Regional management of worsening heart failure: rationale and design of the CHAIN-HF registry
Abstract
Aims
Heart failure (HF) is a progressive disease in which periods of clinical stability are interrupted by episodes of clinical deterioration known as worsening heart failure (WHF). Patients who develop WHF are at high risk of subsequent death, rehospitalization, and excessive healthcare costs. As such, WHF could be seen as a separate disease stage and precursor of advanced HF. Whether WHF has a substantial health, societal, and economic impact evidence regarding its multifactorial nature and the specific barriers in treatment, including advanced HF therapies, remains scarce. The CHAIN-HF registry aims to describe the incidence, characteristics, current treatment, and outcomes of WHF. Additionally, it will promote structured regional collaboration and educate on increasing awareness for WHF and describe the implementation of guideline directed medical therapy and utilization of advanced HF therapies in a collaborative network.
Methods and results
The CHAIN-HF registry is a prospective, observational, and multicentre study from the collaborating hospitals (Rijnmond HF Network) in the Rotterdam area. Unselected and consecutive patients (irrespective of ejection fraction) with a WHF event will be included. Comprehensive data including demographics, co-morbidities, treatment, and in-hospital and post-discharge outcomes will be collected. Notably, data on socio-economic status, treatment decisions, and referral for advanced HF therapies will be included.
Conclusions
CHAIN-HF will be the first prospective, dedicated WHF registry in a collaborative network of hospitals that will provide robust real-world evidence on the incidence, characteristics, and outcomes of WHF. Moreover, it will provide information on of the value of regional collaboration to improve awareness and outcomes of WHF.
Introduction
Heart failure (HF) is a chronic and progressive syndrome that is associated with a high risk of mortality, morbidity, and healthcare resource utilization.1 The overall incidence of HF in Europe is approximately 5/1000 person-years but is expected to increase due to the ageing of the population, improved survival of myocardial infarction, and advances in HF therapy in the last decades.2 Despite these advances, it is still associated with an impaired prognosis, with a 5-year mortality ranging from 45 to 60%.3
For the majority of patients with chronic HF (CHF), periods of stability are interrupted by episodes of clinical deterioration requiring hospitalization and/or intravenous (IV) diuretics known as worsening heart failure (WHF).4, 5 WHF is relative common in HF with reduced ejection fraction (HFrEF): One in six of these patients develops WHF within 18 months following initial diagnosis.1 Compared with new-onset HF (NOHF) and CHF, WHF carries a higher risk of rehospitalisation and mortality and is associated with a lower quality of life.6-9 Therefore, WHF is increasingly being recognized as an inflection point in the clinical course of HF and a separate disease stage with important prognostic value. The occurrence should act as an important signal for the treating physician that the patient is gradually progressing to advanced HF, which requires additional therapies.10 Emphasis on this separate stage within the patient journey and course of HF is essential to improve medical treatment, patient selection, and timing of advanced HF therapies, thereby improving prognosis and quality of life.
Although the health, social, and economic impact of WHF—which is only expected to increase in the forthcoming years—seems clear, reducing its burden remains challenging. Current knowledge is based on data from randomized controlled trials, which are known to include a highly selected population, and retrospective analyses. Though the latter does provide some insights on the real-world status of WHF, they only focus on classical clinical components such as age, comorbidities and biomarkers. However, WHF represents to be a broader problem where clinical management (both medical and advanced therapies) and outcomes are modulated by non-medical factors such as socio-economic status (SES), sex, race, and geography.11 Furthermore, management and outcome could at least partially be determined by barriers related to the doctor and the (regional) healthcare system.10 Clearly, there is an unmet clinical need to design a study addressing these topics in order to inform both clinicians and policymakers. Therefore, we developed the CHAIN-HF registry, a prospective registry of WHF in Greater Rotterdam and neighbouring areas providing care to 1.5 million inhabitants, which will study the use of a regional approach for HF care and potential clinical markers for disease progression that can be used to optimize treatment for WHF patients and improve their access to advanced HF therapies. Most important, CHAIN-HF stimulates collaboration in a regional network with a hub-and-spoke model.
Study design
CHAIN-HF is a prospective, multicentre Dutch registry of unselected and consecutive WHF patients (irrespective of EF). This study has been approved by the ethics committee (MEC-2021-0659) and will be conducted according to the Helsinki declaration with all patients providing informed consent prior to participation.
Study population
All consecutive patients (aged ≥18 year) with chronic HF (>6 months from diagnosis) and a WHF event, defined as at least one incident HF hospitalization in the past 6 months, current unplanned HF admission, or urgent unplanned HF visit (emergency department) with the necessity of IV diuretics will be included in the registry and prospectively followed on clinical endpoints. Importantly hospitalized HF patients were eligible for inclusion if the primary admission diagnosis was HF. Inclusion and exclusion criteria are shown in Tables 1 and 2, respectively. Patients will be included from all hospitals in the Greater Rijnmond area collaborating within the Rijnmond Heart Failure Network (Figure 1). This area consists of 1.5 million inhabitants from which it is estimated 15 000–30 000 have HF (1–2% of the population); from those, one in six (2500–5000) are estimated to develop WHF.1, 2 Using a conservative estimation and considering both exclusion criteria and non-consent, we aim to include 750 patients minimally in the registry. However, this is an observational study with no maximum to the registry.
|
- Abbreviations: ED, emergency department; HF, heart failure; IV, intravenous; NYHA, New York Heart Association.
|
- Abbreviations: LVAD, left ventricular assist device.

CHAIN-HF programme
The CHAIN-HF registry is part of the larger CHAIN-HF programme, which is aimed at creating awareness of WHF and subsequently to offer this high-risk population the best care and improve prognosis. Within this programme, 11 hospitals (1 university and 10 general hospitals) collaborate according to the hub-and-spoke model.12 This model arranges healthcare service delivery into a network containing multiple hospitals offering different services. Depending on the complexity of disease, needs of the patient, and available services, patients are treated in a specific hospital.13 This model is advocated by the ESC and HFA as an appropriate model to manage HF patient due to the broad spectrum of HF, ranging from patients in early stages of the disease to those who progress to more advanced stage.2, 12 In the CHAIN-HF programme, the ‘hub’ centre is the university hospital (Erasmus Medical Center) with short-term and long-term mechanical support (MCS) and heart transplantation capabilities, and the ‘spoke’ centres are the surrounding hospitals offering the full array of HF treatments except for the aforementioned (Figure 2). Additionally, this collaboration incorporates a standardized, regional care pathway developed by the participating centres in line with the 2021 ESC guidelines on acute and chronic HF.2 In context of this programme, there are weekly digital meetings between the HF specialists from each centre and a dedicated HF team from the hub centre (advanced heart failure cardiologist, and if needed a structural heart intervention cardiologist, advanced imaging cardiologist, and cardiothoracic surgeon) to discuss WHF patients with HFrEF. As the main objective of the CHAIN-HF programme is to improve access to advanced HF therapies, HFpEF patients with a WHF event will not be discussed in the weekly meeting. However, in the future, as guidelines-endorsed treatments increase, we will broaden the CHAIN-HF programme to this population. Subsequently, a collective treatment strategy will be proposed, yet further treatment will be left to the discretion of the treating cardiologist. This proposed treatment strategy can, for example, include an advice to optimize guideline-directed medical therapy (GDMT) in the spoke centre, referral for evaluation as outpatient or as inpatient to the hub centre, or the advice to strive for advanced care and/or palliation.
Objectives and endpoints
The objectives of the CHAIN-HF programme were based on multiple levels. From the organizational perspective, the aims were (i) to develop a strong regional collaboration utilizing the hub-and-spoke principle and (ii) to design a structured regional care programme including a regional heart team to manage patients with WHF. Scientific aims of the CHAIN-HF registry include the following: (i) to describe the incidence, demographic, and clinical characteristics (both medical and non-medical) of WHF in the Rijnmond area; (ii) to characterize the current treatment of patients with WHF and study the change in treatment that are and are not included in the CHAIN-HF programme; (iii) to identify patient characteristics associated with poor health outcomes, and need for advanced HF therapies, in WHF patients; (iv) to study the prognosis of patients with WHF that are and are not included in the CHAIN-HF programme; and (v) additional goals of CHAIN-HF including the improvement of the uptake of GDMT in patients with WHF and increased awareness and utilization of advanced HF therapies.
The primary endpoints are 1-year all-cause mortality, 1-year cardiovascular mortality, number of HF-related hospitalizations (WHF events), and the number of referrals for (advanced) HF therapies. Secondary endpoints are 2-year and 5-year mortality and the utilization and outcomes of advanced HF therapies including transcatheter edge-to-edge repair for both mitral and tricuspid valve and left ventricular assist device (LVAD) or listing for heart transplantation. All endpoints will be determined by the investigators as investigator-reported events.
Data collection and follow-up
The proposed time frame for data collection is illustrated in Figure 3. Data will be collected with the use of an electronic case report forms within an electronic data capture system. Baseline data will include demographics, medical history, medication, physical examination, electrocardiogram, echocardiogram, and laboratory results. Both cardiovascular and non-cardiovascular co-morbidities precipitate WHF and increase the risk of rehospitalization.14 Consequently, co-morbidities and precipitating factors will be documented in the registry. Furthermore, if the patient was discussed in the weekly meeting, the advice will also be documented. The latter offers the possibility to study triggers for referral and possible bias in this decision. Explicitly, we will also include data on gender, race, geographic location, and SES as we believe these factors are associated with worse outcomes and non-referral for advanced therapies. Due to the observational nature of this study, SES will be determined at neighbourhood level by using postal codes. This method of deduction has been proven feasible and reliable.15 Furthermore, race (Western vs. non-Western) will be deducted from the medical records. The patient will be contacted in case of ambiguity.
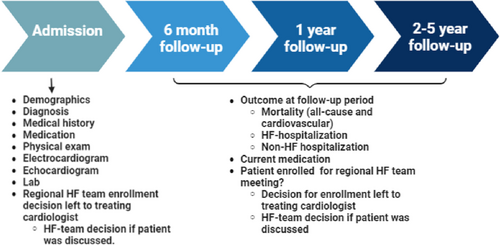
Longitudinal (follow-up) data will be collected after 6 and 12 months and yearly thereafter recording disease outcome measures (survival, repeat hospitalization, referral, treatment changes, and screening and utilization of advanced HF therapies). Follow-up information will be collected at regular follow-up via the medical record from the hospital or via telephone interview if the patient was not seen at the follow-up time. Every effort will be made by the study personnel to obtain follow-up information. In addition, vital status can be supplemented using national reporting databases.
Statistical analysis plan
Categorical variables are presented as frequencies and percentages and compared with the use of the Pearson chi-square test or Fisher's exact test, as appropriate. Continuous variables are presented as means (±SD) (in case of a normal distribution) or medians (IQR) (in case of a skewed distribution) and compared using Student's t-test or analysis of variance (ANOVA). Normality of the distributions is assessed using the Shapiro–Wilk test. A Cox proportional hazards model will be used model to analyse time to first event. The Andersen–Gill method will be used to analyse recurrent hospital admissions. Hospitalization rates and mortality rates will be estimated using the Kaplan–Meir method, and P-values will be calculated using the log-rank method. A two-sided alpha level of 0.05 is used for all superiority testing. All statistical analysis are performed with the use of Statistical Package for Social Sciences (SPSS, Chicago, Illinois) software version 25.0.
Discussion
Despite its dismal prognosis and public health importance, the characteristics and outcomes of patients with WHF are currently poorly defined. Furthermore, evidence regarding this high-risk population is primarily derived from randomized trials and registries focusing on acute HF (Table 3). The CHAIN-HF registry is the first large-scale, multicentre dedicated WHF registry that will study the characteristics, current treatment, and outcomes of patients with WHF. Additionally, it will evaluate the effects of regional collaboration and a structured programme aimed to increase awareness of WHF, its treatment, and signal function for advanced HF therapies. Importantly, CHAIN-HF will collect data on WHF along the full spectrum of EF. Knowledge on these factors and understanding of the barriers in the current treatment of these patients is critical to improve outcome in this high-risk population and reduce the impact of WHF.
| Name of the registry | EHFS II29 | EAHFE30 | PINNCLE1 | OPTIMIZE-HF31 | BIOSTAT-CHF32 | VICTORIA Simultaneous Registry33 |
|---|---|---|---|---|---|---|
| Author | Harjola | Garcia | Butler | Malik | Núñez | Ezekowitz |
| Year of publication | 2010 | 2019 | 2019 | 2019 | 2020 | 20201 |
| Location | Europe | Spain | USA | USA | Europe | Canada and USA |
| Population | AHF | AHF | WHF | WHF (CHF with recent hospitalization) | AHF and outpatient WHF | WHF (EF < 45%) |
| Type of study | Prospective | Retrospective | Retrospective | Retrospective | Prospective | Retrospective |
| HF phenotype | HFrEF and HFpEF | NR | HFrEF | HFrEF and HFpEF | HFrEF and HFpEF | HFrEF |
| Cohort size | 1860 | 5359 | 1851 | 2768 | 2356 | 2056 |
| Age, years (mean, SD) | NR | 80.0 (9.6) | 70.6 ± 12.8 | NR | 69 ± 12 | 70 (59–80) |
| Female (%) | NR | 57.3 | 37.5 | 51.8 | 26.1 | 32.8 |
| LVEF, % (mean, SD) | NR | NR | 31.0 ± 9.8 | NR | NA | 25 (20–35) |
| Readmission WHF (%) | NR | NR | 70 | 51.7 | NR | NR |
| In-hospital mortality WHF (%) | NR | 7.7 | NR | NR | NR | 2.9 |
| All-cause mortality WHF (%) | 23.2 | 32.9 | 22.5 | 40.3 | NR | NR |
| Follow-up | 1 year | 1 year | 2 years | 1 year | 1 year | 1 year |
| Limitation of the registry | No dedicated WHF registry | No dedicated WHF registry | Retrospective data source and only includes HFrEF | Retrospective | No dedicated WHF and no subgroup analysis of the different HF phenotypes | Retrospective data source and no crude data for the long-term outcome |
- Abbreviations: AHF, acute HF; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; NR, not reported; WHF, worsening HF.
WHF is increasingly being recognized as a distinct entity, which is reflected by the increasing number of WHF trials. Examples of such dedicated WHF trials are the VICTORIA, GALACTIC-HF, and SOLOIST-WHF trials.16-18 The results from these trials highlight the high risk in these patients, as the primary endpoint (composite of cardiovascular death and HF hospitalization) in the control group occurred more frequent compared with other HFrEF landmark trials such as PARADIGM-HF, DAPA-HF, or EMPEROR-REDUCED (38.5%, 39.1%, and 76.3% vs. 26.5%, 21.2%, and 24.7%, respectively).16-21 These outcomes are amplified by evidence from real-world registries. In contrast to this study, these registries are either retrospective, not primarily dedicated to WHF, or only performed in a specific subset of patients (e.g. HFrEF) (Table 3). An additional example of a retrospective WHF registry is the CHART–HF study. The purpose of this recent initiated registry is to evaluate the use of GDMT and to address the barriers towards GDMT in the WHF with reduced ejection fraction (EF).22
For risk stratification, it is important to disentangle WHF from acute HF (AHF) as AHF encompasses both the first manifestation of NOHF and acute decompensated CHF.2 The latter is used synonymously for WHF in the above-mentioned AHF registries. Disentanglement of these entities is important because of the significant difference in prognosis between WHF and NOHF. Greene et al. found that all-cause mortality was significantly higher in WHF (8.1% vs. 13.7%),9 demonstrating that NOHF patients have a significant gain from GDMT, whereas WHF patients most likely deteriorate despite GDMT or on maximum tolerated doses. Similarly, a Danish observational study confirmed that all-cause mortality or HF readmissions in the WHF cohort were significantly higher compared with the NOHF cohort (HR 1.84, 95% CI 1.69–1.93).6 Likewise, is the difference in prognosis between WHF and CHF. The IN-HF registry showed that WHF is associated with a higher 1-year all-cause mortality and HF hospitalizations (27.7% vs 5.9% and 21.4% vs 8.8%).7 In addition, it is important to note that WHF does not only increase the risk of mortality and HF hospitalizations but also is associated with an increase in symptom burden and healthcare resource utilization and costs.8 These results emphasize that WHF is a separate disease stage that needs a platform of recognition.
With the above-mentioned results, the impact of WHF seems clear; however, changing its outcomes is challenging due to its multifactorial nature, the presence of co-morbidities, and the remaining barriers in the implementation of current treatment (including advanced HF therapies for HFrEF). WHF encompasses a broader problem at least partly determined by non-medical factors related to the patient, caregiver, and/or the healthcare system. HF represents the endpoint of numerous cardiovascular pathophysiological processes in a ‘chain of events’, which are modifiable throughout the disease trajectory. A meta-analysis by Hawkins et al. revealed that lower SES, which included measures like education, occupation, and area level indicators, was associated with an increased incidence of HF, higher rehospitalization rates, and impaired survival.11 By examining the role of SES in WHF, CHAIN-HF may reveal further modifiable pathways that are amenable to preventive strategies. These pathways are especially important for the WHF population with HFpEF for whom we still have little evidence regarding effective therapy. At the other end, management and outcomes may partly be determined by the doctor and the healthcare system-related factors. These possible factors include inadequate GDMT prescription and insufficient titration of GDMT for HFrEF, not recognizing WHF as an important infliction point in the patient journey and the lack of knowledge concerning additional treatment options.
Despite the poor prognosis of HF, the use of GDMT in HFrEF is unacceptably low, and target doses are often not realized.1, 23, 24 Although the use of GDMT in Europe is relative high, the average dose of GDMT is still lower than recommended in the guidelines.24 Furthermore, the CHECK-HF registry provided evidence that variation in GDMT use is not only present between different countries but could also within the same country. This underlines the need for a regional approach that might improve unequivocal treatment.25 The impact of suboptimal GDMT is highlighted by McCullough et al., which showed that not receiving optimal GDMT resulted in a 29% increase in morality compared with those who receive optimal GDMT.26 Although novel therapies demonstrating efficacy in the WHF population are becoming increasingly available, determining barriers for implementation of current pharmacotherapy is even more relevant.16, 17
Even when GDMT is successfully implemented, the clinical course of HF can challenge experienced clinicians to correctly identify WHF and its trajectory towards advanced HF and consequently defer referral for appropriate therapies. A recent pooled analysis from nine advanced HF centres revealed that most patients are still referred in a late stage of disease and mainly while being hospitalized requiring inotropic support (INTERMACS class 2 or 3). This critically ill status explains why a substantial part (36%) was deemed too ill for durable LVAD implantation. Medical decision-making is in line with reports showing poor outcomes in higher INTERMACS profiles.27 Given the number of patients still being withheld lifesaving treatment, there is still a need to define appropriate triggers for discussion with an advanced HF centre. Using WHF as a marker of disease progression, as advocated by our programme, offers the possibility to recognize potential candidates in an earlier stage, thereby improving their outcomes. Additionally, clear referral patterns are needed, which are imbedded within a strong regional collaboration. For this reason, the European HF guidelines advocate to collaborate according to the hub-and-spoke principle. Yet, no scientific data regarding efficacy have been published.2, 12 Hence, CHAIN-HF will fill this knowledge gap by being the first registry prospectively reporting on the use of this model of care within the WHF and advanced HF spectrum.
There are several features that highlight the merit of this registry. First, the definition used in the CHAIN-HF registry is broader than the traditional hospitalization-based definition including unplanned emergency department visits with the need for IV diuretics and both elective hospitalization with at least one heart failure hospitalization in the past 6 months and current unplanned HF admission. Although it is important to note that to date there is no consensus on the definition of WHF, this definition is in line with the definition of WHF used in the latest landmark trials. A requirement for the current definition is the diagnosis of CHF, thus excluding NOHF as explained above. Furthermore, this definition has no reference to the location of care (outpatient or inpatient) and implies the assumption of worsening symptoms despite optimal therapy.5 Regarding the former, there is accumulating evidence that even outpatient oral intensification of diuretic therapy is associated with an increased risk of morbidity and mortality.28 These results suggest that the biological changes associated with the progression of disease should be considered instead of the location of care. However, collection of outpatient data on oral diuretic therapy change is challenging, particularly in a multicentre study. Therefore, oral outpatient intensification was not used as a criterion for WHF event in this study. Regarding the latter, it is assumed that patients have worsened despite GDMT. However, there is no consensus on what level of background therapy is required to differentiate WHF from undertreated HF. In addition, there is no guide on how to define those patients with worsening symptoms while being treated suboptimal due to intolerance or absolute or relative contraindications and those patients with a specific trigger for worsening of symptoms, which is common in clinical practice. For these reasons, all these factors will be included as variables within the CHAIN-HF registry offering the possibility to further shape the definition of WHF.
Generally, HF is stratified into three phenotypes according to the EF. Due to the overlapping pathophysiological processes, the main focus of clinical research has been dedicated to HFrEF. As a consequence, there is paucity of evidence in the other two groups, especially in WHF. Nevertheless, this lack of evidence has major implications for the real-world HF-= population in which these groups represent approximately half of the population, and this is expected to increase in the forthcoming years.2 Therefore, CHAIN-HF included all HF patients irrespective of the EF providing a true real-world population. Finally, the most important beneficial feature is, that CHAIN-HF is the first prospective registry that will assess WHF as a broader problem beyond traditional patient characteristics. It will also evaluate the impact of different non-medical factors and the role of a structured regional approach (hub-and-apoke principle) to reduce barriers in the current treatment of WHF patients.
Conclusions
CHAIN-HF will be the first prospective, dedicated WHF registry that will provide robust real-world evidence on the incidence, characteristics, and outcomes of WHF. Additionally, it will provide insight in the value of regional collaboration according to the hub-and-apoke principle to improve awareness and outcomes of WHF.
Acknowledgements
The authors would like to thank all the heart failure cardiologist and nurses of the participating hospitals for their contribution to the CHAIN-HF registry.
Conflict of interest
Dr Brugts has received speaker and consulting fees from Abbott, Novartis, Bayer, Boehringer, and AstraZeneca unrelated to this work. Furthermore, he reported an ISS study grand from Abbott. Dr Manintveld reported consulting fees from Abbott, AstraZeneca, Boehringer Ingelheim, and Novartis. Additionally, he is the president of the working group of heart failure of the Dutch Society of Cardiology. D. Emans reported speaker fees from Vifor international, AstraZeneca, Boehringer Ingelheim, and Novartis. She is also a study domain leader for heart failure in Check@home (study for population screening cardiovascular disease/renal disease/atrial fibrillation/heart failure).
Funding
CHAIN-HF is an investigator-initiated study. Database management, quality control, and data analyses are coordinated by the Erasmus Medical Center (Rotterdam, The Netherlands). The investigators will have full access to all of the data in this study. The study is partially supported by an independent research grant (investigator-sponsored studies (ISS) programme) from Abbott. The sponsor had no role in the data extraction process or analyses, the writing of the manuscript, or the decision to submit the manuscript for publication.




