Heart failure with preserved ejection fraction phenogroup classification using machine learning
Abstract
Aims
Heart failure (HF) with preserved ejection fraction (HFpEF) is a complex syndrome with a poor prognosis. Phenotyping is required to identify subtype-dependent treatment strategies. Phenotypes of Japanese HFpEF patients are not fully elucidated, whose obesity is much less than Western patients. This study aimed to reveal model-based phenomapping using unsupervised machine learning (ML) for HFpEF in Japanese patients.
Methods and results
We studied 365 patients with HFpEF (left ventricular ejection fraction >50%) as a derivation cohort from the Nara Registry and Analyses for Heart Failure (NARA-HF), which registered patients with hospitalization by acute decompensated HF. We used unsupervised ML with a variational Bayesian–Gaussian mixture model (VBGMM) with common clinical variables. We also performed hierarchical clustering on the derivation cohort. We adopted 230 patients in the Japanese Heart Failure Syndrome with Preserved Ejection Fraction Registry as the validation cohort for VBGMM. The primary endpoint was defined as all-cause death and HF readmission within 5 years. Supervised ML was performed on the composite cohort of derivation and validation. The optimal number of clusters was three because of the probable distribution of VBGMM and the minimum Bayesian information criterion, and we stratified HFpEF into three phenogroups. Phenogroup 1 (n = 125) was older (mean age 78.9 ± 9.1 years) and predominantly male (57.6%), with the worst kidney function (mean estimated glomerular filtration rate 28.5 ± 9.7 mL/min/1.73 m2) and a high incidence of atherosclerotic factor. Phenogroup 2 (n = 200) had older individuals (mean age 78.8 ± 9.7 years), the lowest body mass index (BMI; 22.78 ± 3.94), and the highest incidence of women (57.5%) and atrial fibrillation (56.5%). Phenogroup 3 (n = 40) was the youngest (mean age 63.5 ± 11.2) and predominantly male (63.5 ± 11.2), with the highest BMI (27.46 ± 5.85) and a high incidence of left ventricular hypertrophy. We characterized these three phenogroups as atherosclerosis and chronic kidney disease, atrial fibrillation, and younger and left ventricular hypertrophy groups, respectively. At the primary endpoint, Phenogroup 1 demonstrated the worst prognosis (Phenogroups 1–3: 72.0% vs. 58.5% vs. 45%, P = 0.0036). We also successfully classified a derivation cohort into three similar phenogroups using VBGMM. Hierarchical and supervised clustering successfully showed the reproducibility of the three phenogroups.
Conclusions
ML could successfully stratify Japanese HFpEF patients into three phenogroups (atherosclerosis and chronic kidney disease, atrial fibrillation, and younger and left ventricular hypertrophy groups).
Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) has a complex aetiology and has been increasing in multiple ethnicities with a variety of lifestyles and related phenotypes.1 Most previous studies have failed to show effective treatment for HFpEF other than sodium–glucose cotransporter 2 (SGLT2) inhibitors; thus, a ‘one size fits all’ concept is not acceptable in HFpEF management.2, 3 Many cardiologists have recognized the need for subgroup-dependent therapies.4, 5
Recently, phenotyping using machine learning (ML), which makes it possible to clarify complex phenotypes from data patterns in multi-dimensional datasets, has been applied in this field.6 Prior studies have succeeded in stratifying HFpEF into several phenotypes in multiple cohorts that included a small number of Asian but not Japanese patients.7-12
The distribution of patient population characteristics was significantly different between Western and Eastern countries, especially in terms of mean body mass index (BMI) and the incidence of obesity. In the recent HFpEF worldwide registry, the Asian patient population demonstrated lower BMI, a frequent incidence of atrial fibrillation (AF), poor kidney function, and a history of HF admission.13 The difference in obesity and disease distribution might result in differences in phenotyping results and cardiovascular event rates. Therefore, we considered that HFpEF phenomapping in Japanese and Asian populations is needed to understand their pathology and optimal treatment.
This study aimed to develop model-based phenotyping using unsupervised clustering for HFpEF in Japanese people.
Methods
Study population
This study included two HF cohorts: the Nara Registry and Analyses for Heart Failure (NARA-HF) study and the Japanese Heart Failure Syndrome with Preserved Ejection Fraction Registry (JASPER). The NARA-HF study was used as a derivation model, JASPER, and the composite cohort combining NARA-HF and JASPER was used as a validation cohort.
The NARA-HF study is a dynamic prospective cohort study that conformed to the principles outlined in the Declaration of Helsinki and was approved by the ethics committee of Nara Medical University (Approval No. 624). All participants provided written informed consent. All 1448 patients recruited met the Framingham criteria for emergency admission due to acute decompensated HF (ADHF) (either acute new-onset or acute-on-chronic HF) between January 2007 and December 2018. The inclusion and exclusion criteria have been previously reported.14 For the HFpEF cohort, we excluded patients with left ventricular ejection fraction (LVEF) <50% at discharge (n = 852). From the remaining 596 patients, we excluded 231 patients because of in-hospital death (n = 24), haemodialysis at discharge (n = 78), right HF (n = 12), constrictive pericarditis (n = 1), adult congenital heart disease (n = 9), severe valvular disease (n = 71), and estimated glomerular filtration rate (eGFR) <15 mL/min/1.73 m2 (n = 36). The remaining 365 patients satisfied the European Society of Cardiology guideline definition of HFpEF at admission.15 Finally, 365 HFpEF patients from NARA-HF cohort were analysed who had discharge data and 5 year mortality rates (Figure 1).
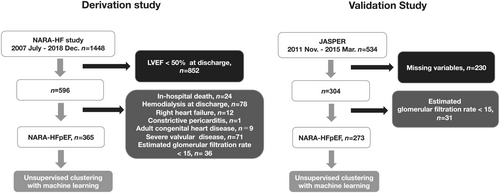
The details of JASPER have been previously described.16 A total of 534 hospitalized patients with HFpEF from 15 university or teaching hospitals were registered from November 2011 to March 2015. Patients with missing variables (n = 230) and an eGFR < 15 mL/min/1.73 m2 at discharge (n = 31) were excluded. Finally, we selected 273 patients for the validation cohort (Figure 1).
We calculated eGFR using the following formula: eGFR (mL/min/1.73 m2) in men = 194 * serum creatinine level(mg/dL)−1.094 * age−0.287 and eGFR (mL/min/1.73 m2) in women = (194 * serum creatinine level (mg/dL)−1.094 * age−0.287) * 0.739.17
Clinical phenogroup assignment
A variational Bayesian–Gaussian mixture model (VBGMM) was used to determine clusters of clinical phenotypes. VBGMM is a statistical technique used in the ML domain for an unknown number of clusters.18 The core idea is based on VBGMM, which allows the computation of the posterior distribution of the Gaussian clusters for each instance. In contrast to other ML learning algorithms, in which the number of clusters is predefined, the proper number is estimated via the sparseness of the Dirichlet before the mixture weights.19 We adopted VBGMM because this method has been recommended when the number of clusters is unknown or when the number of data is <10 000 in the ML domain. The second advantage is that redundant clusters are identified due to the small expected values of the mixing coefficients and are therefore eliminated from the original cluster. Therefore, redundant clusters are less likely to be generated, and clustering with less noise might be possible.19 In medical biology, VBGMM is currently used in image recognition and is known to represent clusters.19-22
The VBGMM algorithm used 18 singular-value continuous and 6 binary discrete variables. These clinical variables were selected because of their general clinical use and ease of routine practice, considering known associations with adverse outcomes in HFpEF, a missing values rate of <20%, and a correlation coefficient of <80%. The selected variables were documented in Supporting Information, Table S1.
In this study, the optimal number of clusters was three as suggested by the VBGMM in the probability distribution derived from automatic relevance determination (Supporting Information, Figure S1A). To validate the probability distribution of VBGMM, the Bayesian information criterion (BIC) was calculated. The minimum BIC score of between two and nine clusters also predicted that the optimal number of clusters was three (Supporting Information, Figure S1B). Additionally, we performed principal component analysis (PCA), a singular-value decomposition (SVD) identifying an orthogonal change in the dataset. PCA is a traditional dimensionality reduction method frequently used in the ML domain. Supporting Information, Figure S2 plots the two-dimensional space of the first two principal components and is colour coded by three phenogroups. PCA illustrated some overlaps between the three phenogroups. Therefore, we decided that the optimal number of phenogroups was three.
To validate the results of the phenomapping of NARA-HF, we performed three different validations: (i) matching the rate of stratification using another unsupervised ML algorithm, (ii) applying VBGMM to another HFpEF cohort as an external validation, and (iii) ensuring the reproducibility with supervised ML.
(i) We applied hierarchical clustering to NARA-HF as another unsupervised ML algorithm. Hierarchical clustering is a representative unsupervised algorithm and has been used in previous HFpEF phenomapping.7, 12, 23 The advantage of hierarchical clustering is that it does not require a pre-specified number of clusters. Only the 18 standardized continuous variables from the 24 used in VBGMM were selected to apply hierarchical clustering. We stratified NARA-HF patients into three phenogroups by hierarchical clustering with Ward's method based on the dendrogram (Supporting Information, Figure S3). The three phenogroups were labelled as Phenogroups A–C. The matching rate between VBGMM and hierarchical clustering was analysed.
(ii) VBGMM was also adopted using JASPER as the derivation, divided into three phenogroups, and their characteristics were confirmed.
(iii) We applied the supervised ML method as a random forest (RF) method, which is generally used in the ML classification domain. The RF method builds decision trees and uses their majority vote for classification. The RF algorithm was trained by the NARA-HF cohort and VBGMM phenomapping labels (RF-NARA). To validate the reproducibility of the NARA-HF phenomapping, NARA-HF and JASPER combined cohorts were used as the composite cohort and used as the test dataset, classified using RF-NARA and VBGMM, and the three phenotypes mapped by VBGMM were compared with those conducted by RF-NARA by accuracy score and F1-measure score, calculated by (2 × precision × recall)∕(precision + recall).
VBGMM, hierarchical clustering, and RF were performed in Python (Version 3.6.5), scikit-learn package 0.19.1, NumPy package 1.14.3, pandas 0.23.0, scipy, and matplotlib 2.2.2 in the Jupyter Notebook (4.4.0). Before analysis, continuous missing values were imputed for ML using SVD in JMP 14.3.0 because the ML algorithm did not accept missing values. All missing data were considered missing at random. Given a matrix with missing values, the missing entries were imputed using a low-rank SVD approximation. SVD provides better imputation for small and large datasets compared with other algorithms.24
Outcomes of interest
In the NARA-HF study, the primary endpoint was defined as all-cause death and HF readmission within 5 years after registration. As a secondary outcome, cardiovascular death was defined as death due to HF, myocardial infarction, sudden death, stroke, or vascular diseases such as aortic dissection. We defined all-cause mortality as the primary endpoint because the cardiovascular death in elderly HF patients was hard to distinguish from other causes of death affected by their comorbidities. We checked the medical records to determine vital status and cause of death. When this information was unavailable in the medical records, we contacted patients or their families. If the patient had died, we interviewed his or her family about the institute where he or she died. We then asked the physician and confirmed the patient's cause of death. In the validation cohort of JASPER, we defined the primary and secondary endpoints as described above for 2 years because of differences in study design and mean follow-up duration.
Statistical analysis
Statistical analyses were performed using JMP Version 14.3.0 (SAS Institute, Cary, NC, USA). All values are expressed as mean ± standard deviation or as median with an inter-quartile range for continuous variables and counts and percentages for categorical variables. Continuous variables were compared using parametric one-way analysis of variance or the non-parametric Kruskal–Wallis test based on the normality of a variable's distribution. Categorical data were evaluated using Pearson's χ2 test. Statistical significance was set at P < 0.05.
Results
Classification of heart failure with preserved ejection fraction in derivation study
We divided the derivation cohort into three phenogroups (1–3) on the basis of the VBGMM probability distribution, the minimum BIC, and PCA plot (Supporting Information, Figures S1–S3).
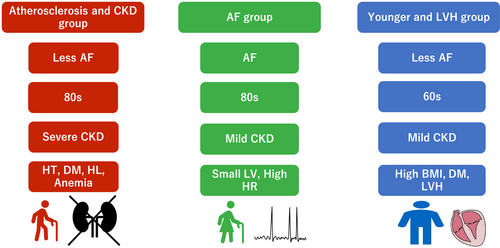
A comparison of the baseline characteristics in NARA-HF among the three clusters is shown in Tables 1 and 2. Phenogroup 1 was older (mean age 78.9 ± 9.1 years) and predominantly male and had a high incidence of hypertension, diabetes mellitus (DM), hyperlipidaemia, old myocardial infarction, and anaemia at discharge. Also, Phenogroup 1 showed the highest levels of brain natriuretic peptide (BNP) and C-reactive protein (CRP) among the three phenotypes. This phenogroup also had worse kidney function. The feature of Phenogroup 1 is characterized by atherosclerotic vascular diseases and related organ damage such as old myocardial infarction and chronic kidney disease (CKD). Phenogroup 2 had a higher incidence of women and older individuals and the lowest BMI among the phenogroups. Phenogroup 2 also had the highest incidence of AF and the lowest incidence of atherosclerotic factors. The features of Phenogroup 2 were summarized as AF and aged women (mean age 78.8 ± 9.7 years). Phenogroup 3 was the youngest and predominantly male. BMI was the highest. Echocardiography revealed the highest incidence of left ventricular hypertrophy (LVH) (47.5%). The characteristics of Phenogroup 3 were as follows: youngest, obese, and LVH. A summary of the characteristics of the three phenotypes in the derivation cohort is illustrated in Figure 2. We named the three phenogroups as the atherosclerosis and CKD group, the AF group, and the younger and LVH group.
| Phenogroup 1 (n = 125) | Phenogroup 2 (n = 200) | Phenogroup 3 (n = 40) | P value | |
|---|---|---|---|---|
| Demographics on admission | ||||
| Age (years) | 78.9 ± 9.1 | 78.8 ± 9.7 | 63.5 ± 11.2 | <0.0001 |
| Male, n (%) | 72 (57.6) | 85 (42.5) | 23 (57.5) | 0.0164 |
| Clinical characteristics on admission | ||||
| BMI at admission (kg/m2) | 23.40 ± 3.60 | 22.78 ± 3.94 | 27.46 ± 5.85 | <0.0001 |
| HR (b.p.m.) | 86.06 ± 26.04 | 92.97 ± 31.62 | 103.50 ± 29.73 | <0.0001 |
| SBP (mmHg) | 149.06 ± 31.08 | 147.29 ± 32.68 | 175.78 ± 40.26 | <0.0001 |
| DBP (mmHg) | 79.20 ± 20.76 | 79.70 ± 19.98 | 108.62 ± 27.18 | <0.0001 |
| NYHA, n (%) | <0.0001 | |||
| II | 12 (10) | 25 (13) | 3 (8) | |
| III | 69 (55) | 90 (45) | 11 (28) | |
| IV | 44 (35) | 85 (43) | 26 (65) | |
| Medical history on admission | ||||
| Hypertension, n (%) | 116 (92.8) | 145 (72.5) | 32 (80.0) | <0.0001 |
| Diabetes mellitus, n (%) | 68 (54.4) | 74 (37.0) | 16 (40.0) | 0.0079 |
| Hyperlipidaemia, n (%) | 61 (48.8) | 55 (27.5) | 21 (52.5) | <0.0001 |
| History of myocardial infarction, n (%) | 50 (40.0) | 11 (5.5) | 9 (22.5) | <0.0001 |
| Atrial fibrillation, n (%) | 49 (39.2) | 113 (56.5) | 16 (40.0) | 0.005 |
| Anaemia at discharge, n (%) | 53 (42.5) | 45 (22.5) | 1 (2.5) | <0.0001 |
| Echocardiographic data on admission | ||||
| LVEF (%) | 60.4 ± 10.5 | 60.3 ± 12.1 | 54.5 ± 14.5 | 0.0156 |
| LVDd (mm) | 48.8 ± 0.66 | 46.7 ± 0.53 | 49.1 ± 1.19 | 0.0273 |
| LVDs (mm) | 32.9 ± 0.65 | 31.1 ± 0.53 | 35.7 ± 1.17 | 0.0009 |
| Left ventricular hypertrophy, n (%) | 40 (32) | 19 (9.5) | 19 (47.5) | <0.0001 |
| IVST (mm) | 11.6 ± 2.3 | 10.3 ± 1.76 | 13.2 ± 3.8 | <0.0001 |
| PWT (mm) | 11.2 ± 1.9 | 10.2 ± 1.7 | 12.5 ± 2.7 | <0.0001 |
| LAD (mm) | 45.4 ± 8.7 | 44.8 ± 8.8 | 43.0 ± 6.3 | 0.429 |
- BMI, body mass index; DBP, diastolic blood pressure; HR, heart rate; IVST, interventricular septal thickness; LAD, left arterial diameter; LVDd, left ventricular end-diastolic diameter; LVDs, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association functional classification; PWT, posterior wall thickness; SBP, systolic blood pressure.
| Phenogroup 1 (n = 125) | Phenogroup 2 (n = 200) | Phenogroup 3 (n = 40) | P value | |
|---|---|---|---|---|
| HbA1c (%) | 6.15 ± 0.95 | 6.10 ± 1.04 | 6.09 ± 1.03 | 0.9194 |
| BNP (pg/dL) | 412.1 ± 406.4 | 183 ± 147.1 | 212.7 ± 177.4 | <0.0001 |
| CRP (mg/dL) | 1.07 ± 1.86 | 0.71 ± 1.01 | 0.56 ± 0.72 | 0.0285 |
| Haemoglobin (g/dL) | 10.31 ± 1.56 | 11.39 ± 1.89 | 13.64 ± 1.73 | <0.0001 |
| Serum renin (pg/mL) | 6.47 ± 7.99 | 7.51 ± 13.0 | 9.31 ± 14.2 | 0.5332 |
| Serum aldosterone (mg/dL) | 109.7 ± 9.5 | 102.5 ± 7.3 | 145.0 ± 14.3 | 0.0316 |
| Serum sodium (mmol/L) | 139.1 ± 2.8 | 138.3 ± 4.3 | 140.2 ± 2.2 | 0.0010 |
| Creatinine (mg/dL) | 1.79 ± 0.54 | 0.94 ± 0.31 | 0.96 ± 0.29 | <0.0001 |
| Blood urea nitrogen (mg/mL) | 41.3 ± 17.6 | 23.1 ± 9.1 | 20.4 ± 8.9 | <0.0001 |
| eGFR | 28.5 ± 9.7 | 55.6 ± 19.3 | 59.8 ± 18.9 | <0.0001 |
| Total protein (g/dL) | 6.53 ± 0.61 | 6.68 ± 0.76 | 7.17 ± 0.54 | <0.0001 |
| Serum albumin (g/dL) | 3.51 ± 0.45 | 3.61 ± 0.44 | 4.03 ± 0.30 | <0.0001 |
| Vital signs | ||||
| Heart rate (b.p.m.) | 68.8 ± 10.8 | 73.2 ± 12.9 | 67.6 ± 8.0 | 0.0007 |
| Systolic blood pressure (mmHg) | 118.5 ± 16.5 | 111.5 ± 14.7 | 122.4 ± 16.3 | <0.0001 |
| Diastolic pressure (mmHg) | 62.6 ± 10.4 | 61.4 ± 9.9 | 67.9 ± 9.0 | 0.0011 |
| Medication at discharge, n (%) | ||||
| ACE inhibitor | 59 (47.2) | 97 (48.5) | 20 (50.0) | 0.9469 |
| Angiotensin receptor blockers | 58 (46.4) | 71 (35.5) | 15 (37.5) | 0.1424 |
| Any RAS blocker | 107 (85.6) | 165 (82.5) | 33 (82.5) | 0.575 |
| MRA | 34 (27.2) | 79 (39.5) | 15 (37.5) | 0.0733 |
| Calcium channel blocker | 69 (55.2) | 67 (33.5) | 14 (35.0) | 0.0004 |
| Diuretics | 109 (87.2) | 154 (77.0) | 25 (62.5) | 0.0024 |
| Digitalis | 7 (5.6) | 18 (9.0) | 2 (5.0) | 0.4381 |
| Antiplatelet therapy | 29 (23.3) | 23 (11.5) | 8 (20.0) | 0.0176 |
| Any anticoagulation therapy | 49 (39.2) | 96 (48.0) | 16 (40.) | 0.2561 |
| Statins | 46 (36.8) | 45 (22.5) | 17 (42.5) | 0.0038 |
| Diabetes drugs | 50 (40) | 49 (24.5) | 8 (20) | 0.0266 |
| SGLT2 inhibitor | 1 (0.008) | 2 (0.01) | 0 (0) | 0.8146 |
| Insulin user | 10 (8.0) | 10 (5.0) | 1 (2.5) | 0.3407 |
- ACE, angiotensin-converting enzyme; BNP, brain natriuretic peptide; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; MRA, mineralocorticoid receptor antagonist; RAS, renin–angiotensin system; SGLT2, sodium–glucose cotransporter 2.
Prognostic relationship between clinical phenotypes and patient outcome
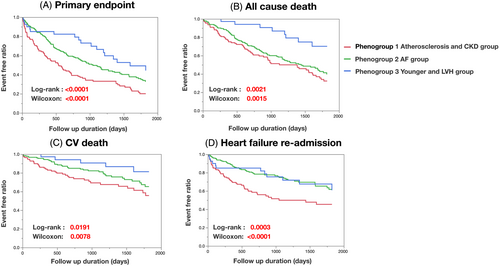
As shown in Table 3, Phenogroup 1 demonstrated a significantly worse prognosis of the primary composite endpoint compared with the other two phenotypes (Phenogroups 1–3: 72.0% vs. 58.5% vs. 45%, P = 0.0036). As secondary endpoints, the incidences of all-cause death, cardiovascular death, or HF readmission within the 5 years were also significantly higher in Phenogroup 1 than in the other two groups (Table 3). When comparing Phenogroups 2 and 3, the occurrence of all-cause death tended to be higher in Phenogroup 2 than Phenogroup 3. In contrast, the rates of cardiovascular death and HF readmission were similar between the two phenogroups despite older age and worse kidney function in Phenogroup 2. The Kaplan–Meier survival curves of the primary composite endpoint and each secondary endpoint for 5 years are illustrated in Figure 3.
| 5 year clinical outcome | Phenogroup 1 (n = 119) | Phenogroup 2 (n = 184) | Phenogroup 3 (n = 39) | P value |
|---|---|---|---|---|
| Primary endpoint, n (%) | 90 (72.0) | 117 (58.5) | 18 (45) | 0.0036 |
| Secondary endpoint | ||||
| All-cause death, n (%) | 62 (51.7) | 88 (44.0) | 8 (20) | 0.0021 |
| Cardiovascular death, n (%) | 35 (28.0) | 39 (19.5) | 5 (12.5) | 0.0467 |
| Heart failure re-hospitalization, n (%) | 53 (42.4) | 51 (25.5) | 12 (30.0) | 0.0061 |
- Primary endpoint included all-cause death and heart failure re-hospitalization.
Hierarchical clustering in NARA-HF
The characteristics of the three phenogroups stratified by hierarchical clustering are documented in Supporting Information, Table S2 and are summarized as follows: Phenogroup A was older with predominantly atherosclerotic factors and CKD, Phenogroup B was predominantly female with AF, and Phenogroup C was younger with relatively high BMI. These characteristics were similar to the VBGMM phenomapping results. From the above matching features, we determined that VBGMM Phenogroups 1–3 corresponded to the hierarchical clustering Phenogroups A–C, respectively. Similar to the previous report that compared the result of orthogonal two unsupervised ML algorithms, the matching rate between these two algorithms is illustrated in Supporting Information, Figure S4. The percentage of matched individuals in VBGMM (% cluster in VBGMM) of Phenogroup 1 and Phenogroup A was 69.6%, of Phenogroup 2 and Phenogroup B was 49.0%, and of Phenogroup 3 and Phenogroup C was 77.5%. However, Phenogroup 2 and Phenogroup C did not show complete agreement regarding the prevalence of LVH, which may have caused the difference between the clustering algorithms. Phenogroup C included only 29.3% of Phenogroup 3 and 40.6% of Phenogroup 2.
External validation of the phenogroups in the JASPER cohort
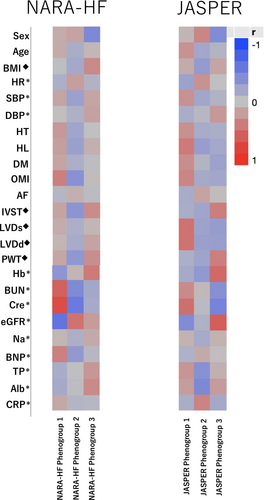
For external validation, we validated our results using data from the JASPER cohort. Among the 534 patients with HFpEF in the JASPER study, 273 patients were analysed after excluding 230 patients with missing values for 24 variables used for clustering (Supporting Information, Table S1) and 31 patients with eGFR < 15 mL/min/1.73 m2 (Figure 1). The major differences in baseline characteristics between the derivation and validation cohorts are shown in Supporting Information, Table S3. After phenomapping by VBGMM using the same 24 variables as in the derivation analyses, we succeeded in dividing the patients into three phenogroups similar to those in the derivation cohort. The characteristics of each phenogroup are presented in Table 4. Phenogroup 1 had a higher incidence of older aged patients, men, and atherosclerotic risk factors and the highest rate of old myocardial infarction and poor kidney function. Phenogroup 2 had the oldest age, lowest BMI, and highest prevalence of women and AF. Phenogroup 3 had the youngest age and highest BMI, relatively better kidney function, and LVH. The characteristics of these three phenogroups in the validation cohort were similar to those in the derivation cohort of the NARA-HF study, which was clearly shown by the correlation heat map of each variable (Figure 4). The variables of sex, age, BMI, heart rate, blood pressure at admission, atherosclerotic risk factors, AF, left ventricular wall thickness, and kidney function showed a similar correlation coefficient pattern between the two cohorts. However, laboratory findings such as CRP, serum total protein (TP), and serum albumin (Alb) did not clarify the correspondence between the two cohorts. However, among the three phenogroups in the validation cohort, the phenogroup of AF showed a significantly worse prognosis of 2 year primary and secondary endpoints than the phenogroup of atherosclerosis and CKD (Supporting Information, Table S4 and Figure S5), which were different from those in the derivation cohort.
| Phenogroup 1 (n = 126) | Phenogroup 2 (n = 74) | Phenogroup 3 (n = 73) | P value | |
|---|---|---|---|---|
| Demographics on admission | ||||
| Age (years) | 78.2 ± 7.9 | 82.6 ± 6.6 | 70.7 ± 11.7 | <0.0001 |
| Male, n (%) | 80 (63.5) | 24 (34.3) | 30 (41.1) | <0.0001 |
| BMI (kg/m2) | 24.6 ± 3.6 | 21.6 ± 4.4 | 25.2 ± 6.0 | <0.0001 |
| Medical history on admission | ||||
| Diabetes, n (%) | 70 (55.6) | 24 (32.4) | 15 (20.6) | <0.0001 |
| Hypertension, n (%) | 113 (89.7) | 49 (66.2) | 49 (67.1) | <0.0001 |
| Hyperlipidaemia, n (%) | 83 (65.9) | 16 (21.6) | 21 (28.8) | <0.0001 |
| Hyperuricaemia, n (%) | 68 (53.9) | 26 (35.1) | 20 (27.4) | 0.0015 |
| Atrial fibrillation, n (%) | 70 (55.6) | 57 (77.0) | 48 (65.8) | 0.0088 |
| History of myocardial infraction, n (%) | 27 (21.4) | 1 (1.4) | 0 (0) | <0.0001 |
| Echocardiographic data on admission | ||||
| LVDd (mm) | 50.3 ± 6.0 | 44.3 ± 5.6 | 43.6 ± 6.5 | <0.0001 |
| LVDs (mm) | 33.6 ± 6.0 | 27.8 ± 4.2 | 27.6 ± 6.5 | <0.0001 |
| LVEF (%) | 59.5 ± 6.7 | 61.0 ± 7.8 | 61.2 ± 7.9 | 0.2004 |
| IVST (mm) | 10.4 ± 1.7 | 9.9 ± 1.9 | 12.3 ± 3.1 | <0.0001 |
| PWT (mm) | 10.3 ± 1.4 | 9.8 ± 1.4 | 11.8 ± 2.7 | <0.0001 |
| LAD (mm) | 47.2 ± 9.2 | 46.4 ± 10.7 | 44.8 ± 8.7 | 0.2813 |
| Laboratory findings at discharge | ||||
| HbA1c (%) | 6.4 ± 1.1 | 6.0 ± 1.0 | 5.9 ± 0.9 | 0.2201 |
| BNP (pg/dL) | 180.1 ± 160.1 | 256.1 ± 289.5 | 212.9 ± 249.8 | 0.0730 |
| CRP (mg/dL) | 0.40 ± 0.53 | 0.94 ± 1.04 | 0.30 ± 0.34 | <0.0001 |
| Haemoglobin (g/dL) | 11.2 ± 1.7 | 10.5 ± 1.5 | 13.2 ± 1.9 | <0.0001 |
| Creatinine (mg/dL) | 1.42 ± 0.47 | 1.15 ± 0.37 | 0.81 ± 0.21 | <0.0001 |
| Blood urea nitrogen (mg/dL) | 34.9 ± 1.3 | 30.6 ± 1.7 | 19.6 ± 1.6 | <0.0001 |
| eGFR at discharge (mL/min/1.73 m2) | 38.1 ± 13.2 | 43.0 ± 15.3 | 64.2 ± 11.9 | <0.0001 |
| Total protein (g/dL) | 7.0 ± 0.6 | 6.4 ± 0.6 | 6.9 ± 0.6 | <0.0001 |
| Serum albumin (mg/dL) | 3.8 ± 0.4 | 3.4 ± 0.4 | 3.9 ± 0.4 | <0.0001 |
| Vital signs at discharge | ||||
| Heart rate (b.p.m.) | 64.1 ± 10.4 | 71.9 ± 11.9 | 66.9 ± 10.1 | <0.0001 |
| Systolic blood pressure (mmHg) | 118.8 ± 14.9 | 109.6 ± 14.5 | 112.1 ± 14.4 | <0.0001 |
| Diastolic pressure (mmHg) | 60.2 ± 11.8 | 61.0 ± 10.0 | 66.5 ± 10.1 | 0.0004 |
- BMI, body mass index; BNP, brain natriuretic peptide; CRP, C-reactive protein; eGFR, estimated glomerular filtration rate; HbA1c, glycated haemoglobin; IVST, interventricular septal thickness; LAD, left arterial diameter; LVDd, left ventricular end-diastolic diameter; LVDs, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; PWT, posterior wall thickness.
Supervised machine learning validation
We validated our results of the present unsupervised phenomapping for NARA-HF and JASPER by supervised ML, an RF algorithm. The composite cohort combining NARA-HF and JASPER was classified into three phenogroups as the derivation by VBGMM. Subsequently, the composite cohort was also classified into three phenogroups by RF-NARA, which was trained by the derivation phenomapping result of NARA-HF. The accuracy and F1-measure scores comparing the three phenogroup labels obtained by VBGMM and RF-NARA to the composite cohort were 0.845 and 0.785, respectively.
Discussion
We stratified HFpEF into three phenogroups based on standard clinical variables using unsupervised ML with the NARA-HF study as a derivation cohort in Japan. Next, we validated the phenomapping of NARA-HF using another unsupervised ML algorithm and a supervised one. Finally, using another Japanese multicentre HFpEF registry, the JASPER study, as a validation cohort, we succeeded in stratifying three phenogroups similar to those in the derivation cohort. Both cohorts included patients who were admitted to the hospital for ADHF. Therefore, the patients were much older than those enrolled in randomized control trials (RCTs), such as the I-Preserved trial and the TOPCAT trial, and the BMI was much lower in Japanese cohorts than in Western populations.2, 8
About the clustering variables
Phenotyping using ML was dependent on the variables used. In the present study, we used standard variables such as BMI, atherosclerotic risk factors, kidney function, AF, LVH, and BNP levels, so that the present phenotyping can be widely applied. Consequently, the three phenogroups stratified in the present study were characterized as atherosclerosis and CKD, AF, and younger and LVH, providing insight on the development of HFpEF. Although the present phenotyping was not entirely consistent with previous studies, these characteristics were commonly observed in each phenogroup in earlier reports in Western countries,7-12 in which patients were more obese than Japanese patients. However, when comparing the phenotype results of the JASPER and NARA-HF studies, the trends in mean values of CRP, TP, and Alb were not completely consistent. These results might not be important variables for the phenomapping of HFpEF using clinical data.
Phenogroup 2 (AF group)
In the phenogroup characterized by AF, the patients were older women and frequently had CKD in both our cohorts and several previous studies' populations, whereas BMI was much lower in patients in Japanese studies than in those in Western countries.7-9 Shah et al. first reported a phenogroup characterized by CKD and AF in ML phenomapping.7 Cohen et al. also presented an older, with stiff arteries, small left ventricles, diastolic function, and AF group.8 Additionally, Jones et al. also reported a distinct HFpEF phenogroup, which showed characteristics of diastolic dysfunction in haemodynamic analysis, similar to Cohen et al.'s AF group.12 The AF group we presented here was very similar to previously reported phenogroups, especially in terms of frequency of AF, lower BMI, older age, and poorer renal function than the other groups. Given that AF is almost equally observed in patients with higher and lower BMI in the Japanese AF cohort,25 it is possible that AF contributes to the development of HFpEF with or without obesity. Considering that AF occurs more frequently in men than in women, it is notable that the incidence of women was higher in this phenogroup of HFpEF.25 Additionally, a phenogroup with AF, predominantly elderly women, and mildly CKD was frequently observed in previous Western studies. Therefore, the phenogroup with elderly, AF, and CKD may be a common HFpEF phenotype worldwide, although their BMI was slightly different.
Phenogroups 1 (atherosclerosis and CKD group) and 3 (younger and LVH group)
Phenogroups with a higher incidence of atherosclerotic risk factors have also been identified in previous reports. Kao et al. reported phenogroups with a high rate of atherosclerotic factors, such as coronary artery disease, DM, and CKD, in patients enrolled in the I-Preserved trial, and these phenogroups with a high risk of atherosclerosis showed the worst outcome.9 Cohen et al. proposed that the phenogroup with high-risk factors for atherosclerosis and obesity had the highest mortality in HFpEF phenotyping using the TOPCAT trial.8 Thus, a phenogroup similar to Phenogroup 1 in the present study was also classified in previous Western cohorts of HFpEF. This phenotype would be similar to one recently reported by Hahn et al.11 and Jones et al.12 that is characterized by a higher N-terminal pro-BNP value and relatively low ejection fraction (EF). However, the characteristics of our atherosclerotic phenogroup were not completely consistent with those of atherosclerotic and obesity phenotypes in previous reports, such as the prevalence of DM. For example, Cohen et al.'s Phenogroup 3 (obese, diabetic, with advanced symptoms) had a DM frequency of 88%, whereas our equivalent Phenogroup 3 had a DM frequency of 40%.8 Subgroup C presented by Kao et al. (elderly patients with a high prevalence of atherosclerotic factor and CKD) also had a DM frequency of 100%, whereas our Phenogroup 1 had a frequency of 68%.9 This may be explained by the difference in lifestyle between Western countries and Japan, which could be represented by the proportion of obesity between our study and previous reports. Generally, obesity is a stronger risk factor for atherosclerotic disease in Western countries than in Japan and other Asian countries. In the present study, patients in the atherosclerosis and CKD phenogroup were not obese, and their cardiac geometry was not hypertrophied. However, patients in the corresponding phenogroups in the I-Preserved and TOPCAT trials were obese and had LVH.8, 9 Our patients in Phenogroup 3 (younger and LVH) were more obese and had the most severe LVH compared with the other two phenogroups. The phenogroup of atherosclerosis in Western countries might include a subgroup of patients who would be classified as Phenogroup 3 if in Japan.
In previous phenotyping of HFpEF using RCTs, there was a phenogroup with relatively normal left ventricular geometry, with a lower proportion of atherosclerotic risk factors and AF.8 In the present study, we were unable to identify this phenogroup. A possible explanation may be the difference in patient recruitment between our study and previous studies. The NARA-HF and JASPER studies are registries of patients with ADHF at unexpected admission to hospitals, whereas the I-Preserved and TOPCAT trials are RCTs that recruited outpatient patients with HFpEF.
Possible treatment strategies by phenotype
Recently, SGLT2 inhibitors were reported to be drugs useful for reducing a composite of cardiovascular death and HF hospitalization in patients with HFpEF.3 However, given that HFpEF is a complex syndrome consisting of different subgroups classified with some common characteristics, phenotype-related treatment strategies should be proposed in the future. Considering the present results, the subgroup of patients characterized with AF would possibly be suitable candidates for treatment with ablation,26, 27 those characterized with atherosclerosis and CKD would be for SGLT2 inhibitors,28 and those characterized with LVH would be for sacubitril–valsartan29 or mineralocorticoid receptor antagonists.8
Limitations
The present study had several limitations. First, the NARA-HF study is a single-centre, relatively small study; therefore, regional and clinical decision bias might be related to the phenotypes and clinical outcomes. Second, because the appropriate number of clusters varies depending on the dataset and clustering method, it is difficult to determine whether the number of clusters currently presented is truly optimal. Third, the selection bias of variables for phenomapping might have affected the results.
Fourth, we could not completely exclude the patients with secondary cardiomyopathy and recovered EF who improved their LVEF during hospitalization in the NARA-HF study. Fifth, we used only a few echo parameters in our analysis, because many echo parameters had 20% or more missing values. This may have affected the present phenomapping result. Sixth, we imputed some missing variables. The largest missing variable was Alb at discharge (deficit rate was 19.5%). Segar et al.10 excluded variables with 10% or more missing values, and the missing values were imputed using the missForest package. Kao et al.9 also imputed missing data for 67% of patients, using 20 multiply imputed datasets. Compared to past studies, the percentages of imputed variables in the current study might be acceptable. However, there might be some bias depending on the imputation algorithm. Finally, the primary composite event rate in the derivation study did not match that in the validation study. This could have resulted from the difference in kidney function and age of each phenogroup between the two cohorts. In the NARA-HF study, the incidence of worse CKD was higher than that in the JASPER study.
In conclusion, we identified three HFpEF phenotypes with different clinical characteristics in Japanese patients with HFpEF, who had lower BMI and incidence of obesity than Western patients. The feature values and comorbidities of each phenogroup such as atherosclerotic risk factors, age, sex, and AF in the present study were similar to those of earlier studies conducted in Western countries, whereas BMI and the rate of obesity did not correspond to these earlier studies. Further studies with a much larger sample size are necessary to further clarify the precise phenotypes in HFpEF, a complex syndrome.
Acknowledgements
We thank Yoko Wada, Yuki Kamada, and Rika Nagao for their support during data collection.
Conflict of interest
Y.S. has received research funds from Otsuka Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd., Takeda Pharmaceutical Co., Ltd., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Bristol-Myers Squibb Company, Actelion Pharmaceuticals Japan Ltd., Kyowa Kirin Co., Ltd., Kowa Pharmaceutical Co. Ltd., Shionogi & Co., Ltd., Dainippon Sumitomo Pharma Co., Ltd., Teijin Pharma Ltd., Chugai Pharmaceutical Co., Ltd., Eli Lilly Japan K.K., Nihon Medi-Physics Co., Ltd., Novartis Pharma K.K., Pfizer Japan Inc., and Fuji Yakuhin Co., Ltd.; research expenses from Novartis Pharma K.K., Roche Diagnostics K.K., Amgen Inc., Bayer Yakuhin, Ltd., Astellas Pharma Inc., and Actelion Pharmaceuticals Japan Ltd.; speakers' bureau/honorarium from Alnylam Japan K.K., AstraZeneca K.K., Otsuka Pharmaceutical Co., Ltd., Kowa Pharmaceutical Co. Ltd., Daiichi Sankyo Co., Ltd., Mitsubishi Tanabe Pharma Corporation, Tsumura & Co., Teijin Pharma Ltd., Toa Eiyo Ltd., Nippon Shinyaku Co., Ltd., Nippon Boehringer Ingelheim Co., Ltd., Novartis Pharma K.K., Bayer Yakuhin Ltd., Pfizer Japan Inc., Bristol-Myers Squibb Company, and Mochida Pharmaceutical Co., Ltd.; and consultation fees from Ono Pharmaceutical Co., Ltd. and Novartis Pharma K.K. The remaining authors have no conflicts of interest to report.




