Association between lactate/albumin ratio and mortality in patients with heart failure after myocardial infarction
Yang Chen and Ke Yang contributed equally to this work.
Abstract
Aims
Lactate/albumin ratio (L/A) is a recognized prognostic index of patients with heart failure (HF) after myocardial infarction (MI). We aim to evaluate the prognostic value of L/A ratio in predicting in-hospital mortality for those patients.
Methods and results
We enrolled qualified patients from Medical Information Mart for Intensive Care IV database for retrospective study. A receiver operating characteristic (ROC) curve of the subjects was applied to determine the predicted value and the best cut-off value of L/A on admission. Univariate/multivariate Cox regression analysis and restricted cubic splines (RCS) were performed to identify the association between hospital admission and hospital mortality. The Kaplan–Meier (KM) method was used to draw the survival curve of the two groups with different L/A levels at admission. L/A values at admission were significantly higher in the death groups than the survival groups [1.36 (1.20) vs. 0.62 (0.36), P < 0.05], and area under the ROC curve [0.780 (95% confidence interval, 0.772–0.827)] was better than other indicators, and the best the cut-off value was 0.671. Data of Cox regression analysis showed that higher L/A value supposed to be an independent risk factor for in-hospital mortality. RCS analysis showed evidence of an increasing trend and a non-linear relationship between L/A and in-hospital mortality (P value was non-linear <0.05). KM survival curves were significantly lower in the high L/A group than the low L/A group (P < 0.001), and the former group had an increased risk of in-hospital mortality compared with the latter one (log rank P < 0.001).
Conclusions
Elevated L/A ratio on admission is an independent predictor of high in-hospital mortality in post-MI heart failure patients, which proved to be better than lactate, Sequential Organ Failure Assessment score and other related indicators.
Introduction
The incidence of myocardial infarction (MI) is increasing year by year, and the number of patients with heart failure (HF) after MI is also gradually elevating. According to statistics, there are approximately 23 million people with HF worldwide by 2017.1 Previous studies have shown that the 30 day and 1 year mortality rates for HF are 10.4% and 22%, respectively.2-4 Moreover, the Framingham Heart Study showed that the incidence of HF 30 day post-infarction increased from 10.0% to 23.1%, and the mortality rate decreased from 12.2% to 4.1% in the time periods 1970 to 1979 and 1990 to 1999, respectively. The incidence of HF 5 year after MI increased from 27.6% to 31.9%, and the mortality rate decreased from 41.1% to 17.3%. Although the mortality rate of heart attack is on a decreasing trend, the incidence of HF is still rapidly increasing.5 Additionally, a Norwegian study enrolled 86 771 patients with first-episode acute heart attack from 2001 to 2009 (follow-up to the end of 2009), and its data shown that the mortality rates were 61.4% and 28.3% in men with and without HF, as well as 70.0% and 39.2% in women, respectively.6 Therefore, it is important to early detected MI patient with HF.
Predictive biomarkers play a vital role in improving management of critical patients with post-MI HF. Elevated lactate levels are associated with mortality, which are widely used for early diagnosis, treatment, and risk stratification of patients with infectious shock.7-9 However, lactate levels are associated with complex changes in the bodies, such as decreased lactate excretion due to liver/renal dysfunction and accelerated glycolysis, which indicated that initial lactate levels could be low.10-13 In fact, there is a high risk for premature death in patients with normal or moderate lactate levels. Importantly, serum albumin, as a negative acute phase protein, also served as a biomarker of prognosis in patients with sepsis.14 Similar to lactate levels, serum albumin levels are affected by a variety of pathological conditions, including inflammation, malnutrition, and hepatic sclerosis.15
Interestingly, the lactate/albumin (L/A) ratio is a well-known biomarker for predicting short-term survival outcomes in patients with septic shock or severe sepsis.16-18 In addition, Shin et al. demonstrated that the L/A ratio was superior to lactate in predicting the area under the receiver operating characteristic (ROC) curve (AUROC) for prognosis in severe sepsis.16 Other studies have also evaluated the validity of the L/A ratio as a predictor. Kong et al. concluded that the L/A ratio was superior to lactate alone in predicting favourable neurological outcomes and survival to hospital discharge after out-of-hospital cardiac arrest.19
However, the role of the L/A ratio in predicting the prognosis of patients with severe post-MI HF remains unclear. Therefore, we conducted a retrospective study to address this key question. We hypothesized that the L/A ratio would predict survival outcomes during hospitalization in patients with severe HF after MI.
Methods
Data source and ethics statement
Medical Information Mart for Intensive Care IV (MIMIC-IV), developed by the MIT Computational Physiology Laboratory, is a public database integrating de-identified clinically anonymous data from patients admitted to Beth Israel Deaconess Medical Center (BIDMC, Boston, MA, USA) from 2008 to 2019. It protects patient privacy so that no informed consent is required for data collection. We completed the Collaborative Institutional Training Initiative (CITI) programme course entitled ‘Data or Specimens Only Research’ in ‘Human Research’ (Name ID: 9652605, Record ID: 39635575), and received permission to access the dataset. We designed and conducted this study in accordance with the relevant guidelines and regulations (Declaration of Helsinki).
Inclusion and exclusion criteria
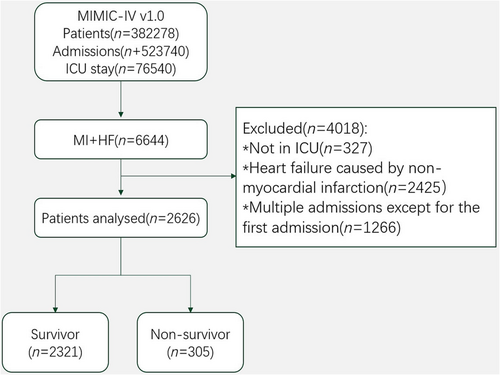
Upon further review, patient samples meeting the following criteria were excluded: non-critically ill patients not stay in intensive care unit (ICU), Patients with diagnoses of HF caused by non-MI were excluded. Critically ill patients diagnosed with MI and HF on admission to the ICU were included in this study.20 For patients with multiple admissions, only initial admission data were used. Details of the selection process employed in this study were presented in Figure 1. Some important points were listed below: (1) all patients enrolled in this study were from the MIMIC-IV database with a diagnosis of MI and HF, and HF patients admitted to the ICU due to old MI were also included; (2) both STEMI and NSTEMI patients were included, either type 1 MI or type 2 MI, and patients with angina pectoris were excluded; (3) for patients with multiple hospitalizations, only the first hospitalization data were selected for the study, and both coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI) were homogeneous in this study; (4) enrolled samples with a diagnosis of HF post-MI were admitted, and samples with HF without MI were excluded; (5) chronic HF was not the exclusion criteria, but patients with chronic HF who were not diagnosed as HF post-MI during this hospitalization will be excluded.
Data extraction
We used Structured Query Language and Navicat software (version 15) to extract the data. For clinical parameters with multiple outcomes during a patient's hospitalization, only the initial outcome was included. The primary factor L/A, was calculated as L/A. Clinical parameters were considered potential confounders, including demographics, comorbidities, laboratory values, vital signs, scoring systems, and treatment. In subsequent analyses, less than 30% missing values were interpolated using multiple imputation methods. The end point of this study is all-cause hospitalization mortality.
Statistical analysis
Baseline characteristics of the survivor and non-survivor samples were compared. Normal distributions were assessed using the Kolmogorov–Smirnov test. Continuous variables were expressed as mean with standard deviation (normal distribution) or median with interquartile range (non-normal distribution). Student's t-test or Mann–Whitney test was used. Categorical variables were expressed as counts (percentages, %) and compared using χ2 tests.
Independent risk factors for in-hospital mortality were identified using univariate and multifactorial Cox regression analyses. Potential risk factors with P values <0.05 in the univariate analysis were included in a multifactorial Cox regression model using backward Wald stepwise variable selection. In addition, variance inflation factor was used to test for collinearity among variables after backward Wald stepwise variable selection, and the remaining variables constituted a multivariate adjusted model.
ROC analysis was used to analyse the relationship between admission L/A and in-hospital mortality. The optimal cut-off value for admission L/A was determined based on Youden's index. AUROC was used to compare admission L/A with other relevant indicators.
RCS method was used to analyse the non-linear relationship between admission L/A and inpatient morbidity and mortality. The RCS does not require the assumption that the relationship between covariates and outcomes is linear, which provides greater flexibility in fitting the data.21 Thus, RCS allows the model to have a more complex relationship between the outcome and the variable of interest based on Cox regression while adjusting for other covariates. The RCS in this study is based on our previously established multivariate adjusted Cox regression model. Splines were defined using four nodes at 25th, 50th, 75th, and 95th percentiles.
The samples in this study were divided into high L/A and low L/A groups based on the best cut-off value for admission. Survival curves were plotted for the two groups using the KM method, and differences between groups were compared (log rank test). Hazard ratio (HR) for in-hospital mortality was calculated using a previously established multivariate adjusted Cox proportional risk model.
P values <0.05 were considered statistically significant and all tests were two-tailed. SPSS software (version 23) was used to calculate the Youden's index and cut-off value. Stata software (version 16) was used to calculate and plot ROC, RCS, and KM.
Results
Overall, 6644 critically ill patients with post-infarction HF were screened in the database. Of these patients, 4018 were excluded because they did not meet the criteria. Thus, the final analysis included 2626 participants. In total, 305 participants (11.6%) died in hospital (Figure 1).
The non-survival group had a higher admission L/A ratio values
In Table 1, the admission L/A values were higher in the death group than the survival group [1.36 (1.22–1.49) vs. 0.62 (0.60–0.63), P < 0.05]. In addition, other variables showed significant differences between the two groups. For example, lactate and troponin T levels were higher in the death group compared with the survival group, but PCI and intra-aortic balloon pump (IABP) ratios were not significantly different.
| Variables | All patients | Survivor | Non-survivor | P value |
|---|---|---|---|---|
| Patients | n = 2626 | n = 2321 | n = 305 | — |
| Demographics | ||||
| Age (years) | 67.48 (67.02–67.95) | 67.22 (66.73–67.71) | 69.50 (68.12–70.87) | 0.002 |
| Gender [male, n (%)] | 1734 (66.0%) | 1545 (66.6%) | 189 (62.0%) | 0.111 |
| BMI (kg/m2) | 28.92 (28.66–29.19) | 28.83 (28.56–29.11) | 29.60 (28.68–20.52) | 0.067 |
| Comorbidities | ||||
| Hypertension [n (%)] | 1166 (44.4%) | 1093 (47.1%) | 73 (23.9%) | <0.001 |
| T2DM [n (%)] | 942 (35.9%) | 844 (36.4%) | 98 (32.1%) | 0.147 |
| Stroke [n (%)] | 741 (28.2%) | 671 (28.9%) | 70 (23.0%) | 0.03 |
| CKD [n (%)] | 1269 (48.3%) | 1136 (48.9%) | 133 (43.6%) | 0.079 |
| AF [n (%)] | 1357 (51.7%) | 1220 (52.6%) | 137 (44.9%) | 0.012 |
| COPD [n (%)] | 382 (14.5%) | 330 (14.2%) | 52 (17.0%) | 0.187 |
| Laboratory variables | ||||
| L/A | 0.70 (0.68–0.73) | 0.62 (0.60–0.63) | 1.36 (1.22–1.49) | <0.001 |
| Lactate (mmol/L) | 2.29 (2.23–2.35) | 2.10 (2.06–2.15) | 3.69 (3.39–3.99) | <0.001 |
| Albumin (g/dL) | 3.45 (3.43–3.47) | 3.51 (3.49–3.54) | 2.95 (2.88–3.02) | <0.001 |
| Creatinine (mg/dL) | 1.72 (1.67–1.77) | 1.66 (1.61–1.72) | 2.15 (2.00–2.30) | <0.001 |
| PO2 (mmHg) | 154.07 (151.58–156.55) | 159.07 (156.40–161.73) | 116.00 (110.79–121.23) | <0.001 |
| PCO2 (mmHg) | 41.63 (41.37–41.88) | 41.56 (41.30–41.82) | 42.14 (41.27–43.01) | 0.147 |
| Haemoglobin (g/dL) | 10.34 (10.29–10.40) | 10.39 (10.34–10.45) | 9.96 (9.79–10.13) | <0.001 |
| WBC (K/uL) | 10.89 (10.69–11.10) | 10.42 (10.23–10.63) | 10.44 (13.65–15.24) | <0.001 |
| PLT (K/uL) | 216.56 (213.42–219.71) | 220.75 (217.48–224.03) | 184.67 (174.72–194.62) | <0.001 |
| Sodium (mmol/L) | 138.30 (138.19–138.42) | 138.31 (138.19–138.42) | 138.31 (137.79–138.84) | 0.970 |
| Potassium (mmol/L) | 4.26 (4.25–4.27) | 4.25 (4.24–4.26) | 4.32 (4.27–4.38) | <0.001 |
| Troponin T (ng/mL) | 0.92 (0.85–0.99) | 0.80 (0.74–0.87) | 1.79 (1.46–2.12) | <0.001 |
| Vital signs | ||||
| SBP (mmHg) | 115.12 (114.59–115.67) | 116.02 (115.47–116.57) | 108.31 (106.45–110.18) | <0.001 |
| DBP (mmHg) | 56.32 (55.81–56.83) | 56.52 (55.96–57.08) | 54.82 (53.79–55.85) | 0.036 |
| Heart rate (bpm) | 84.20 (83.78–84.63) | 83.57 (83.14–84.01) | 89.01 (87.52–90.49) | <0.001 |
| Scoring systems | ||||
| SOFA | 5.13 (5.03–5.23) | 4.80 (4.70–4.90) | 7.63 (7.26–8.01) | <0.001 |
| SIRS | 2.64 (2.61–2.67) | 2.61 (2.58–2.64) | 2.92 (2.83–3.00) | <0.001 |
| Prescription | ||||
| ACEI [n (%)] | 462 (17.59%) | 432 (18.61%) | 32 (10.49%) | <0.001 |
| Digoxin [n (%)] | 82 (3.12%) | 63 (2.71%) | 19 (6.23%) | 0.001 |
| Beta blocker [n (%)] | 925 (35.22%) | 789 (33.99%) | 136 (44.59%) | <0.001 |
| Diuretic [n (%)] | 939 (35.76%) | 775 (33.39%) | 164 (53.77%) | <0.001 |
| Treatments | ||||
| RRT [n (%)] | 250 (9.52%) | 164 (7.07%) | 86 (28.20%) | <0.001 |
| CABG [n (%)] | 935 (35.61%) | 904 (38.95%) | 31 (10.16%) | <0.001 |
| PTCA [n (%)] | 444 (16.91%) | 415 (17.88%) | 29 (9.51%) | <0.001 |
| PCI [n (%)] | 164 (6.25%) | 140 (6.03%) | 24 (7.89%) | 0.213 |
| IABP [n (%)] | 359 (13.67%) | 305 (13.14%) | 54 (17.70%) | 0.029 |
- ACEI, angiotensin-converting-enzyme inhibitors; AF, atrial fibrillation; BMI, body mass index; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; PTCA, percutaneous transluminal coronary angioplasty; RRT, renal replacement therapy; SBP, systolic blood pressure; SIRS, Systemic Inflammatory Response Syndrome; SOFA, Sequential Organ Failure Assessment; T2DM, type 2 diabetes mellitus.
- P values <0.05 are indicated in bold.
High ratio of L/A in hospital is an independent risk factor for hospitalization mortality
By calculating HR and confidence interval (CI) of variables included in this study, univariate Cox regression analysis shows that many variables are related to in-hospital mortality (Table 2). Multivariate Cox regression analysis showed that higher admission L/A (HR, 1.8839; 95% CI, 1.6853–2.1058; P < 0.001), older (HR, 1.0256; 95% CI, 1.0147–1.0367; P < 0.001), higher creatinine (HR, 1.1534; 95% CI, 1.0605–1.2543; P < 0.001), higher white blood cell (HR, 1.0207; 95% CI, 1.0102–1.0314; P < 0.001), higher Troponin T (HR, 1.0867; 95% CI, 1.0473–1.1276; P < 0.001), higher Sequential Organ Failure Assessment (SOFA) score (HR, 1.1216; 95% CI, 1.0790–1.1659; P<0.001), and IABP (HR, 1.1694; 95% CI, 0.8438–1.6206; P = 0.029) are independent risk factors of death in hospital. On the contrary, hypertension (HR, 0.5976; 95% CI, 0.4494–0.7947, P < 0.001) and CABG (HR, 0.3012; 95% CI, 0.2052–0.4423; P < 0.001) are protective factors. Finally, data from the multivariate Cox regression analysis shown that the nine variables related to inpatient mortality constituted a multivariate adjusted Cox regression model, which would be used in the follow-up study.
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| Prognostic variables | HR (95% CIs) | P value | HR (95% CIs) | P value |
| Demographics | ||||
| Age | 1.0167 (1.0069–1.0267) | <0.001 | 1.0256 (1.0147–1.0367) | <0.001 |
| Gender | 1.2166 (.9653–1.5334) | 0.100 | 1.2328 (0.9723–1.5631) | 0.084 |
| BMI | 1.0143 (0.9990–1.0300) | 0.075 | 1.0142 (0.9984–1.0304) | 0.078 |
| Comorbidities | ||||
| Hypertension | 0.3757 (0.2887–0.4887) | <0.001 | 0.5976 (0.4494–0.7947) | <0.001 |
| T2DM | 0.8383 (0.6591–1.0662) | 0.147 | 0.9138 (0.7098–1.1764) | 0.484 |
| Stroke | 0.7475 (0.5723–0.9763) | 0.029 | 1.0031 (0.75951.3249) | 0.982 |
| CKD | 0.8232 (0.6563–1.0325) | 0.091 | 0.7439 (0.5665–0.9767) | 0.033 |
| AF | 0.7452 (0.5945–0.9341) | 0.011 | 0.8487 (0.6646–1.0838) | 0.188 |
| COPD | 1.2250 (0.9089–1.6512) | 0.193 | ||
| Laboratory variables | ||||
| L/A | 2.6080 (2.4117–2.8203) | <0.001 | 1.8839 (1.6853–2.1058) | <0.001 |
| Lactate | 1.4272 (1.3789–1.4772) | <0.001 | ||
| Albumin | 0.2301 (0.1917–0.2762) | <0.001 | ||
| Creatinine | 1.1937 (1.1258–1.2656) | <0.001 | 1.1534 (1.0605–1.2543) | 0.001 |
| PO2 | 0.9868 (0.9843–0.9892) | <0.001 | ||
| PCO2 | 1.0121 (0.9957–1.0287) | 0.159 | ||
| Haemoglobin | 0.8029 (0.7355–0.8765) | <0.001 | ||
| WBC | 1.0323 (1.0263–1.0383) | <0.001 | 1.0207 (1.0102–1.0314) | <0.001 |
| PLT | 0.9934 (0.9917–0.9951) | <0.001 | ||
| Sodium | 0.9992 (0.9609–1.0391) | 0.968 | ||
| Potassium | 2.2664 (1.5826–3.2456) | <0.001 | ||
| Troponin T | 1.1497 (1.1158–1.1847) | <0.001 | 1.0867 (1.0473–1.1276) | <0.001 |
| Vital signs | ||||
| SBP | 0.9637 (0.9568–0.97058) | <0.001 | ||
| DBP | 0.9798 (0.9662–0.9937) | 0.004 | ||
| Heart rate | 1.0402 (1.0303–1.0503) | <0.001 | ||
| Scoring systems | ||||
| SOFA | 1.3269 (1.2862–1.3689) | <0.001 | 1.1216 (1.0790–1.1659) | <0.001 |
| SIRS | 1.6559 (1.4233–1.9266) | <0.001 | 1.1217 (0.9521–1.3215) | 0.170 |
| Prescription | ||||
| ACEI | 0.4898 (0.3360–0.7141) | <0.001 | ||
| Digoxin | 2.1283 (1.3376–3.3863) | <0.001 | 1.4679 (0.9054–2.3800) | 0.120 |
| Beta blocker | 1.4855 (1.1849–1.8623) | <0.001 | ||
| Diuretic | 2.1701 (1.7319–2.7191) | <0.001 | ||
| Treatments | ||||
| RRT | 4.1876 (3.2620–5.3758) | <0.001 | ||
| CABG | 0.1914 (0.1320–0.2774) | <0.001 | 0.3012 (0.2052–0.4423) | <0.001 |
| PTCA | 0.5026 (0.3428–0.7369) | <0.001 | 0.7535 (0.5020–1.1310) | 0.172 |
| PCI | 1.3025 (0.8585–1.9762) | 0.231 | 0.8992 (0.5794–1.3954) | 0.635 |
| IABP | 1.4051 (1.0471–1.8855) | 0.029 | 1.1694 (0.8438–1.6206) | <0.001 |
- ACEI, angiotensin-converting-enzyme inhibitors; AF, atrial fibrillation; BMI, body mass index; CI, confidence interval; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; DBP, diastolic blood pressure; PTCA, percutaneous transluminal coronary angioplasty; RRT, renal replacement therapy; SBP, systolic blood pressure; SIRS, Systemic Inflammatory Response Syndrome; SOFA, Sequential Organ Failure Assessment; T2DM, type 2 diabetes mellitus.
- P values less than 0.05 are indicated in bold.
L/a ratio at admission as a predictor of in-hospital mortality
ROC curves of L/A, lactate, TNT, SOFA, and Systemic Inflammatory Response Syndrome (SIRS) score for predicting in-hospital mortality are plotted in Figure 2. We found that the AUC of admission L/A and SOFA were better than other indicators. DeLong test confirmed that the AUC of admission L/A was superior to admission Sofa [0.800 (95% CI: 0.772–0.828) vs.0.753 (95% CI: 0.721–0.785), P = 0.015]. Therefore, L/A was supposed to be a certain predictive value for hospital mortality. The optimal cut-off value was 0.671, the sensitivity was 76.1%, and the specificity was 72.3% (Maximum Youden's index) (Table 3).

| Variables | AUC | 95% CI | Cut-off value | Sensitivity | Specificity | Youden's index |
|---|---|---|---|---|---|---|
| L/A | 0.780 | 0.772–0.827 | 0.671 | 0.761 | 0.723 | 0.484 |
| Lactate | 0.739 | 0.706–0.772 | 2.219 | 0.695 | 0.696 | 0.391 |
| TNT | 0.623 | 0.588–0.658 | 1.056 | 0.384 | 0.807 | 0.191 |
| SOFA | 0.753 | 0.721–0.785 | 8.5 | 0.770 | 0.673 | 0.443 |
| SIRS | 0.617 | 0.585–0.650 | 2.980 | 0.669 | 0.541 | 0.210 |
- AUC, area under the curve; CI, confidence interval; L/A, lactate/albumin ratio; SIRS, Systemic Inflammatory Response Syndrome; SOFA, Sequential Organ Failure Assessment.
Higher admission L/A ratio indicates higher in-hospital mortality
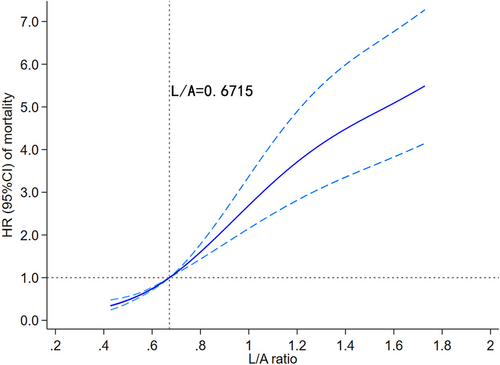
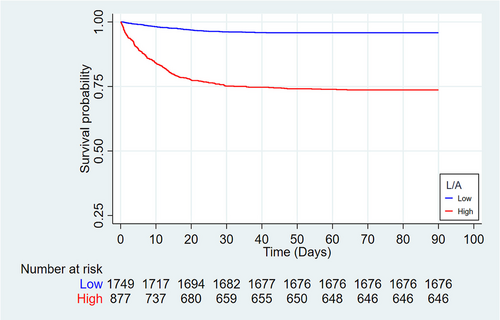
Visual models of L/A and in-hospital mortality were established using RCS. In multivariate analysis, the model adjusted for cofounders with P < 0.05. In Figure 3, HR curve showed an upward trend, indicating that the risk of in-hospital death increased with the elevate of L/A. According to the optimal cut-off value, the samples were divided into the high admission L/A group (L/A ≥ 0.671, n = 877) and the low admission L/A group (L/A < 0.671, n = 1749), and the KM curves were made for different groups. In Figure 4, the survival curve of the high-admission L/A group was significantly lower than that of the low-admission L/A group, and the difference between the two groups was verified by log rank test (P < 0.001). Multivariate Cox regression based on the established model showed that higher L/A admission was associated with an increased risk of in-hospital death compared with lower L/A admission (HR = 3.869, 95% CI: 2.919–5.129; P < 0.001). In conclusion, higher L/A levels predict higher in-hospital mortality.
Discussion
In this study, we used the open-source MIMIC-IV database to evaluate the role of the L/A ratio in predicting outcomes in critically ill patients with HF. Results showed a linear relationship between the L/A ratio and the risk of all-cause mortality during hospitalization in critically ill patients with post-MI HF. Notably, the higher levels of L/A ratio, the higher risk of death.
With the increasing incidence of MI, the number of patients with post-MI HF has also increased year by year. Such patients have poor prognosis and a large economic burden. Therefore, predicting the outcome of these patients can help clinicians make adaptive clinical decisions. Previous studies have shown that lactate can predict survival outcomes in patients with acute HF.22, 23 However, lactate levels are affected by multiple factors. For example, liver or kidney dysfunction may lead to clearance barriers and contributed to abnormally elevated lactate. Shin et al. evaluated the value of the L/A ratio in predicting prognosis in patients with severe sepsis.16 It was found that the AUROC of the L/A ratio was higher than that of lactate. In addition, the AUROC of the L/A ratio remained higher than that of lactate in patients with impaired lactate clearance. Therefore, the L/A ratio reflected the inverse changes caused by two different mechanisms, and it was more accurate in predicting the prognosis of critically ill patients, such as post-MI HF. Moreover, for patients with the same lactate level, the use of the L/A ratio can further identify high risk.16 Wang et al. evaluated the predictive role of the L/A ratio in the risk of death in 54 patients with multiple organ dysfunction syndrome (MODS) caused by severe sepsis or septic shock.18 It was found that the L/A ratio was associated with MODS and risk of death. In addition, a study by Lichtenauer et al. showed that the L/A ratio was helpful in risk stratification of patients with sepsis.17 Therefore, monitoring the L/A ratio is expected to better manage critically ill patients with post-MI HF in clinical practice.
In critically ill patients with HF after MI, there was a linear relationship between the L/A ratio and the risk of all-cause death. Considerable reasons were listed: (i) patients with HF after MI were often accompanied by haemodynamic instability, and the level of lactic acid increased under insufficient perfusion and hypoxia, the increase of lactate level is related to the increased risk of in-hospital death in patients with acute decompensated HF23; (2) severe decrease of ejection fraction is common in HF after MI. In this kind of patients, long-term and extensive use of diuretics during treatment can easily lead to insufficient tissue perfusion and anaerobic metabolism, resulting in an increase in lactate level; (3) hypoalbuminemia caused by liver congestion is common in patients with HF. According to statistics, 14% of patients with acute HF have hypoalbuminaemia, and the prognosis of patients with hypoalbuminaemia is poor.24, 25 In our study, the high L/A ratio reflected increased levels of lactate and hypoalbuminaemia, which increased the risk of all-cause death in patients with HF after severe MI.
Analysis of the baseline characteristics of this study showed that the admission L/A values were higher in the death group than the survivor group. Then Cox regression analysis showed that higher admission L/A values were an independent risk factor for in-hospital mortality. Moreover, ROC curves showed that the AUC of admission L/A ratio was better than that of lactate, troponin T, SOFA, and SIRS. The AUC of admission L/A = 0.8, indicating that admission L/A ratio has considerable predictive value for in-hospital mortality. Finally, RCS models and KM curves for samples from different levels of admission L/A groups indicated that higher levels of admission L/A ratio were associated with higher in-hospital mortality.
This study reported the value of admission L/A in predicting in-hospital mortality in patients with HF after MI. The relatively large population of patients reduced the selection bias in this research. Moreover, admission L/A has the characteristics of fast, cheap, and easy to obtain in clinic.
Conclusions
In this study, we found that admission L/A was an independent predictor of in-hospital mortality in patients admitted to ICU with HF after MI, and high admission L/A level was associated with high mortality. Adequate attention and close monitoring should be provided throughout hospitalization for patients with elevated L/A on admission.
Limitations of the study
First, although these results provided strong evidences for the predictive value of admission L/A ratio in patients with post-MI HF, the relatively small amount of data replaced by the multiple imputation methods in this retrospective, single-centre study may have resulted in a loss of practical significance. Second, this study investigated the relationship between patient admission L/A levels and prognosis, and it was impossible to assess the prognostic impact of dynamic changes in this ratio. Third, out-of-hospital mortality and other important outcomes could not be investigated because of the absence of out-of-hospital mortality data in the MIMIC-IVv1.0 database deserve further study in the future.
Conflict of interest
The authors declare no competing financial interest.
Funding
This work was supported by the National Natural Science Foundation of China (81370214, 82102667).




