Is ischaemic heart failure an autoimmune disease?
Inflammation and autoimmunity have been implicated beyond doubt in the pathology of myocarditis and inflammatory cardiomyopathy.1, 2 Increasing evidence now also points towards the existence of a self-perpetuating cycle of autoimmunity and inflammation (Figure 1) in post-ischaemic heart failure. This vicious cycle starts with tissue damage in the myocardium causing acute inflammation, which may—in genetically or environmentally susceptible individuals—develop into an autoimmune response, triggering more, now autoimmune-mediated, damage. Chronic myocardial inflammation as manifestation of established anti-heart autoimmunity then contributes to the progression of heart failure through broad immune-dysregulation, local inflammation and fibrosis, remodelling, and structural changes.3
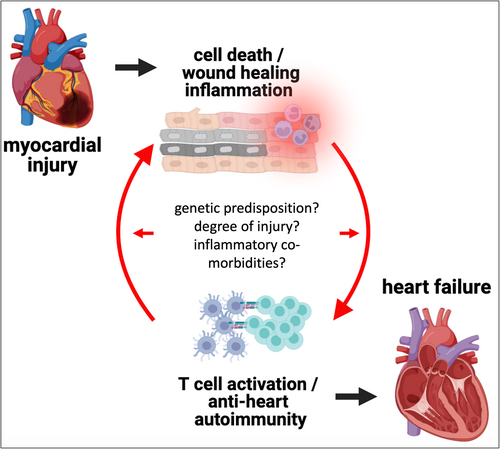
Source: Image created with BioRender.com.
Massive necrotic cell death after an acute myocardial infarction (AMI) provides an obvious inflammatory insult in heart failure with reduced ejection fraction (HFrEF). While such a definite trigger is difficult to identify in heart failure with preserved ejection fraction, cardiomyocyte cell death still occurs, as indicated by raised high-sensitivity troponin T levels.4 Necrosis releases cellular constituents, some of them inflammatory in themselves (‘alarmins’), and others carrying antigenic peptides capable of inducing antigen-specific autoimmune responses. While in hindsight a lot of signs have pointed towards an autoimmune component of post-ischaemic HFrEF for a long time, this has gathered attention only recently and begs the question ‘Should we be looking at post-ischaemic HFrEF through the eyes of an immunologist?’
To attempt an answer, we first need to agree on the definition of autoimmunity and autoimmune disease. Tolerance against self-antigens is normally ensured through tightly controlled selection processes during T cell development (‘central tolerance’) and ‘peripheral tolerance’ mechanisms for T cell clones escaping central tolerance. Yet, central tolerance against, for example, cardiac myosin is missing.5 Consequently, if peripheral tolerance mechanisms fail, get interrupted on purpose (e.g. through immune-checkpoint inhibitor cancer therapy), or are overwhelmed by excessive inflammation (e.g. after severe tissue damage due to MI), cardiac myosin-specific T cells can be activated to attack cardiomyocytes. Due to ‘epitope spreading’, auto-reactive T cells will subsequently develop against a wide range of other cardiac antigens.
To label a disease as autoimmune, a set of criteria (Rose–Witebsky's postulates) have been suggested6: (1) auto-antibodies must be detectable in all cases of the disease; (2) the disease must be experimentally reproducible by immunization with the antigen; (3) experimental disease must present with immunopathological lesions comparable to the original disease in humans; and (4) the disease phenotype must be transferable by serum or lymphoid cells.
- Auto-antibodies must be detectable in all cases of the disease.
A wealth of data exists on the presence and potential role of anti-heart auto-antibodies in various forms of heart failure, unsurprisingly in particular in heart failure of infectious or inflammatory origin.1 However, auto-antibodies are also found in patients with ischaemic HFrEF, as well as rodent models of experimental MI.7, 8 The most prominent, and receiving renewed attention recently, are anti-myosin antibodies.9 Due to the lack of central tolerance to cardiac myosin,5 its release from dying cardiomyocytes in large quantities can activate T cells, which induce B cell maturation and antibody production. However, several other cardiomyocyte auto-antibody targets have been identified with a range of effects, including cardiac troponin,10 or β1-adrenergic receptors.11 Activating auto-antibodies against β-adrenergic receptors exist in approximately 10% of patients with ischaemic cardiomyopathy and are associated with reduced cardiac function.12 Ongoing clinical trials aim at B cell depletion to prevent left ventricular dysfunction and cardiac remodelling after AMI.13 Importantly, while many studies understandably assess auto-antibodies in the circulation, their pathological roles likely play out in the myocardium,8 where they may be able to cause functional effects or trigger complement- and/or cell-mediated cytotoxicity, a classical phenomenon of established autoimmune-mediated tissue injury. The presence of auto-reactive T cells14 and the enlargement of heart-draining lymph nodes after AMI and during heart failure8 are additional indirect indicators of auto-antibody production.
- The disease must be experimentally reproducible by immunization with the antigen.
Here again, we need to return to cardiac myosin. Immunization with cardiac myosin provokes an anti-heart immune response that leads to myocardial infiltration, inflammation, and injury, and is thus used as a standard animal model of myocarditis [experimental autoimmune myocarditis (EAM)]. Cardiac functional defects develop downstream of acute myocarditis just as they do in some human patients.15 While the goal of inducing EAM generally is a severe clinically manifest myocarditis, prolonged milder treatment is likely to lead to initially subclinical cardiac inflammation causing functional effects over time.
Animal models of ischaemic heart failure, largely induced through AMI surgeries, develop anti-heart auto-antibodies and myocardial inflammation8 entirely without active immunization. In this case, the AMI itself provides all necessary components of immunization. Immunization in a nutshell relies on antigenic peptides in combination with an adjuvant to activate the immune response. An AMI provides both; (1) plenty of the antigenic peptide released from necrotic cardiomyocytes16 and (2) a highly inflammatory environment caused by tissue damage as endogenous adjuvant.17
- Experimental disease must present with immunopathological lesions comparable to the original disease in humans.
- The disease phenotype must be transferable by serum or lymphoid cells.
Autoimmune diseases can be studied by transferring immune cells or serum from diseased individuals to healthy recipients. If the transfer causes disease manifestations in the recipient, autoimmune mechanisms are at play. Myocarditis can be induced in experimental animals through adoptive transfer of serum or lymphocytes from animals with existing myocarditis.15 Most importantly, lymphocytes isolated from mice with ischaemic heart disease are able to induce a cardiac phenotype in healthy recipient rats and mice, including inflammatory infiltration,20 fibrosis, and functional decline.21
Clearly there are very few studies that directly tested this postulate, but these do indicate transferability of disease phenotype, which allows us to carefully consider this postulate as fulfilled. Signs of autoimmunity are thus widely detectable in HFrEF, and a pathological role of auto-reactivity against the myocardium is feasible. However, whether autoimmunity is indeed causative, and the actual degree of its contribution to post-ischaemic decline of cardiac function remains to be fully elucidated. The difficulty with defining an autoimmune aetiology of heart failure underscores yet again that heart failure is simply not a uniform condition,2 and development of post-ischaemic autoimmunity likely also depends on several factors, including genetic predisposition to autoimmunity, the severity of the initial tissue damage, and co-morbidities exacerbating inflammation (Figure 1). These too are phenomena often debated for classical autoimmune diseases. Another clear hint towards a critical overlap in underlying mechanisms between autoimmune diseases and heart failure is the recent successes in using anti-inflammatory drugs in heart disease.2 Therefore, while it might still be too early to globally call all ischaemic heart failure an autoimmune disease, a more inter-disciplinary approach, including the immunologist's perspective, is certainly warranted.
Conflict of interest
None declared.




