Urea nitrogen-to-albumin ratio predicts ventricular aneurysm formation in ST-segment elevation myocardial infarction
Abstract
Aims
Left ventricular aneurysm (LVA) is an important complication of acute myocardial infarction. The aim of this study was to investigate the possible predictive value of blood urea nitrogen-to-albumin ratio (BAR) for the LVA formation in acute ST-segment elevation myocardial infarction (STEMI) patients who underwent primary percutaneous coronary intervention (PCI).
Methods and results
A total of 1123 consecutive patients with STEMI were prospectively enrolled. The clinical and laboratory data were compared between LVA group and non-LVA group. Multivariable logistic regression analysis was performed to assess the independent risk factors of LVA formation. Predictive power of BAR and composite variable for LVA formation were assessed using receiver operating characteristic curve. LVA was detected in 162 patients (14.4%). The BAR was significantly higher in patients with LVA [0.16 (0.13–0.19) vs. 0.13 (0.10–0.17), P < 0.001]. Multivariable logistic regression analysis revealed that left ventricular ejection fraction (LVEF) [odds ratio (OR) = 0.865, P < 0.001], culprit vessel–left anterior descending artery (LAD) (OR = 4.705, P < 0.001), and BAR (OR = 2.208, P = 0.018) were all independent predictors for LVA formation. The predictive value of BAR remained significant even after cross-validation by splitting population into training set (OR = 1.957, P = 0.034) and validation set (OR = 1.982, P = 0.039). The maximal length and width of LVA were significantly increased in patients with BAR ≥ 0.15 when compared with BAR < 0.15 (3.37 ± 1.09 vs. 2.92 ± 0.93, P = 0.01, for maximal length, and 2.20 ± 0.55 vs. 1.85 ± 0.63, P = 0.001, for maximal width). The discriminant power of BAR for LVA is 0.723, which is superior to both blood urea nitrogen (C statistic = 0.586, P < 0.001) and albumin (C statistic = 0.64, P < 0.001). The combination of BAR, LVEF, and culprit vessel–LAD could significantly increase the predictive ability (C statistic = 0.874, P < 0.001, for vs. BAR). Subgroup analysis of age, sex, hypertension, diabetes, smoking, LVEF, serum albumin, multiple-vessel disease, and Gensini score had no effect on the association between BAR and risk of LVA formation (P < 0.05 for all subgroups).
Conclusions
A higher BAR was an independent predictor for LVA formation in STEMI patients with primary PCI.
Introduction
Left ventricular aneurysm (LVA), characterized by the outward expansion of the infarcted myocardium during systole and diastole,1 is a prevalent and severe complication of acute myocardial infarction (AMI).2, 3 Numerous studies have demonstrated that LVA can increase the risk of arrhythmias,4 thrombo-embolic phenomena,4 heart failure (HF),5 and cardiac rupture6 and ultimately lead to a poor prognosis for AMI patients.7 Given the high incidence rate (10–38% of AMI patients)8 and the catastrophic complications associated with LVA, it is imperative to identify the risk factors contributing to its formation, which is crucial for early prevention and treatment.
Blood urea nitrogen (BUN) is the major product of protein metabolism in the human body and is primarily eliminated by the kidney.9 BUN has been identified as a biomarker for renal function and was linked to the prognosis of various diseases, such as acute aortic dissection,10 pancreatitis,11 and acute intracerebral haemorrhage.12 Recently, there has been growing attention towards the role of BUN in predicting cardiovascular events. A cohort study involving 353 HF patients found that persistently high BUN levels during hospitalization were associated with an increased risk of cardiovascular death and HF readmission.13 Additionally, a study conducted by Yano et al. demonstrated that higher BUN levels were independently associated with an increased risk of all-cause death and HF readmission.14
Serum albumin (ALB), synthesized by the liver as preproalbumin and converted to proalbumin in the lumen of the endoplasmic reticulum of hepatocytes, is the most abundant circulating protein.15 It is widely recognized that ALB reflects the nutritional status of the body.16 Additionally, ALB possesses anti-inflammatory, antioxidant, anticoagulant, anti-platelet aggregation, colloid osmotic pressure maintenance, and transport properties for various endogenous and exogenous substances.17-20 Numerous studies have demonstrated a strong association between ALB and cardiovascular diseases (CVDs). Low serum ALB in the remote phase after AMI was shown to be a valuable predictor of long-term adverse outcomes.21 In a cohort of 1726 systolic HF patients, hypoalbuminaemia was independently associated with an increased risk of death.22 Similarly, Liu et al. also identified hypoalbuminaemia as an independent predictor of death in patients with HF with preserved ejection fraction.23
The BUN-to-ALB ratio (BAR), which is the mathematical ratio of BUN and ALB, has been identified as a novel prognostic factor associated with various diseases, such as ST-elevation myocardial infarction,24 pulmonary embolism,25 sepsis,9 and lung cancer.26 However, no study has been conducted to investigate the association between BAR and LVA formation. In this study, we aimed to assess the predictive value of BAR for the risk of LVA formation in the Chinese population.
Methods
Study population
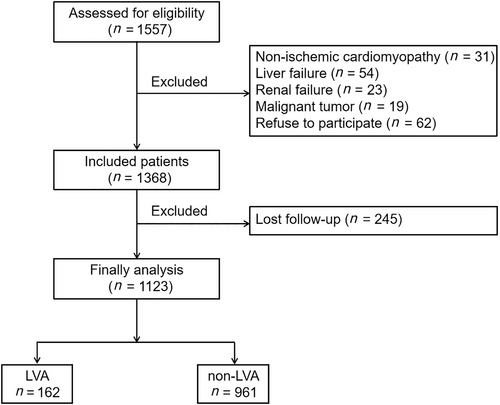
A total of 1557 consecutive acute ST-segment elevation myocardial infarction (STEMI) patients who underwent primary percutaneous coronary intervention (PPCI) from 2018 to 2023 in Central China Fuwai Hospital were enrolled in our study. Acute STEMI was defined according to the fourth universal definition of myocardial infarction27: typical chest pain lasting longer than 30 min, with new ST-segment elevation at the J point in at least two contiguous leads of >2 mm (0.2 mV) in men or >1.5 mm (0.15 mV) in women on admission electrocardiogram, and an increase in cardiac enzyme levels, which was defined as being above the 99th percentile cut-off point for cardiac troponin I (cTnI) or creatine kinase–myocardial band isoenzyme (CK-MB). Patients were excluded if they had non-ischaemic cardiomyopathy (including hypertrophic and dilated cardiomyopathy), congenital heart disease, liver or renal failure, malignant tumours or a life expectancy <1 year, and active infection, received thrombolytic therapy prior to admission, or were lost to follow-up. Finally, 1123 patients were included in our study (Figure 1). The study complied with the principles of the Declaration of Helsinki and received approval from the Review Board of Central China Fuwai Hospital. All participants provided informed consent.
Percutaneous coronary intervention procedure and definition
Prior to PPCI, all patients received aspirin (300 mg loading dose followed by 100 mg daily), clopidogrel (600 mg loading dose followed by 75 mg daily), or ticagrelor (180 mg loading dose followed by 90 mg twice daily). Additionally, an intravenous bolus of unfractionated heparin (UFH) was administered at a dose of 70 U/kg of body weight. The PPCI was performed using either the standard radial or femoral approach, in line with current guideline recommendations. A stent was deployed in the culprit artery of all patients. During the percutaneous coronary intervention (PCI) procedure, the operator had the discretion to decide on the use of balloon before or after dilatation, the type of stents (bare metal or drug-eluting), and the application of thrombus aspiration. The glycoprotein IIb/IIIa receptor inhibitor tirofiban was initiated during the PCI procedure, as per the operator's judgement, with a 10 μg/kg intracoronary bolus followed by a 0.15 μg/kg/min intravenous infusion. A residual stenosis of <10% in the culprit lesion after procedure was considered indicative of a technically successful stent implantation. The results of coronary angiograms, as well as the extent and degree of stenosis of each major coronary artery vessel, were assessed and confirmed by two independent experts. Upon discharge, medical therapy was prescribed based on the patient's individual condition and guideline recommendations for secondary prevention.
Definitions
The diagnosis of LVA was made in accordance with the protocol outlined in the Coronary Artery Surgery Study (CASS).28 The criteria for LVA diagnosis included the following: (i) bulging of the left ventricular wall during both diastole and systole, exhibiting either akinesia or dyskinesia; (ii) clear demarcation of the infarcted segment; and (iii) absence of trabeculation in the affected segment. After admission, we conducted two-dimensional transthoracic echocardiography (TTE) within 3 days, 1 month, and 6 months during follow-up. LVA was diagnosed using TTE at 6 months of follow-up. Diabetes mellitus was defined as a fasting plasma glucose level >7.0 mmol/L, a post-prandial blood glucose level >11.1 mmol/L, a glycated haemoglobin (HbA1c) level >6.5%, or treatment for diabetes mellitus. Hypertension was defined as treatment for hypertension prior to admission or a blood pressure measurement exceeding 140/90 mmHg. Smoking history was defined as having smoked more than 2 pack-years and/or having smoked within the past year.
The Gensini score was calculated using the method developed by Celebi et al.29 In summary, a severity score was assigned to each coronary stenosis based on the degree of narrowing: 1 point for ≤25% narrowing, 2 points for 26–50% narrowing, 4 points for 51–75% narrowing, 8 points for 76–90% narrowing, 16 points for 91–99% narrowing, and 32 points for total occlusion. Additionally, each coronary stenosis score was multiplied by a factor that considered the importance of the lesion's position in the coronary circulation (5 for the left main coronary artery; 2.5 for the proximal segment of the left anterior descending coronary artery; 2.5 for the proximal segment of the circumflex artery; 1.5 for the mid-segment of the left anterior descending coronary artery; 1.0 for the right coronary artery, the distal segment of the left anterior descending coronary artery, the posterolateral artery, or the obtuse marginal artery; and 0.5 for the other segments). The sum of the scores for each coronary segment yielded the Gensini score.
Data collection
Venous blood samples were collected from all patients upon admission to the emergency room before PPCI, as well as 8–12 h PPCI. To determine the peak value of cardiac enzyme, blood samples for troponin I (TnI), CK-MB, creatine kinase (CK), and lactate dehydrogenase (LDH) were obtained from a peripheral vein, after admission to the intensive coronary care unit, every 12 h during the first 48 h, and every 24 h during the rest of the stay in the intensive coronary care unit. Haematological parameters, including white blood cell count, neutrophil count, platelet count, and lymphocyte count, were measured using an automated blood cell counter.
Statistical analysis
Statistical analysis was performed using SPSS Version 13.0 (SPSS Inc., Chicago, IL) for Windows (Microsoft Corporation, Redmond, WA). Normally distributed quantitative data were presented as mean ± standard deviation (SD), while abnormally distributed quantitative data were presented as median [inter-quartile range (IQR)]. Categorical variables were presented as numbers (per cent). The best cut-off value of BAR to stratify patients into two groups was determined to be 0.15 according to the receiver operating characteristic (ROC) curve analysis. Differences among groups were examined using χ2 test, one-way analysis of variance, or Mann–Whitney U test, where appropriate. Univariate logistic regression analysis was performed to assess the association between different variables and the risk of LVA formation. All factors with P < 0.05 in the univariate analysis were included in the multiple logistic regression analysis. All comparisons were two-sided, and a significance level of P < 0.05 was considered statistically significant.
Results
Subjects' characteristics
A total of 1123 acute STEMI patients who underwent PPCI were enrolled in this study. The population was divided into two groups: the LVA group (N = 162) and the non-LVA group (N = 961). The detailed baseline demographics and clinical characteristics of the patients in the two groups are presented in Table 1. Compared with the non-LVA group, the LVA group was older and more likely to be female. Meanwhile, the LVA group had higher level of diastolic blood pressure (DBP), heart rate, aspartate aminotransferase (AST), globulin (GLB), BUN, low-density lipoprotein (LDL), apolipoprotein B (ApoB), LDH, peak cTnI, D-dimer, BAR, and Gensini score and a higher proportion of aldosterone receptor blocker use, thiazide or loop diuretic use, multiple-vessel disease, and culprit vessel–left anterior descending artery (LAD) (P < 0.05). Conversely, patients with LVA had lower proportions of smokers and lower levels of left ventricular ejection fraction (LVEF), lymphocyte count, haemoglobin, ALB, and triglyceride (TG) compared with those without LVA (P < 0.05).
| Whole cohort (N = 1123) | Non-LVA patients (N = 961) | LVA patients (N = 162) | P value | |
|---|---|---|---|---|
| Demographics | ||||
| Age (years) | 61.2 ± 12.8 | 60.5 ± 12.7 | 65.0 ± 12.8 | <0.001 |
| Male, n (%) | 904 (80.5) | 786 (81.8) | 118 (72.8) | 0.009 |
| Hypertension, n (%) | 612 (54.5) | 524 (54.5) | 88 (54.3) | 0.972 |
| Diabetes, n (%) | 358 (31.9) | 300 (31.2) | 58 (35.8) | 0.243 |
| Smoking, n (%) | 606 (54.0) | 534 (55.6) | 72 (44.4) | 0.009 |
| LVEF (%) | 54.0 ± 8.6 | 55.9 ± 7.2 | 43.2 ± 8.0 | <0.001 |
| SBP (mmHg) | 129.2 ± 20.7 | 129.1 ± 21.1 | 129.6 ± 18.3 | 0.75 |
| DBP (mmHg) | 81.1 ± 14.4 | 80.5 ± 14.8 | 84.3 ± 11.7 | 0.002 |
| Heart rate (b.p.m.) | 83.5 ± 16.3 | 82.7 ± 16.0 | 88.8 ± 16.9 | 0.001 |
| Laboratory parameters | ||||
| White blood cell count (× 109/L) | 9.7 (7.8–12.2) | 9.8 (7.8–12.1) | 9.7 (7.7–12.5) | 0.512 |
| Red blood cell count (× 109/L) | 4.5 ± 0.7 | 4.5 ± 0.7 | 4.4 ± 0.6 | 0.425 |
| Neutrophil count (× 109/L) | 7.6 (5.8–10.0) | 7.5 (5.7–9.8) | 7.9 (6.0–10.3) | 0.103 |
| Platelet count (× 109/L) | 223.4 ± 65.3 | 223.2 ± 66.3 | 224.9 ± 58.8 | 0.759 |
| Lymphocyte count (× 109/L) | 1.3 (1.0–1.8) | 1.4 (1.0–1.8) | 1.2 (0.8–1.7) | <0.001 |
| Haemoglobin (g/L) | 138.4 ± 21.4 | 139.0 ± 21.6 | 134.9 ± 20.0 | 0.024 |
| Sodium (mmol/L) | 139.1 ± 3.1 | 139.2 ± 3.1 | 138.8 ± 3.4 | 0.179 |
| Potassium (mmol/L) | 4.0 ± 0.5 | 4.0 ± 0.5 | 4.0 ± 0.5 | 0.09 |
| ALT (U/L) | 28.2 (17.9–48.6) | 28.0 (18.0–47.3) | 31.5 (17.7–56.0) | 0.237 |
| AST (U/L) | 59.2 (25.8–164.1) | 57.8 (25.0–155.0) | 69.3 (26.4–241.5) | 0.032 |
| ALB (g/L) | 41.0 ± 5.3 | 41.4 ± 5.2 | 39.0 ± 5.5 | <0.001 |
| GLB (g/L) | 27.7 ± 4.9 | 27.6 ± 4.9 | 28.4 ± 4.7 | 0.04 |
| HbA1c (%) | 6.0 (5.6–6.9) | 6.0 (5.6–6.9) | 6.1 (5.6–7.6) | 0.091 |
| Cr (μmol/L) | 72.2 (62.0–88.0) | 71.8 (62.0–88.0) | 74.4 (62.7–87.9) | 0.316 |
| BUN (μmol/L) | 5.5 (4.4–6.9) | 5.4 (4.4–6.8) | 5.9 (4.7–7.3) | 0.003 |
| UA (μmol/L) | 381.3 ± 108.2 | 382.0 ± 102.1 | 377.0 ± 139.3 | 0.662 |
| TC (mmol/L) | 4.7 ± 1.2 | 4.7 ± 1.2 | 4.8 ± 1.1 | 0.1 |
| TG (mmol/L) | 1.4 (0.9–2.1) | 1.4 (0.9–2.2) | 1.3 (0.8–1.7) | 0.001 |
| HDL (mmol/L) | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.761 |
| LDL (mmol/L) | 3.1 ± 1.1 | 3.1 ± 1.1 | 3.3 ± 1.1 | 0.019 |
| ApoA1 (g/L) | 1.1 ± 0.2 | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.134 |
| ApoB (g/L) | 1.0 ± 0.3 | 1.0 ± 0.3 | 1.1 ± 0.3 | 0.038 |
| LDH (U/L) | 316.0 (202.0–583.5) | 294.0 (199.3–545.0) | 472.0 (244.0–755.5) | <0.001 |
| Peak cTnI (ng/mL) | 20.5 (3.7–50.0) | 17.9 (3.6–47.4) | 26.8 (5.0–50.0) | 0.003 |
| D-dimer (μg/mL) | 0.4 (0.2–0.7) | 0.4 (0.2–0.6) | 0.5 (0.3–1.0) | <0.001 |
| BAR | 0.14 (0.10–0.17) | 0.13 (0.10–0.17) | 0.16 (0.13–0.19) | <0.001 |
| Medication at hospital discharge | ||||
| Statin, n (%) | 1074 (95.6) | 923 (96.0) | 151 (93.2) | 0.102 |
| Beta-blockers, n (%) | 1066 (94.9) | 916 (95.3) | 150 (92.6) | 0.144 |
| ACE inhibitors or ARB, n (%) | 817 (72.8) | 704 (73.3) | 113 (69.8) | 0.354 |
| Aldosterone receptor blockers, n (%) | 350 (31.2) | 270 (28.1) | 80 (49.4) | <0.001 |
| Thiazide or loop diuretic, n (%) | 376 (33.5) | 283 (29.4) | 93 (57.4) | <0.001 |
| Coronary artery disease | ||||
| Gensini score | 72 (47–92) | 67 (45–90) | 89 (69–106) | <0.001 |
| Multiple-vessel disease, n (%) | 730 (65.0) | 612 (63.7) | 118 (72.8) | 0.023 |
| Culprit vessel–LAD, n (%) | 568 (50.6) | 438 (45.6) | 130 (80.2) | <0.001 |
- ACE, angiotensin-converting enzyme; ALB, albumin; ALT, alanine aminotransferase; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; BAR, blood urea nitrogen-to-albumin ratio; BUN, blood urea nitrogen; Cr, creatinine; cTnI, cardiac troponin I; DBP, diastolic blood pressure; GLB, globulin; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; LAD, left anterior descending artery; LDH, lactate dehydrogenase; LDL, low-density lipoprotein; LVA, left ventricular aneurysm; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; UA, uric acid.
According to the criterion of maximum Youden index, we determined the optimal cut-off value for BAR at 0.15 to stratify patients into BAR < 0.15 and BAR ≥ 0.15 groups using ROC curve analysis. As shown in Table 2, the group with BAR ≥ 0.15 was found to be older, more likely to be female, and had higher levels of HbA1c, creatinine (Cr), BUN, uric acid (UA), D-dimer, and Gensini score, as well as a higher prevalence of hypertension, diabetes, thiazide or loop diuretic use, multiple-vessel disease, and LVA formation. On the contrary, patients with a BAR ≥ 0.15 exhibited lower frequencies of smoking and beta-blocker use. Additionally, they had lower levels of LVEF, systolic blood pressure (SBP), DBP, white blood cell count, red blood cell count, platelet count, lymphocyte count, haemoglobin, alanine transaminase (ALT), ALB, total cholesterol (TC), TG, LDL, and ApoB when compared with those with a BAR < 0.15.
| BUN/ALB < 0.15 (N = 682) | CRP/ALB ≥ 0.15 (N = 441) | P value | |
|---|---|---|---|
| Demographics | |||
| Age (years) | 57.4 ± 12.7 | 66.9 ± 10.7 | <0.001 |
| Male, n (%) | 578 (84.8) | 326 (73.9) | <0.001 |
| Hypertension, n (%) | 320 (46.9) | 292 (66.2) | <0.001 |
| Diabetes, n (%) | 194 (28.4) | 164 (37.2) | 0.002 |
| Smoking, n (%) | 384 (56.3) | 222 (50.3) | 0.046 |
| LVEF (%) | 55.6 ± 7.4 | 51.6 ± 9.5 | <0.001 |
| SBP (mmHg) | 131.2 ± 20.2 | 126.0 ± 21.0 | <0.001 |
| DBP (mmHg) | 82.6 ± 14.2 | 78.7 ± 14.6 | <0.001 |
| Heart rate (b.p.m.) | 83.4 ± 14.5 | 83.8 ± 18.8 | 0.707 |
| Laboratory parameters | |||
| White blood cell count (× 109/L) | 10.1 (8.1–12.15) | 9.2 (7.5–12.6) | 0.01 |
| Red blood cell count (× 109/L) | 4.6 ± 0.6 | 4.2 ± 0.7 | <0.001 |
| Neutrophil count (× 109/L) | 7.7 (5.9–9.8) | 7.2 (5.5–10.2) | 0.566 |
| Platelet count (× 109/L) | 229.8 ± 67.2 | 213.5 ± 61.0 | <0.001 |
| Lymphocyte count (× 109/L) | 1.4 (1.1–1.9) | 1.2 (0.9–1.7) | <0.001 |
| Haemoglobin (g/L) | 143.5 ± 18.7 | 130.4 ± 22.7 | <0.001 |
| Sodium (mmol/L) | 139.1 ± 2.7 | 139.2 ± 3.6 | 0.645 |
| Potassium (mmol/L) | 4.0 ± 0.4 | 4.1 ± 0.5 | 0.072 |
| ALT (U/L) | 29.3 (18.9–49.8) | 26.2 (15.8–46.0) | 0.021 |
| AST (U/L) | 58.5 (25.0–149.1) | 61.8 (26.4–171.6) | 0.464 |
| ALB (g/L) | 42.7 ± 4.8 | 38.3 ± 5.0 | <0.001 |
| GLB (g/L) | 27.8 ± 4.8 | 27.6 ± 5.0 | 0.582 |
| HbA1c (%) | 5.9 (5.5–6.6) | 6.2 (5.7–7.6) | <0.001 |
| Cr (μmol/L) | 67.7 (58.9–78.5) | 83.5 (68.2–110.5) | <0.001 |
| BUN (μmol/L) | 4.7 (4.0–5.3) | 7.3 (6.5–8.9) | <0.001 |
| UA (μmol/L) | 370 ± 97 | 399 ± 122 | <0.001 |
| TC (mmol/L) | 4.8 ± 1.3 | 4.5 ± 1.0 | <0.001 |
| TG (mmol/L) | 1.6 (1.0–2.5) | 1.3 (0.8–1.7) | <0.001 |
| HDL (mmol/L) | 1.0 ± 0.3 | 1.0 ± 0.3 | 0.393 |
| LDL (mmol/L) | 3.2 ± 1.2 | 2.9 ± 0.9 | <0.001 |
| ApoA1 (g/L) | 1.1 ± 0.2 | 1.1 ± 0.2 | 0.316 |
| ApoB (g/L) | 1.1 ± 0.3 | 1.0 ± 0.2 | <0.001 |
| LDH (U/L) | 300 (197–573) | 351 (219–585) | 0.053 |
| Peak cTnI (ng/mL) | 21.1 (3.9–50) | 19.7 (3.5–50) | 0.363 |
| D-dimer (μg/mL) | 0.3 (0.2–0.5) | 0.5 (0.3–0.9) | <0.001 |
| Medication at hospital discharge | |||
| Statin, n (%) | 648 (95.0) | 426 (96.6) | 0.204 |
| Beta-blockers, n (%) | 658 (96.5) | 408 (92.5) | 0.003 |
| ACE inhibitors or ARB, n (%) | 507 (74.3) | 310 (70.3) | 0.137 |
| Aldosterone receptor blockers, n (%) | 208 (30.5) | 142 (32.2) | 0.548 |
| Thiazide or loop diuretic, n (%) | 211 (30.9) | 165 (37.4) | 0.025 |
| Coronary artery disease | |||
| Gensini score | 68 (45–90) | 79 (51–96) | <0.001 |
| Multiple-vessel disease, n (%) | 418 (61.3) | 312 (70.7) | 0.001 |
| Culprit vessel–LAD, n (%) | 348 (51.0) | 220 (50.0) | 0.682 |
| LVA, n (%) | 60 (8.8) | 102 (23.1) | <0.001 |
- ACE, angiotensin-converting enzyme; ALB, albumin; ALT, alanine aminotransferase; ApoA1, apolipoprotein A1; ApoB, apolipoprotein B; ARB, angiotensin receptor blocker; AST, aspartate aminotransferase; BAR, blood urea nitrogen-to-albumin ratio; BUN, blood urea nitrogen; Cr, creatinine; CRP, C-reactive protein; cTnI, cardiac troponin I; DBP, diastolic blood pressure; GLB, globulin; HbA1c, glycated haemoglobin; HDL, high-density lipoprotein; LAD, left anterior descending artery; LDH, lactate dehydrogenase; LDL, low-density lipoprotein; LVA, left ventricular aneurysm; LVEF, left ventricular ejection fraction; SBP, systolic blood pressure; TC, total cholesterol; TG, triglyceride; UA, uric acid.
Predictors of left ventricular aneurysm formation
Univariate logistic regression analysis was conducted to assess the predictive value of all variables for the formation of LVA in acute STEMI patients. As shown in Table 3, a total of 21 variables were found to be associated with the LVA formation. These variables included age, gender, smoking, LVEF, DBP, heart rate, haemoglobin, neutrophil count, lymphocyte count, HbA1c, AST, ALB, GLB, TG, LDH, peak cTnI, BUN, Gensini score, multiple-vessel disease, culprit vessel–LAD, and BAR (<0.15/≥0.15). Subsequently, these 21 variables were included in a multivariate logistic regression analysis, which revealed that only LVEF [odds ratio (OR) = 0.865, 95% confidence interval (CI) = 0.839–0.891, P < 0.001], culprit vessel–LAD (OR = 4.705, 95% CI = 2.634–8.405, P < 0.001), and BAR (OR = 2.208, 95% CI = 1.144–4.262, P = 0.018) remained significantly associated with the risk of LVA formation.
| Univariate logistic regression analysis | Multivariate logistic regression analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | 1.029 | 1.015–1.043 | <0.001 | |||
| Gender | 1.663 | 1.134–2.439 | 0.009 | |||
| Smoking | 0.638 | 0.457–0.892 | 0.009 | |||
| LVEF | 0.85 | 0.83–0.87 | <0.001 | 0.865 | 0.839–0.891 | <0.001 |
| DBP | 1.013 | 1.002–1.025 | 0.021 | |||
| Heart rate | 1.021 | 1.011–1.031 | <0.001 | |||
| Haemoglobin | 0.988 | 0.981–0.996 | 0.004 | |||
| Neutrophil count | 1.053 | 1.004–1.103 | 0.033 | |||
| Lymphocyte count | 0.691 | 0.544–0.879 | 0.003 | |||
| HbA1c | 1.196 | 1.096–1.305 | <0.001 | |||
| AST | 1.002 | 1.001–1.003 | <0.001 | |||
| ALB | 0.903 | 0.874–0.933 | <0.001 | |||
| GLB | 1.036 | 1.002–1.071 | 0.039 | |||
| TG | 0.741 | 0.623–0.880 | <0.001 | |||
| LDH | 1.001 | 1.001–1.001 | <0.001 | |||
| Peak cTnI | 1.011 | 1.003–1.018 | 0.004 | |||
| BUN | 1.072 | 1.025–1.121 | 0.002 | |||
| Gensini score | 1.013 | 1.008–1.017 | <0.001 | |||
| Multiple-vessel disease | 1.618 | 1.096–2.390 | 0.015 | |||
| Culprit vessel–LAD | 5.867 | 3.723–9.244 | <0.001 | 4.705 | 2.634–8.405 | <0.001 |
| BAR (<0.15/≥0.15) | 3.119 | 2.209–4.405 | <0.001 | 2.208 | 1.144–4.262 | 0.018 |
- ALB, albumin; AST, aspartate aminotransferase; BAR, blood urea nitrogen-to-albumin ratio; BUN, blood urea nitrogen; CI, confidence interval; DBP, diastolic blood pressure; GLB, globulin; HbA1c, glycated haemoglobin; LAD, left anterior descending artery; LDH, lactate dehydrogenase; LVEF, left ventricular ejection fraction; MI, myocardial infarction; OR, odds ratio; TG, triglyceride.
Additionally, we split the population into two groups, including the training set (562 patients) and the validation set (561 patients). Multivariate logistic regression analysis indicated that BAR was independently associated with the risk of LVA formation in both training and validation sets (OR = 1.957, 95% CI = 1.053–3.635, P = 0.034, for training set; OR = 1.982, 95% CI = 1.036–3.789, P = 0.039, for validation set) (Tables 4 and 5).
| Univariate logistic regression analysis | Multivariate logistic regression analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Gender | 1.029 | 1.010–1.049 | 0.003 | |||
| Smoking | 0.526 | 0.329–0.838 | 0.007 | |||
| LVEF | 0.856 | 0.829–0.884 | <0.001 | 0.877 | 0.844–0.912 | <0.001 |
| DBP | 1.02 | 1.004–1.037 | 0.014 | |||
| Heart rate | 1.016 | 1.002–1.031 | 0.025 | |||
| ALT | 1.005 | 1.000–1.009 | 0.038 | |||
| AST | 1.002 | 1.001–1.003 | <0.001 | |||
| ALB | 0.915 | 0.875–0.957 | <0.001 | |||
| LDH | 1.001 | 1.001–1.002 | <0.001 | |||
| Peak cTnI | 1.014 | 1.003–1.024 | 0.008 | |||
| D-dimer | 1.231 | 1.039–1.459 | 0.016 | |||
| Gensini score | 1.009 | 1.003–1.015 | 0.003 | |||
| Culprit vessel–LAD | 7.918 | 4.089–15.334 | <0.001 | 6.509 | 3.004–14.102 | <0.001 |
| BAR (<0.15/≥0.15) | 2.679 | 1.673–4.290 | <0.001 | 1.957 | 1.053–3.635 | 0.034 |
- ALB, albumin; ALT, alanine aminotransferase; AST, aspartate aminotransferase; BAR, blood urea nitrogen-to-albumin ratio; CI, confidence interval; DBP, diastolic blood pressure; LAD, left anterior descending artery; LDH, lactate dehydrogenase; LVEF, left ventricular ejection fraction; MI, myocardial infarction; OR, odds ratio.
| Univariate logistic regression analysis | Multivariate logistic regression analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | 1.74 | 1.009–3.002 | 0.046 | |||
| Gender | 1.03 | 1.009–1.050 | 0.005 | |||
| LVEF | 0.84 | 0.811–0.870 | <0.001 | 0.857 | 0.824–0.891 | <0.001 |
| Heart rate | 1.026 | 1.012–1.040 | <0.001 | |||
| Haemoglobin | 0.984 | 0.973–0.995 | 0.004 | |||
| Lymphocyte count | 0.588 | 0.394–0.879 | 0.01 | |||
| HbA1c | 1.316 | 1.154–1.500 | <0.001 | |||
| ALB | 0.888 | 0.847–0.932 | <0.001 | |||
| TG | 0.670 | 0.508–0.883 | 0.004 | |||
| BUN | 1.159 | 1.079–1.246 | <0.001 | |||
| Gensini score | 1.017 | 1.010–1.023 | <0.001 | |||
| Culprit vessel–LAD | 4.326 | 2.304–8.121 | <0.001 | 3.143 | 1.503–6.576 | 0.002 |
| BAR (<0.15/≥0.15) | 3.696 | 2.220–6.152 | <0.001 | 1.982 | 1.036–3.789 | 0.039 |
- ALB, albumin; BAR, blood urea nitrogen-to-albumin ratio; BUN, blood urea nitrogen; CI, confidence interval; HbA1c, glycated haemoglobin; LAD, left anterior descending artery; LVEF, left ventricular ejection fraction; MI, myocardial infarction; OR, odds ratio; TG, triglyceride.
Relationship between blood urea nitrogen-to-albumin ratio and clinical parameters of left ventricular aneurysm
In order to assess the correlation between BAR and the size of LVA, we compared the maximal length and width of LVA between patients grouped by BAR. Figure 2 illustrates that the maximal length and width were significantly increased in patients with BAR ≥ 0.15 compared with those with BAR < 0.15 (2.92 ± 0.93 vs. 3.37 ± 1.09 for maximal length and 1.85 ± 0.63 vs. 2.20 ± 0.55 for maximal width).

Discriminative power analysis
Subsequently, we conducted ROC analysis to evaluate the discriminative power of the following variables in predicting the risk of LVA formation: BUN, ALB, BAR, and the composite variable (BAR combined with LVEF and culprit vessel–LAD). Figure 3 and Table 6 showed that the average areas under the curve (AUCs) for BUN, ALB, BAR, and the composite variable were 0.586 (95% CI = 0.553–0.617), 0.640 (95% CI = 0.609–0.671), 0.723 (95% CI = 0.693–0.751), and 0.874 (95% CI = 0.851–0.894), respectively. The AUC of BAR for predicting the risk of LVA formation is significantly higher than both BUN (P < 0.001) and ALB (P = 0.003), and the composite variable exhibited the best predictive value (P < 0.001).
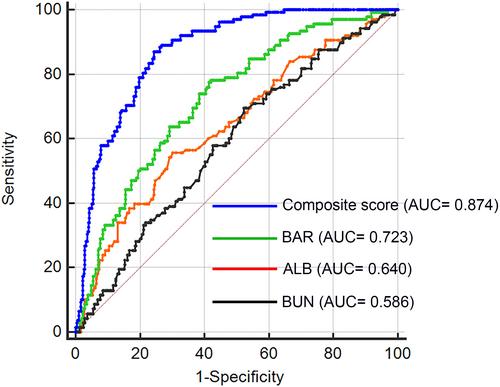
| AUC | SE | 95% CI | |
|---|---|---|---|
| BUN | 0.586 | 0.0255 | 0.553–0.617 |
| ALB | 0.64 | 0.0261 | 0.609–0.671 |
| BAR | 0.723 | 0.0224 | 0.693–0.751 |
| Composite variable | 0.874 | 0.0136 | 0.851–0.894 |
- ALB, albumin; AUC, area under the curve; BAR, blood urea nitrogen-to-albumin ratio; BUN, blood urea nitrogen; CI, confidence interval; ROC, receiver operating characteristic; SE, standard error.
Subgroup analysis
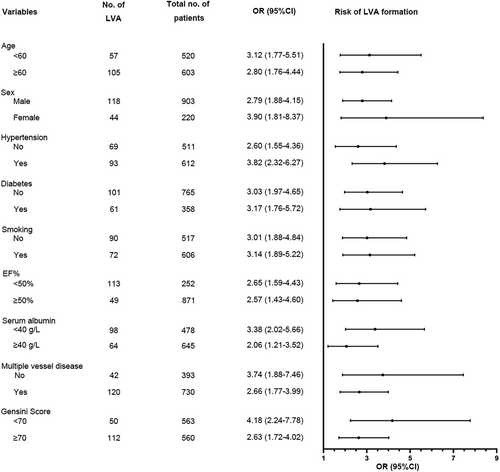
To further investigate whether BAR remained an independent predictor for LVA formation in specific subgroups, we performed an exploratory subgroup analysis of age, sex, hypertension, diabetes, smoking, LVEF, serum ALB, multiple-vessel disease, and Gensini score. The forest plot displayed that BAR was an independent predictive factor in all subgroups (Figure 4).
Discussion
We prospectively analysed 1123 acute STEMI patients who underwent PPCI to examine the correlation between BAR and the risk of LVA formation. Logistic regression analysis demonstrated that a higher BAR was an independent predictor for LVA formation. The predictive value remained significant in both the training and validation sets. Stratification by age, sex, hypertension, diabetes, smoking, LVEF, serum ALB, multiple-vessel disease, and Gensini score did not significantly affect the association between BAR and LVA formation. Among patients with LVA, the maximal length and width in those with BAR ≥ 0.15 were significantly increased compared with those with BAR < 0.15. Furthermore, BAR exhibited greater predictive power for LVA formation than BUN and ALB, while the composite variable showed the best predictive value.
LVA is characterized by the outward expansion of the infarcted myocardium during systole and diastole.1 Previous studies have shown that patients with LVA have a worse prognosis compared with those without LVA.7 With advancements in the treatment of acute STEMI, the prevalence of LVA after AMI has decreased from 10–30% to 8–15%.29 In our study, the incidence of LVA in acute STEMI patients who underwent PPCI was 14.4%, which aligns with findings from previous reports.7, 8
The BUN serves as the marker of renal function, with its concentration determined by the balance of excretion and reabsorption in the kidney. Reabsorption of BUN is closely linked to water reabsorption at the distal nephron under the influence of antidiuretic hormone,30 which is affected by angiotensin II. Elevated BUN levels are associated with the activation of the renin–angiotensin–aldosterone and sympathetic nerve systems, which can result from renal hypoperfusion due to hypovolaemia, renovascular disease, or reduced cardiac output. Therefore, it can be hypothesized that BUN levels are likely to increase considering the increased sympathetic activity due to heightened neurohumoral activity in patients with AMI, renal hypoperfusion resulting from reduced cardiac output, and the activated renin–angiotensin system. Additionally, previous studies have demonstrated BUN as the independent prognostic factor for many CVDs. A prospective cohort study conducted by Horiuchi et al. found that a high level of BUN is a significant predictor for in-hospital mortality in patients with AMI.31 Additionally, Richter et al. demonstrated that circulating BUN on admission is an independent predictor of long-term cardiovascular mortality in AMI patients.32 In our study, the level of BUN in patients with LVA was significantly higher compared with those without LVA, which implied the importance of BUN in LVA formation.
Serum ALB, synthesized by the liver, plays an important role in both acute and chronic inflammation by transporting many substrates. Previous studies have highlighted the importance of inflammation in AMI in both human and animals.33-36 Hypoalbuminaemia is a known marker of inflammation.37 In the present study, patients with LVA exhibited lower level of ALB than those without LVA, suggesting that inflammation may play a crucial role in this association. Furthermore, previous study has demonstrated that low serum ALB levels during the late phase following AMI were a valuable predictor of long-term adverse outcomes.21 In a perspective cohort study of 2253 patients with AMI, Yoshioka et al. found that low serum ALB on admission was independently associated with an increased risk of HF or cardiovascular death. And this prediction effect remained significant even in the remote phase after AMI.38 Collectively, these findings indicate that ALB is closely associated with LVA formation after AMI as a marker of inflammation.
Recently, BAR has been used as a new biomarker to assess the prognosis of various diseases and is an important prognostic factor for death in several diseases, such as pulmonary embolism,25 sepsis,9 and lung cancer.26 Moreover, numerous studies have identified BAR as an independent predictor for mortality risk of AMI.30, 39 In present study, we observed significantly higher BAR levels in patients with LVA. Specifically, the group with BAR ≥ 0.15 had a higher proportion of LVA compared with the group with BAR < 0.15. Besides, the Gensini score was significantly higher in the group with BAR ≥ 0.15 while the LVEF was opposite, which indicated that increased BAR presented more serious coronary artery stenosis and myocardial injury.
Numerous predictive factors for the risk of LVA formation in AMI patients have been identified in previous studies. In a prospective cohort study including 1519 patients with STEMI, Celebi et al. found that plasma N-terminal pro-B-type natriuretic peptide level at admission among other variables provided valuable predictive information regarding the development of LVA.29 In a case–control study, Feng et al. found that single-vessel disease, decreased glomerular filtration rate (GFR), and abnormal ferritin were independent predictors of LVA formation.4 Furthermore, a prospective cohort study with 942 acute anterior myocardial infarction patients who underwent PPCI revealed that longer symptom-to-balloon time, higher initial and residual SYNTAX score, lower LVEF, and persistent ST-segment elevation were independent predictors of LVA formation.7 However, these studies primarily focused on single variables, most of which had only modest or small effects on LVA prediction. Besides, there are still some disagreements regarding these risk factors. In our study, we used BAR to predict LVA formation for the first time, and the OR for LVA formation in higher BAR reached up to 2.208. Additionally, LVEF was also an independent predictor for LVA formation in our study, which is consistent with the results from Wang et al.8 It is widely accepted that the size of LVA reflected the extent of myocardial injury. Our research revealed that patients with BAR ≥ 0.15 demonstrated a significant increase in maximal length and width compared with those with BAR < 0.15, underscoring the critical role of BAR in myocardial injury. Furthermore, BAR proved to be a more potent predictor for LVA formation than BUN and ALB, with the composite variable yielding the highest predictive value.
Our study has several limitations. Firstly, the diagnosis of LVA was according to ultrasonographic examination, which is not considered the gold standard for LVA detection. However, this method is widely used in clinical practice and epidemiological studies due to its availability and non-invasiveness. Additionally, whether increased C-reactive protein (CRP) and ALB are bystanders, causal factors, or consequences of LVA formation cannot be concluded from the results of our study. Finally, it is important to note that this analysis was conducted at a single centre. In order to obtain more robust results, future work should include multicentre cohort study.
In conclusion, our study showed that increased BAR was independently associated with the formation of LVA in patients with STEMI undergoing PPCI. The combination of BAR, LVEF, and culprit vessel–LAD could substantially enhance the ability to distinguish LVA formation. Further studies on the involvement of BAR in LVA formation will not only expand the authors' understanding of the mechanism of LVA but also assist in the future development of new prevention and treatment strategies for the disease.
Conflict of interest
None declared.
Funding
None.




