical University due to retrospective nature of the study.
Outcomes and definition
The primary outcomes were 1-month mortality and 1-year mortality. The secondary outcomes were 3-month mortality and 6-month mortality. Mortality was defined as a death that occurred within 1 month/3 months/6 months/1 year from the date of patient admission. The maximum follow-up period for patients was 1 year, and patients were followed up by telephone after discharge from the hospital. The HALP was defined as haemoglobin level (g/L) × albumin level (g/L) × lymphocyte count (109/L) /platelet count (109/L).21 The PNI was calculated as albumin level (g/L) + 5 × lymphocyte count (109/L).22 In addition, the Meta-Analysis Global Group in Chronic Heart Failure (MAGGIC-HF) risk score, one of the commonly used heart failure risk scores in clinical practice, was applied.23 The MAGGIC-HF was calculated by summing the contribution of each risk factor for each patient to obtain a total integer value for that patient.23 The parameters used in the HALP score were measured in the same blood sample during the same time period of hospitalization. Fasting blood was collected from patients on the morning of the second day of hospitalization.
Data collection
Clinical data were collected including age (<75 and ≥75 years), sex (female and male), body mass index (BMI, <25 kg/m2 and ≥25 kg/m2), smoking (yes and no), drink alcohol (yes and no), SBP on admission (mmHg), diastolic blood pressure (DBP) on admission (mmHg), heart rate on admission (b.p.m.), respiratory rate on admission (b.p.m.), temperature on admission (°C), SBP at discharge (mmHg), DBP at discharge (mmHg), heart rate at discharge (b.p.m.), respiratory rate at discharge (b.p.m.), temperature at discharge (°C), acute myocardial infarction (yes and no), atrial fibrillation (paroxysmal, chronic, and no), hypertension (yes and no), diabetes mellitus (yes and no), hyperlipidaemia (yes and no), permanent pacemaker (yes and no), anaemia (yes and no), NYHA (II, III and IV), haemoglobin (g/L), albumin (g/L), lymphocytes (109/L), platelets (109/L), total cholesterol (mmol/L), triglyceride (mmol/L), low-density lipoprotein cholesterol (LDL-C) (mmol/L), high-density lipoprotein cholesterol (HDL-C) (mmol/L), alanine transaminase (U/L), aspartate aminotransferase (U/L), C-reactive protein (CRP, mg/L), direct bilirubin (μmol/L), indirect bilirubin (μmol/L), urea nitrogen (mmol/L), serum creatinine (μmol/L), prothrombin time (second), partial thromboplastin time (second), thrombin time (second), fibrinogen (g/L), D-Dimer (mg/L), aspirin (yes and no), clopidogrel (yes and no), ticagrelor (yes and no), rivaroxaban (yes and no), dabigatran (yes and no), statin (yes and no), beta-block (yes and no), ACEI (yes and no), ARBs (yes and no), angiotensin receptor neprilysin inhibitor (ARNI, yes and no), furosemide/tolasemide (yes and no), spironolactone (yes and no), digoxin (yes and no), dapagliflozin (yes and no), antibiotic (yes and no), ventilator use (yes and no), left ventricular ejection fraction (LVEF, %), and length of hospital stay (day). Smoking was defined as having smoked at least 20 packs of cigarettes in a lifetime or at least one cigarette a day for at least 1 year. Drinking was defined as drinking alcohol once a week. Information on the patient's disease history was obtained from medical records.
Statistical analysis
Continuous variables were presented as mean ± standard deviation (SD) or median and quartiles [M (Q1, Q3)], and the t-test or Wilcoxon rank sum test was used for comparison between groups. Categorical variables were expressed as numbers and percentages [n (%)], and the chi-square test was utilized for comparison between groups.
The missing value of the covariates was filled by the ‘PROC MI’ procedure of SAS software. Difference analysis was performed before and after data imputation. Covariates that were statistically significant in the univariable logistic regression analysis were adjusted as confounders (Table S1). The associations of HALP, PNI, CRP, and MAGGIC-HF with risk of mortality (1-month mortality, 3-month mortality, 6-month mortality, and 1-year mortality) in patients with heart failure were assessed by the multivariable logistic regression analysis. Stratified analyses were conducted based on NYHA (II/III, IV) stage and LVEF (<50%, ≥50%). Odds ratio (OR) with 95% confidence intervals (CI) were reported. The area under the receiver operating characteristic (ROC) curve (AUC) was used to evaluate the ability of HALP, PNI, CRP, and MAGGIC-HF in predicting mortality in patients with heart failure. All statistical analyses were performed by SAS 9.4 software (SAS Institute Inc., Cary, NC, USA) and R 4.2.1 software. A value of P < 0.05 was regarded as statistically significant.
Results
Characteristics of patients
There were 756 patients with heart failure collected between 2019 and 2021. A total of 730 patients were included in this study after excluding 26 patients including 7 patients with end-stage malignancies, 14 patients who received immunosuppressive therapy in the 6 months before admission, and 5 patients who gave up treatment. Table 1 presents a comparison of the characteristics of patients who died and survived. Of these patients, 61 (8.36%) died within 1 month, 67 (9.18%) died within 3 months, 71 (9.73%) died within 6 months, and 77 (10.55%) died within 1 year. The median length of hospital stay for patients was 9.00 (6.00, 12.00) days. There were 520 (71.23%) patients aged ≥75 years old and 437 (59.86%) male patients. The distribution of the NYHA was stage II in 166 (22.74%) cases, stage III in 175 (23.97%) cases, and stage IV in 389 (53.29%) cases, and there were no cases of stage I. For the distribution of LVEF, 282 (38.63%) patients had LVEF <50%, 421 (57.67%) patients had LVEF ≥50%, and 27 (3.70%) patients had unknown LVEF. The median CRP level was 4.51 (1.06, 20.40) mg/L. The mean MAGGIC-HF score of the patients was 25.62 ± 6.67. The median HALP and PNI scores of the patients were 39.31 (19.93, 94.21) and 43.98 (39.55, 53.70), respectively.
| Variables | Total (n = 730) | Survivors (n = 653) | Non-survivors (n = 77) | P |
|---|---|---|---|---|
| Length of hospital stay, days, M (Q1, Q3) | 9.00 (6.00, 12.00) | 9.00 (7.00, 12.00) | 7.00 (3.00, 11.00) | 0.002 |
| Age, years, n (%) | 0.102 | |||
| <75 | 210 (28.77) | 194 (29.71) | 16 (20.78) | |
| ≥75 | 520 (71.23) | 459 (70.29) | 61 (79.22) | |
| Sex, n (%) | 0.147 | |||
| Female | 293 (40.14) | 268 (41.04) | 25 (32.47) | |
| Male | 437 (59.86) | 385 (58.96) | 52 (67.53) | |
| Body mass index, kg/m2, n (%) | <0.001 | |||
| <25 | 132 (18.08) | 107 (16.39) | 25 (32.47) | |
| ≥25 | 598 (81.92) | 546 (83.61) | 52 (67.53) | |
| Smoking, n (%) | 0.713 | |||
| No | 497 (68.08) | 446 (68.30) | 51 (66.23) | |
| Yes | 233 (31.92) | 207 (31.70) | 26 (33.77) | |
| Drink alcohol, n (%) | 0.791 | |||
| No | 642 (87.95) | 575 (88.06) | 67 (87.01) | |
| Yes | 88 (12.05) | 78 (11.94) | 10 (12.99) | |
| SBP on admission, mmHg, mean ± SD | 132.22 ± 28.76 | 133.67 ± 28.81 | 120.00 ± 25.42 | <0.001 |
| DBP on admission, mmHg, mean ± SD | 77.59 ± 18.30 | 78.55 ± 18.41 | 69.49 ± 15.14 | <0.001 |
| Heart rate on admission, b.p.m., mean ± SD | 88.18 ± 46.51 | 88.19 ± 48.20 | 88.17 ± 28.60 | 0.997 |
| Respiratory rate on admission, b.p.m., mean ± SD | 21.34 ± 6.75 | 21.05 ± 5.38 | 23.77 ± 13.47 | 0.083 |
| Temperature on admission, °C, mean ± SD | 36.41 ± 1.38 | 36.40 ± 1.44 | 36.52 ± 0.51 | 0.146 |
| SBP at discharge, mmHg, mean ± SD | 112.40 ± 25.08 | 115.49 ± 18.61 | 86.19 ± 47.77 | <0.001 |
| DBP at discharge, mmHg, mean ± SD | 65.95 ± 15.91 | 68.02 ± 12.19 | 48.40 ± 28.34 | <0.001 |
| Heart rate at discharge, b.p.m., mean ± SD | 72.45 ± 18.66 | 73.14 ± 14.30 | 66.66 ± 39.32 | 0.156 |
| Respiratory rate at discharge, b.p.m., mean ± SD | 17.71 ± 4.32 | 18.02 ± 3.20 | 15.04 ± 9.10 | 0.006 |
| Temperature at discharge, °C, mean ± SD | 35.53 ± 5.61 | 36.29 ± 1.87 | 29.11 ± 15.02 | <0.001 |
| Acute myocardial infarction, n (%) | 0.012 | |||
| No | 508 (69.59) | 464 (71.06) | 44 (57.14) | |
| Yes | 222 (30.41) | 189 (28.94) | 33 (42.86) | |
| Atrial fibrillation, n (%) | 0.055 | |||
| No | 451 (61.78) | 410 (62.79) | 41 (53.25) | |
| Paroxysmal | 91 (12.47) | 75 (11.49) | 16 (20.78) | |
| Chronic | 188 (25.75) | 168 (25.73) | 20 (25.97) | |
| Hypertension, n (%) | 0.541 | |||
| No | 289 (39.59) | 261 (39.97) | 28 (36.36) | |
| Yes | 441 (60.41) | 392 (60.03) | 49 (63.64) | |
| Diabetes mellitus, n (%) | 0.946 | |||
| No | 500 (68.49) | 447 (68.45) | 53 (68.83) | |
| Yes | 230 (31.51) | 206 (31.55) | 24 (31.17) | |
| Hyperlipidaemia, n (%) | 0.346 | |||
| No | 682 (93.42) | 612 (93.72) | 70 (90.91) | |
| Yes | 48 (6.58) | 41 (6.28) | 7 (9.09) | |
| Permanent pacemaker, n (%) | 0.263 | |||
| No | 623 (85.34) | 554 (84.84) | 69 (89.61) | |
| Yes | 107 (14.66) | 99 (15.16) | 8 (10.39) | |
| Anaemia, n (%) | 0.879 | |||
| No | 555 (76.03) | 497 (76.11) | 58 (75.32) | |
| Yes | 175 (23.97) | 156 (23.89) | 19 (24.68) | |
| NYHA stage, n (%) | 0.003 | |||
| II | 166 (22.74) | 151 (23.12) | 15 (19.48) | |
| III | 175 (23.97) | 167 (25.57) | 8 (10.39) | |
| IV | 389 (53.29) | 335 (51.30) | 54 (70.13) | |
| Haemoglobin, g/L, mean ± SD | 120.95 ± 24.70 | 120.95 ± 24.52 | 120.90 ± 26.31 | 0.984 |
| Lymphocytes,109/L, M (Q1, Q3) | 1.40 (0.91, 3.40) | 1.45 (0.96, 3.80) | 1.06 (0.58, 1.47) | <0.001 |
| Platelets,109/L, M (Q1, Q3) | 165.50 (125.00, 218.00) | 164.00 (125.00, 217.00) | 173.00 (127.00, 224.00) | 0.477 |
| Total cholesterol, mmol/L, mean ± SD | 3.53 (2.88, 4.27) | 3.55 (2.91, 4.27) | 3.45 (2.77, 4.18) | 0.324 |
| Triglyceride, mmol/L, M (Q1, Q3) | 1.06 (0.78, 1.43) | 1.07 (0.78, 1.43) | 1.01 (0.76, 1.64) | 0.722 |
| HDL-C, mmol/L, M (Q1, Q3) | 0.99 (0.82, 1.19) | 1.00 (0.83, 1.19) | 0.91 (0.78, 1.15) | 0.124 |
| LDL-C, mmol/L, M (Q1, Q3) | 2.14 (1.64, 2.74) | 2.15 (1.68, 2.74) | 2.06 (1.51, 2.67) | 0.150 |
| Alanine transaminase, U/L, M (Q1, Q3) | 23.00 (15.00, 41.00) | 22.00 (15.00, 39.00) | 34.00 (16.00, 73.00) | 0.002 |
| Aspartate aminotransferase, U/L, M (Q1, Q3) | 32.00 (22.00, 66.00) | 31.00 (22.00, 59.00) | 50.00 (26.00, 165.00) | <0.001 |
| Albumin, g/L, mean ± SD | 35.80 (32.80, 38.40) | 35.90 (32.80, 38.60) | 35.00 (31.50, 37.60) | 0.080 |
| Direct bilirubin, μmol/L, M (Q1, Q3) | 3.50 (2.20, 5.70) | 3.50 (2.20, 5.40) | 4.20 (2.40, 7.90) | 0.131 |
| Indirect bilirubin, μmol/L, M (Q1, Q3) | 12.00 (8.50, 18.00) | 12.10 (8.60, 18.00) | 10.10 (7.30, 15.70) | 0.034 |
| Urea nitrogen, mmol/L, M (Q1, Q3) | 8.13 (6.10, 11.22) | 7.83 (5.94, 10.62) | 10.20 (7.90, 15.68) | <0.001 |
| Serum creatinine, μmol/L, M (Q1, Q3) | 86.30 (68.00, 124.20) | 84.00 (67.00, 118.40) | 124.00 (83.40, 187.00) | <0.001 |
| Prothrombin time, second, M (Q1, Q3) | 13.10 (12.20, 14.50) | 13.10 (12.20, 14.40) | 13.60 (12.20, 15.70) | 0.130 |
| Partial thromboplastin time, second, M (Q1, Q3) | 30.20 (27.10, 34.10) | 30.10 (27.10, 34.00) | 30.50 (27.50, 35.40) | 0.620 |
| Thrombin time, second, M (Q1, Q3) | 17.40 (16.50, 18.30) | 17.30 (16.60, 18.30) | 17.40 (16.50, 18.20) | 0.745 |
| Fibrinogen, g/L, M (Q1, Q3) | 3.14 (2.50, 3.92) | 3.13 (2.50, 3.90) | 3.23 (2.49, 4.05) | 0.627 |
| D-Dimer, mg/L, M (Q1, Q3) | 71.28 (1.23, 1030.00) | 20.04 (1.14, 940.00) | 550.00 (3.40, 2840.00) | <0.001 |
| Aspirin, n (%) | 0.047 | |||
| No | 437 (59.86) | 399 (61.10) | 38 (49.35) | |
| Yes | 293 (40.14) | 254 (38.90) | 39 (50.65) | |
| Clopidogrel, n (%) | <0.001 | |||
| No | 400 (54.79) | 372 (56.97) | 28 (36.36) | |
| Yes | 330 (45.21) | 281 (43.03) | 49 (63.64) | |
| Ticagrelor, n (%) | 0.098 | |||
| No | 628 (86.03) | 557 (85.30) | 71 (92.21) | |
| Yes | 102 (13.97) | 96 (14.70) | 6 (7.79) | |
| Rivaroxaban, n (%) | <0.001 | |||
| No | 589 (80.68) | 515 (78.87) | 74 (96.10) | |
| Yes | 141 (19.32) | 138 (21.13) | 3 (3.90) | |
| Dabigatran, n (%) | <0.001 | |||
| No | 571 (78.22) | 499 (76.42) | 72 (93.51) | |
| Yes | 159 (21.78) | 154 (23.58) | 5 (6.49) | |
| Statin, n (%) | 0.014 | |||
| No | 314 (43.01) | 291 (44.56) | 23 (29.87) | |
| Yes | 416 (56.99) | 362 (55.44) | 54 (70.13) | |
| Beta-block, n (%) | 0.395 | |||
| No | 308 (42.19) | 279 (42.73) | 29 (37.66) | |
| Yes | 422 (57.81) | 374 (57.27) | 48 (62.34) | |
| ACEI, n (%) | 0.097 | |||
| No | 572 (78.36) | 506 (77.49) | 66 (85.71) | |
| Yes | 158 (21.64) | 147 (22.51) | 11 (14.29) | |
| ARB, n (%) | <0.001 | |||
| No | 539 (73.84) | 468 (71.67) | 71 (92.21) | |
| Yes | 191 (26.16) | 185 (28.33) | 6 (7.79) | |
| ARNI, n (%) | <0.001 | |||
| No | 300 (41.10) | 250 (38.28) | 50 (64.94) | |
| Yes | 430 (58.90) | 403 (61.72) | 27 (35.06) | |
| Furosemide/tolasemide, n (%) | 0.019 | |||
| No | 139 (19.04) | 132 (20.21) | 7 (9.09) | |
| Yes | 591 (80.96) | 521 (79.79) | 70 (90.91) | |
| Spironolactone, n (%) | 0.565 | |||
| No | 458 (62.74) | 412 (63.09) | 46 (59.74) | |
| Yes | 272 (37.26) | 241 (36.91) | 31 (40.26) | |
| Digoxin, n (%) | 0.044 | |||
| No | 376 (51.51) | 328 (50.23) | 48 (62.34) | |
| Yes | 354 (48.49) | 325 (49.77) | 29 (37.66) | |
| Dapagliflozin, n (%) | 0.237 | |||
| No | 604 (82.74) | 544 (83.31) | 60 (77.92) | |
| Yes | 126 (17.26) | 109 (16.69) | 17 (22.08) | |
| Antibiotic, n (%) | 0.815 | |||
| No | 322 (44.11) | 289 (44.26) | 33 (42.86) | |
| Yes | 408 (55.89) | 364 (55.74) | 44 (57.14) | |
| Ventilator use, n (%) | <0.001 | |||
| No | 617 (84.52) | 582 (89.13) | 35 (45.45) | |
| Yes | 113 (15.48) | 71 (10.87) | 42 (54.55) | |
| LVEF, % | 0.011 | |||
| <50% | 282 (38.63) | 241 (36.91) | 41 (53.25) | |
| ≥50% | 421 (57.67) | 389 (59.57) | 32 (41.56) | |
| Unknown | 27 (3.70) | 23 (3.52) | 4 (5.19) | |
| PNI, M (Q1, Q3) | 43.98 (39.55, 53.70) | 44.50 (39.80, 56.95) | 40.45 (36.25, 44.70) | <0.001 |
| CRP, mg/L, M (Q1, Q3) | 4.51 (1.06, 20.40) | 3.95 (0.98, 17.33) | 12.79 (2.61, 44.27) | <0.001 |
| MAGGIC-HF, mean ± SD | 25.62 ± 6.67 | 25.22 ± 6.60 | 29.00 ± 6.32 | <0.001 |
| HALP, M (Q1, Q3) | 39.31 (19.93, 94.21) | 41.02 (21.67, 110.48) | 23.73 (12.83, 44.15) | <0.001 |
- ACEI, angiotensin converting enzyme inhibitors; ARB, angiotensin receptor blocker; ARNI, angiotensin receptor neprilysin inhibitor; CRP, C-reactive protein; DBP, diastolic blood pressure; HALP, haemoglobin, albumin, lymphocytes, and platelets; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; LVEF, left ventricular ejection fraction; MAGGIC-HF, Meta-Analysis Global Group in Chronic Heart Failure risk score; NYHA, New York Heart Association functional classification; PNI, prognostic nutritional index; SBP, systolic blood pressure.
Association between haemoglobin, albumin, lymphocytes, and platelets and mortality
Table 2 shows the association of HALP, PNI, CRP, and MAGGIC-HF with mortality in patients with heart failure. Both high HALP (OR = 0.989, 95% CI: 0.982–0.996) and PNI (OR = 0.981, 95% CI: 0.966–0.996) scores were correlated with a reduced risk of 1-month mortality in patients with heart failure, whereas high CRP levels (OR = 1.008, 95% CI: 1.004–1.013) and MAGGIC-HF (OR = 1.092, 95% CI: 1.046–1.140) scores were associated with an increased risk of 1-month mortality. The above results were also observed in the association of HALP, PNI, CRP, and MAGGIC-HF with 3-month mortality, 6-month mortality, and 1-year mortality in patients with heart failure (P < 0.05). After adjustment for confounders, only high HALP scores were still associated with a reduced risk of 1-month mortality (OR = 0.978, 95% CI: 0.963–0.992), 3-month mortality (OR = 0.983, 95% CI: 0.971–0.995), 6-month mortality (OR = 0.983, 95% CI: 0.972–0.995), and 1-year mortality (OR = 0.987, 95% CI: 0.977–0.997) in patients with heart failure.
| Variables | Univariable model | Multivariable model | ||
|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | |
| 1-month mortality | ||||
| HALP | 0.989 (0.982–0.996) | 0.004 | 0.978 (0.963–0.992) | 0.003 |
| CRP | 1.008 (1.004–1.013) | <0.001 | 1.007 (0.998–1.016) | 0.107 |
| PNI | 0.981 (0.966–0.996) | 0.015 | 0.988 (0.971–1.006) | 0.202 |
| MAGGIC-HF | 1.092 (1.046–1.140) | <0.001 | 1.022 (0.961–1.087) | 0.481 |
| 3-month mortality | ||||
| HALP | 0.989 (0.983–0.996) | 0.003 | 0.983 (0.971–0.995) | 0.006 |
| CRP | 1.008 (1.004–1.012) | <0.001 | 1.004 (0.996–1.012) | 0.313 |
| PNI | 0.981 (0.967–0.996) | 0.011 | 0.992 (0.976–1.008) | 0.308 |
| MAGGIC-HF | 1.089 (1.046–1.135) | <0.001 | 1.024 (0.968–1.083) | 0.408 |
| 6-month mortality | ||||
| HALP | 0.989 (0.983–0.996) | 0.002 | 0.983 (0.972–0.995) | 0.005 |
| CRP | 1.007 (1.003–1.012) | <0.001 | 1.003 (0.995–1.010) | 0.494 |
| PNI | 0.980 (0.966–0.995) | 0.007 | 0.989 (0.975–1.004) | 0.161 |
| MAGGIC-HF | 1.086 (1.044–1.131) | <0.001 | 1.025 (0.972–1.081) | 0.365 |
| 1-year mortality | ||||
| HALP | 0.989 (0.983–0.996) | 0.001 | 0.987 (0.977–0.997) | 0.009 |
| CRP | 1.007 (1.002–1.011) | 0.002 | 1.001 (0.994–1.009) | 0.713 |
| PNI | 0.979 (0.965–0.993) | 0.004 | 0.991 (0.977–1.005) | 0.215 |
| MAGGIC-HF | 1.097 (1.055–1.140) | <0.001 | 1.045 (0.993–1.100) | 0.094 |
- Multivariable model of HALP, CRP, and PNI adjusted for BMI, SBP (admission and discharge), DBP (admission and discharge), respiratory rate (admission and discharge), temperature on admission, heart rate at discharge, acute myocardial infarction, atrial fibrillation, direct bilirubin, serum creatinine, D-Dimer, aspirin, clopidogrel, dabigatran, statin, ARB, ARNI, furosemide/tolasemide, digoxin, and ventilator use. Multivariable model of MAGGIC-HF adjusted for SBP (discharge), DBP (admission and discharge), respiratory rate (admission and discharge), temperature on admission, heart rate at discharge, acute myocardial infarction, atrial fibrillation, direct bilirubin, D-Dimer, aspirin, clopidogrel, dabigatran, statin, ARNI, furosemide/tolasemide, digoxin, and ventilator use.
- CI, confidence interval; CRP, C-reactive protein; HALP, haemoglobin, albumin, lymphocytes, and platelets; MAGGIC-HF, Meta-Analysis Global Group in Chronic Heart Failure risk score; OR, odds ratio; PNI, prognostic nutritional index.
The association between HALP and mortality was stratified by NYHA (II/III, IV) and LVEF (<50%, ≥50%) (Table 3). In patients with different NYHA stages, high HALP scores may be correlated with a reduced risk of 6-month mortality (OR = 0.978, 95% CI: 0.957–1.000, P = 0.045) and 1-year mortality (0.978, 95% CI: 0.957–1.000, P = 0.045) in patients with stage II/III, and high HALP scores were associated with a reduced risk of 1-month mortality (OR = 0.952, 95% CI: 0.912–0.994) in patients with stage IV. Among patients with different LVEFs, high HALP scores were correlated with a reduced risk of 1-month mortality (OR = 0.956, 95% CI: 0.921–0.993), 3-month mortality (OR = 0.960, 95% CI: 0.931–0.990), 6-month mortality (OR = 0.964, 95% CI: 0.936–0.992), and 1-year mortality (OR = 0.970, 95% CI: 0.945–0.996) only in patients with LVEF ≥50%, but not in patients with LVEF <50% (P > 0.05).
| Populations | Outcomes | Univariable model | Multivariable model | ||
|---|---|---|---|---|---|
| OR (95% CI) | P | OR (95% CI) | P | ||
| NYHA stage II/III | 1-month mortality | 0.985 (0.968–1.003) | 0.103 | 0.818 (0.622–1.075) | 0.149 |
| 3-month mortality | 0.986 (0.971–1.002) | 0.087 | 0.985 (0.953–1.019) | 0.394 | |
| 6-month mortality | 0.985 (0.969–1.001) | 0.064 | 0.978 (0.957–1.000) | 0.045 | |
| 1-year mortality | 0.985 (0.969–1.001) | 0.064 | 0.978 (0.957–1.000) | 0.045 | |
| NYHA stage IV | 1-month mortality | 0.987 (0.978–0.997) | 0.008 | 0.952 (0.912–0.994) | 0.025 |
| 3-month mortality | 0.989 (0.980–0.997) | 0.007 | 0.987 (0.969–1.004) | 0.135 | |
| 6-month mortality | 0.989 (0.981–0.997) | 0.006 | 0.986 (0.968–1.003) | 0.109 | |
| 1-year mortality | 0.989 (0.982–0.996) | 0.004 | 0.990 (0.976–1.003) | 0.127 | |
| LVEF <50% | 1-month mortality | 0.989 (0.978–0.999) | 0.035 | 0.985 (0.964–1.006) | 0.154 |
| 3-month mortality | 0.990 (0.981–0.999) | 0.029 | 0.992 (0.981–1.004) | 0.197 | |
| 6-month mortality | 0.990 (0.981–0.998) | 0.021 | 0.991 (0.979–1.003) | 0.135 | |
| 1-year mortality | 0.990 (0.982–0.998) | 0.018 | 0.992 (0.982–1.002) | 0.132 | |
| LVEF ≥50% | 1-month mortality | 0.984 (0.969–0.999) | 0.040 | 0.956 (0.921–0.993) | 0.019 |
| 3-month mortality | 0.984 (0.969–0.999) | 0.036 | 0.960 (0.931–0.990) | 0.010 | |
| 6-month mortality | 0.984 (0.969–0.999) | 0.033 | 0.964 (0.936–0.992) | 0.011 | |
| 1-year mortality | 0.984 (0.970–0.998) | 0.025 | 0.970 (0.945–0.996) | 0.024 | |
- CI, confidence interval; HALP, haemoglobin, albumin, lymphocytes, and platelets; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association functional classification; OR, odds ratio.
- Multivariable model adjusted for BMI, SBP (admission and discharge), DBP (admission and discharge), respiratory rate (admission and discharge), temperature on admission, heart rate at discharge, acute myocardial infarction, atrial fibrillation, direct bilirubin, serum creatinine, D-Dimer, aspirin, clopidogrel, dabigatran, statin, ARB, ARNI, furosemide/tolasemide, digoxin, and ventilator use.
Predictive ability of haemoglobin, albumin, lymphocytes, and platelets for mortality in patients with heart failure
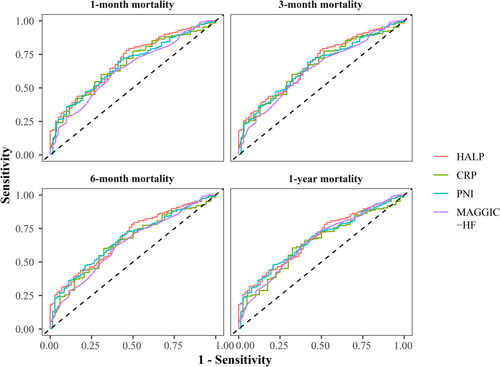
Table 4 presents the AUC values of HALP, PNI, CRP, and MAGGIC-HF in predicting 1-month mortality, 3-month mortality, 6-month mortality, and 1-year mortality in patients with heart failure. The AUC values of HALP, PNI, CRP, and MAGGIC-HF in predicting 1-month mortality in patients with heart failure were 0.699 (95% CI: 0.634–0.764), 0.682 (95% CI: 0.618–0.747), 0.668 (95% CI: 0.594–0.741), and 0.643 (95% CI: 0.574–0.713), respectively. The AUC values for HALP, PNI, CRP, and MAGGIC-HF to predict 1-year mortality in patients with heart failure were 0.677 (95% CI: 0.619–0.735), 0.666 (95% CI: 0.608–0.723), 0.638 (95% CI: 0.572–0.704), and 0.654 (95% CI: 0.591–0.717), respectively. Similarly, HALP had a slightly higher AUC values than PNI, CRP, and MAGGIC-HF for predicting 3-month mortality (0.685 vs. 0.665 vs. 0.653 vs. 0.641) and 6-month mortality (0.684 vs. 0.670 vs. 0.643 vs. 0.637). The ROC curve of the HALP, PNI, CRP, and MAGGIC-HF for predicting 1-month mortality, 3-month mortality, 6-month mortality, and 1-year mortality in patients with heart failure was shown in Figure 1.
| Outcomes | Variable | AUC (95% CI) |
|---|---|---|
| 1-month mortality | HALP | 0.699 (0.634–0.764) |
| CRP | 0.668 (0.594–0.741) | |
| PNI | 0.682 (0.618–0.747) | |
| MAGGIC-HF | 0.643 (0.574–0.713) | |
| 3-month mortality | HALP | 0.685 (0.622–0.747) |
| CRP | 0.653 (0.582–0.724) | |
| PNI | 0.665 (0.603–0.728) | |
| MAGGIC-HF | 0.641 (0.574–0.707) | |
| 6-month mortality | HALP | 0.684 (0.624–0.745) |
| CRP | 0.643 (0.574–0.713) | |
| PNI | 0.670 (0.609–0.73) | |
| MAGGIC-HF | 0.637 (0.571–0.702) | |
| 1-year mortality | HALP | 0.677 (0.619–0.735) |
| CRP | 0.638 (0.572–0.704) | |
| PNI | 0.666 (0.608–0.723) | |
| MAGGIC-HF | 0.654 (0.591–0.717) |
- AUC, the area under the receiver operating characteristic curve; CRP, C-reactive protein; HALP, haemoglobin, albumin, lymphocytes, and platelets; PNI, prognostic nutritional index.
In addition, the association between HALP components and mortality in patients with heart failure and the predictive effect of HALP components on mortality were analysed. In multivariable analyses, no associations were found between haemoglobin, albumin, lymphocytes, platelets, and mortality (1-month, 3-month, 6-month, and 1-year mortality) in patients with heart failure (Table S2). Lymphocytes had the higher AUC values than haemoglobin, albumin, and platelets for predicting 1-month mortality (0.629 vs. 0.509 vs. 0.555 vs. 0.500) and 1-year mortality (0.591 vs. 0.512 vs. 0.559 vs. 0.526) (Table S3).
Discussion
This study evaluated the association between the new index HALP and mortality in patients with heart failure. HALP is a combined indicator of response to inflammation and nutritional status. Our results found that high HALP scores were associated with a reduced risk of 1-month mortality, 3-month mortality, 6-month mortality, and 1-year mortality in patients with heart failure. HALP has a predictive value for short- and long-term mortality in heart failure patients, with an AUC of 0.699 and 0.677 for 1-month mortality and 1-year mortality prediction, respectively.
Previous studies have reported an association between HALP and other diseases.5, 6 Tian et al. reported that acute ischaemic stroke patients with high HALP scores had a lower risk of adverse outcomes at 90 days and 1 year.6 HALP is calculated from haemoglobin, albumin, lymphocytes, and platelets and reflects the patient's malnutrition, immune response, and prognosis. Previous studies have analysed the associations between haemoglobin,24 albumin,25 lymphocytes,26 and platelet levels27 and patients with heart failure. Our study investigated the relationship between combined index HALP and short- and long-term mortality in patients with heart failure. Our study found that high HALP was associated with a reduced risk of 1-month mortality and 1-year mortality in patients with heart failure, which was consistent with previous studies.19 Compared with previous studies,19 our study had a larger sample, adjusted for the effects of disease history and treatment, and was compared with the clinically used MAGGIC-HF score. Our study compared the predictive ability of HALP with the inflammatory index CRP, the prognostic nutritional index PNI, and the commonly used clinical score MAGGIC-HF for mortality in heart failure patients. The result showed that HALP had a slightly higher AUC than PNI, CRP, and MAGGIC-HF for predicting 1-month mortality and 1-year mortality. Both CRP and PNI have been reported to be associated with mortality in patients with heart failure.13, 28 Moreover, other inflammatory markers such as neutrophil-lymphocyte ratio (NLR) and platelet-lymphocyte ratio (PLR) have been reported to be correlated with prognosis in patients with heart failure,29, 30 but the predictive effect of these markers is limited.19 More simple indicators associated with prognosis in heart failure patients may need to be explored in the future.
Heart failure is a chronic progressive clinical syndrome caused by structural or functional cardiac abnormalities. Possible pathogenetic mechanisms of heart failure includes increased haemodynamic load, ischaemia-related dysfunction, ventricular remodelling, the excessive neurohumoral stimulation, abnormal myocyte calcium cycling, excessive or inadequate proliferation of the extracellular matrix, accelerated apoptosis, and genetic mutations.1, 31, 32 Systemic inflammation has an important role in the pathogenetic progression of heart failure.33 Inflammation contributes to the pathogenesis of heart failure through multiple mechanistic pathways involving the release of pro-inflammatory cytokines, activation of the innate and humoral immune systems, endothelial inflammation, and inflammatory mediators produced by the gastrointestinal tract, spleen, and adipose tissue.33 Specifically, the haemodynamic stress of heart failure triggers the release of a series of pro-inflammatory cytokines that compromise the cardiac structure and function and induce skeletal muscle breakdown.33 The HALP score contains indicators of immune response and nutritional status. Inflammation is involved in the pathogenesis of heart failure patients while nutritional status has an important role in the prognosis of patients. This may explain the ability of the HALP score to predict the prognosis of patients with heart failure. However, the AUC of HALP in our study for predicting 1-month mortality and 1-year mortality in heart failure patients was 0.699 and 0.677, respectively, suggesting that HALP alone may have limited predictive ability for prognosis in heart failure patients. Future studies may need to combine HALP with other indicators such as LVEF, NT-proBNP, and high-sensitivity troponin.
This study explored the relationship between HALP and the risk of mortality in patients with heart failure. Then, the predictive ability of HALP, PNI, CRP, and MAGGIC-HF for mortality in heart failure patients was compared. These results may provide additional evidence for the relationship between HALP and mortality in patients with heart failure. However, our study has several limitations. First, this study was a single-centre study that may have selection bias. Stronger evidence from multicentre studies is needed. Second, the data for the calculation of HALP in this study were derived from a single measurement, and the effect of dynamic changes in patient HALP score during hospitalization on mortality is unknown. Third, we only analysed 1-year mortality due to data limitations, while epidemiological studies have shown up to 75% of 5-year mortality in patients with heart failure. Further studies may need to analyse the predictive value of HALP for 5-year mortality in heart failure patients.
Our results found that high HALP was associated with a reduced risk of 1-month mortality and 1-year mortality in patients with heart failure. HALP has a slightly higher predictive ability than PNI, CRP, and MAGGIC-HF for mortality in heart failure patients. HALP may be a potential marker for predicting mortality in patients with heart failure. Future studies may need to explore the impact of the dynamic trajectory of HALP on longer-term mortality in patients with heart failure.
Funding
This study was supported by the Scientific Research Project of Jiangsu Provincial Health Commission (No. M2022075).
Conflict of interest
None declared.




