Beta-blocker use and mortality among patients with systolic heart failure and pacemaker rhythm
Abstract
Aims
Beta-blockers are proven to improve survival among patients with heart failure with reduced ejection fraction. Their efficacy in patients with heart failure with reduced ejection fraction and pacemaker devices has not been demonstrated. Our aim was to test the hypothesis that beta-blocker therapy is associated with improved survival in patients with chronic heart failure and a pacemaker rhythm on electrocardiogram (ECG).
Methods and results
This is a post hoc analysis from the GISSI-HF randomized clinical trial. We evaluated efficacy of beta-blockers by creating Cox proportional hazards models adjusting for pacemaker rhythm and heart rate, among other variables. Interactions between pacemaker rhythm, heart rate, and beta-blocker were also examined. Of the 6975 patients enrolled in the GISSI-HF trial, 813 (11.7%) had a pacemaker rhythm on baseline ECG. Of these 813 patients, 511 (62.9%) were receiving beta-blocker therapy. The effect of beta-blocker therapy on mortality was assessed using multivariable Cox proportional hazards adjusted for 27 co-variates. In the whole cohort, beta-blocker therapy was significantly associated with reduced mortality (hazard ratio 0.79 [0.72–0.87], P < 0.001), without interaction between beta-blockers, pacemaker rhythm and heart rate. Beta-blocker therapy was beneficial in the sub-group restricted to baseline pacemaker rhythm (hazard ratio 0.62 [0.49–0.79], P < 0.001).
Conclusions
Beta-blocker therapy is associated with improved survival among patients with heart failure and a pacemaker rhythm on ECG. Further studies are necessary to analyse differences between atrial and ventricular pacemakers.
Introduction
Since the initial findings from the Metoprolol in Dilated Cardiomyopathy trial in 1993, beta-blocker therapy has been foundational to the management of patients with heart failure with reduced ejection fraction (HFrEF).1 While not specifically excluded from the core clinical trials demonstrating improved mortality from beta-blockers, patients with a pacemaker device were not well represented. The benefit derived from beta-blocker therapy is not simply due to heart rate lowering, but though inhibiting adrenergic signalling, the anti-arrhythmic properties and prevention of cardiac remodelling.2 Yet chronic pacing is known to be a risk factor for heart failure and may limit the effectiveness of beta-blocker therapy.3 Accordingly, it is unknown whether beta-blocker therapy is beneficial in patients with a pacemaker device. We sought to evaluate this question in a post hoc analysis of the GISSI-HF trial.
Methods
Study population
The study population is from the GISSI-HF randomized clinical trial. This trial was originally designed to test the effects of n-3 polyunsaturated fatty acid and rosuvastatin on mortality among patients with symptomatic heart failure. Full details of the trial design, recruitment and primary outcomes have been reported previously.4 Briefly, between 2002 and 2005, across 356 centres in Italy, patients were recruited if they had symptomatic heart failure with New York Heart Association (NYHA) Class of II or greater, with an ejection fraction (EF) measured within 3 months of enrolment. If the subject's EF was >40%, they required documentation of a hospitalization for heart failure within the previous year. Subjects were excluded if they had acute coronary syndrome or revascularization within 1 month, upcoming cardiac surgery, a congenital or valvular aetiology for heart failure, significant liver disease, pregnancy or lactating status, non-cardiac co-morbidity, which was felt to significantly shorten life expectancy or current or recent treatment with investigational drugs. In addition, some patients were excluded from the statin arm of the trial if their creatinine was >2.5 mg/dL, alanine aminotransferase or aspartate aminotransferase >1.5 times upper limit of normal or elevated creatinine phosphokinase. Participating sites' ethics committees approved the GISSI-HF trial, and all patients provided informed consent.
Exposures and outcomes
Upon enrolment in GISSI-HF, all subjects underwent a baseline electrocardiogram (ECG) and physical examination. Pacemaker rhythm was defined as the presence of atrial or ventricular paced beats on ECG. Medication use information was collected by self-report. The outcomes for our study are (i) all-cause mortality and (ii) cardiovascular death or cardiovascular hospitalization.
Statistical analysis
Baseline characteristics of the overall cohort were compared based on presence of pacemaker rhythm on the ECG performed upon enrolment. Baseline characteristics of the sub-group of subjects with a pacemaker were compared based on treatment with a beta-blocker. Student's t-test and the Chi-square test were used as appropriate to compare differences in baseline characteristics. Cox proportional hazards models for both outcomes were adjusted for the following variables known to impact mortality in HFrEF: age, gender, body mass index, systolic blood pressure, heart rate, NYHA class, ischaemic heart disease, history of diabetes, history of bypass surgery, history of percutaneous coronary intervention, history of implantable cardiac defibrillator (ICD), history of pacemaker, history of stroke, history of peripheral arterial disease, history of atrial fibrillation, history of chronic obstructive pulmonary disease, history of cancer, EF, QRS duration, haemoglobin, creatinine, sodium, beta-blocker use, ACE/ARB use, mineralocorticoid receptor antagonist use, loop diuretic use, thiazide diuretic use and allopurinol use. We created Cox models using the full cohort, and models restricted to those with a pacemaker rhythm at baseline. We performed sub-group analyses based on EF using 40% as a cut-off, and another sub-group analysis with participants who had a history of atrial fibrillation. In an attempt to remove subjects who potentially had a cardiac resynchronization therapy defibrillator (CRT-D) device, we created a Cox model restricted to patients with a pacemaker, without an ICD. We evaluated for interactions between beta-blocker use, pacemaker, and heart rate in separate and combined models using the full cohort. All statistical analyses were performed in RStudio (version 2021.09.0, Boston, MA, USA) using the ‘survival’ package.
Results
Of the 6975 subjects in the GISSI-HF trial database, 813 (11.7%) had a pacemaker rhythm on baseline ECG. Of these 813 patients, 511 (62.9%) were receiving beta-blocker therapy. Over a median follow-up of 3.9 (3.0–4.5) years, there were 1970 deaths in the full study cohort.
Baseline characteristics of the study cohort stratified by pacemaker rhythm are reported in Table 1. Subjects with a pacemaker rhythm were on average more likely to be older, male, have a higher NYHA class, have a history of stroke, diabetes, and atrial fibrillation. Furthermore, on average, their systolic blood pressure was lower, their EF was lower, and they were more likely to be receiving a mineralocorticoid receptor antagonist and loop diuretic.
| Overall (n = 6975) | No pacemaker rhythm (n = 6162) | Pacemaker rhythm (n = 813) | P-value | |
|---|---|---|---|---|
| Age (years), mean (SD) | 67 (11) | 67 (11) | 71 (9) | <0.001 |
| Male, n, (%) | 5459 (78.3) | 4793 (77.8) | 666 (81.9) | 0.008 |
| BMI (kg/m2), mean (SD) | 27 (4) | 27 (5) | 26 (4) | <0.001 |
| Systolic blood pressure (mmHg), mean (SD) | 126 (18) | 127 (18) | 121 (16) | <0.001 |
| Heart rate (b.p.m.), mean (SD) | 72 (13) | 73 (14) | 72 (10 | 0.028 |
| NYHA Class, n (%) | <0.001 | |||
| 2 | 4425 (63.4) | 4033 (65.4) | 392 (48.2) | |
| 3 | 2365 (33.9) | 1972 (32.0) | 393 (48.3) | |
| 4 | 185 (2.7) | 157 (2.5) | 28 (3.4) | |
| Ischaemic aetiology, n (%) | 3467 (49.7) | 3041 (49.4) | 426 (52.4) | 0.110 |
| Previous stroke, n (%) | 346 (5.0) | 281 (4.6) | 65 (8.0) | <0.001 |
| Diabetes, n (%) | 1974 (28.3) | 1733 (28.1) | 241 (29.6) | 0.388 |
| Previous CABG, n, (%) | 1271 (18.2) | 1099 (17.8) | 172 (21.2) | 0.024 |
| Previous PCI, n (%) | 866 (12.4) | 775 (12.6) | 91 (11.2) | 0.285 |
| ICD, n (%) | 497 (7.1) | 275 (4.5) | 222 (27.3) | <0.001 |
| History of atrial fibrillation, n (%) | 1325 (19.0) | 1092 (17.7) | 233 (28.7) | <0.001 |
| PAD, n (%) | 610 (8.7) | 526 (8.5) | 84 (10.3) | 0.101 |
| COPD, n (%) | 1533 (22.0) | 1346 (21.8) | 187 (23.0) | 0.481 |
| History of cancer, n (%) | 256 (3.7) | 229 (3.7) | 27 (3.3) | 0.643 |
| EF (%), mean (SD) | 33 (9) | 33 (8) | 31 (8) | <0.001 |
| Haemoglobin (g/dL), mean (SD) | 13.7 (1.7) | 13.8 (1.6) | 13.4 (1.7) | <0.001 |
| Creatinine (mg/dL), mean (SD) | 1.20 (0.48) | 1.18 (0.45) | 1.38 (0.65) | <0.001 |
| Sodium (mmol/L), mean (SD) | 140 (4) | 140 (4) | 140 (4) | 0.100 |
| Beta-blocker use, n (%) | 4522 (64.8) | 4011 (65.1) | 511 (62.9) | 0.223 |
| ACE/ARB use, n (%) | 6520 (93.5) | 5779 (93.8) | 741 (91.1) | 0.005 |
| MRA use, n (%) | 2740 (39.3) | 2354 (38.2) | 386 (47.5) | <0.001 |
| Loop diuretic use, n (%) | 6073 (87.1) | 5317 (86.3) | 756 (93.0) | <0.001 |
| Thiazide diuretic use, n (%) | 402 (5.8) | 366 (5.9) | 36 (4.4) | 0.097 |
| Allopurinol use, n (%) | 1480 (21.2) | 1246 (20.2) | 234 (28.8) | <0.001 |
- Comparison tests were Student's t-test for continuous variables and χ2 test for categorical variables.
- ACE/ARB, angiotensin converting enzyme inhibitor or angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass graft surgery; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; ICD, implantable cardiac defibrillator; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention.
Baseline characteristics of subjects with a pacemaker rhythm stratified by use of beta-blocker therapy are reported in Table 2. Subjects with a pacemaker rhythm treated with a beta-blocker were more likely to be younger, have a lower NYHA class, have an ICD, and less likely to have peripheral arterial disease or chronic obstructive pulmonary disease. Additionally, they had a lower systolic blood pressure and EF. The use of guideline-directed medical therapy was similar between both groups.
| Overall (n = 813) | No beta-blocker (n = 302) | Beta-blocker (n = 511) | P-value | |
|---|---|---|---|---|
| Age (years), mean (SD) | 71 (9) | 73 (8) | 69 (9) | <0.001 |
| Male, n (%) | 666 (81.9) | 244 (80.8) | 422 (82.6) | 0.585 |
| BMI (kg/m2), mean (SD) | 26 (4) | 26 (4) | 26 (4) | 0.896 |
| Systolic blood pressure (mmHg), mean (SD) | 121 (16) | 124 (16) | 120 (16) | 0.001 |
| Heart rate (b.p.m.), mean (SD) | 72 (10) | 73 (10) | 70 (10) | <0.001 |
| NYHA Class, n (%) | <0.001 | |||
| 2 | 392 (48.2) | 116 (38.4) | 276 (54.0) | |
| 3 | 393 (48.3) | 170 (56.3) | 223 (43.6) | |
| 4 | 28 (3.4) | 16 (5.3) | 12 (2.3) | |
| Ischaemic aetiology, n (%) | 426 (52.4) | 163 (54.0) | 263 (51.5) | 0.536 |
| Previous stroke, n (%) | 65 (8.0) | 30 (9.9) | 35 (6.8) | 0.152 |
| Diabetes, n (%) | 241 (29.6) | 96 (31.8) | 145 (28.4) | 0.342 |
| Previous CABG, n (%) | 172 (21.2) | 62 (20.5) | 110 (21.5) | 0.805 |
| Previous PCI, n (%) | 91 (11.2) | 32 (10.6) | 59 (11.5) | 0.764 |
| ICD, n (%) | 222 (27.3) | 49 (16.2) | 173 (33.9) | <0.001 |
| History of atrial fibrillation, n (%) | 233 (28.7) | 99 (32.8) | 134 (26.2) | 0.055 |
| PAD, n (%) | 84 (10.3) | 42 (13.9) | 42 (8.2) | 0.014 |
| COPD, n (%) | 187 (23.0) | 100 (33.1) | 87 (17.0) | <0.001 |
| History of cancer, n (%) | 27 (3.3) | 9 (3.0) | 18 (3.5) | 0.830 |
| EF (%), mean (SD) | 31 (8) | 32 (10) | 30 (7) | <0.001 |
| QRS duration (ms), mean (SD) | 157 (38) | 156 (40) | 157 (36) | 0.721 |
| Haemoglobin (g/dL), mean (SD) | 13.4 (1.7) | 13.3 (1.8) | 13.4 (1.6) | 0.580 |
| Creatinine (mg/dL), mean (SD) | 1.38 (0.65) | 1.40 (0.78) | 1.37 (0.56) | 0.626 |
| Sodium (mmol/L), mean (SD) | 140 (4) | 140 (4) | 140 (4) | 0.834 |
| ACE/ARB use, n (%) | 741 (91.1) | 269 (89.1) | 472 (92.4) | 0.142 |
| MRA use, n (%) | 386 (47.5) | 141 (46.7) | 245 (47.9) | 0.784 |
| Loop diuretic use, n (%) | 756 (93.0) | 283 (93.7) | 473 (92.6) | 0.634 |
| Thiazide diuretic use, n (%) | 36 (4.4) | 21 (7.0) | 15 (2.9) | 0.012 |
| Allopurinol use, n (%) | 234 (28.8) | 89 (29.5) | 145 (28.4) | 0.800 |
- Baseline characteristics of GISSI-HF trial participants restricted to those with pacemaker rhythm on ECG stratified by beta-blocker treatment. Comparison tests were Student's t-test for continuous variables and χ2 test for categorical variables.
- ACE/ARB, angiotensin converting enzyme inhibitor or angiotensin receptor blocker; BMI, body mass index; CABG, coronary artery bypass graft surgery; COPD, chronic obstructive pulmonary disease; EF, ejection fraction; ICD, implantable cardiac defibrillator; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association; PAD, peripheral arterial disease; PCI, percutaneous coronary intervention.
Kaplan–Meier survival analysis restricted to subjects with a pacemaker rhythm demonstrates better survival associated with beta-blocker therapy (Figure 1). Similarly, in subjects without a pacemaker, beta-blocker therapy was associated with improved survival (Figure 1).
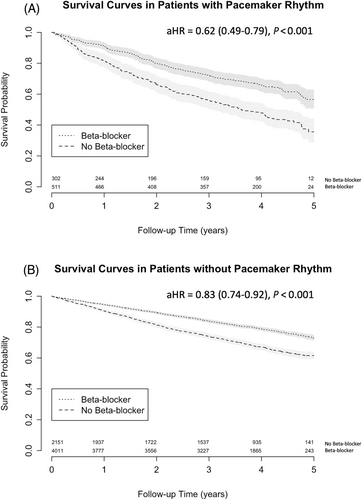
In a Cox proportional hazards model using the whole cohort, beta-blocker therapy was significantly associated with improved mortality after adjustment for pacemaker rhythm and other co-variates as listed above (hazard ratio [HR] 0.79 [0.72–0.87], P < 0.001), without interaction between beta-blockers and pacemaker rhythm (P = 0.275) or beta-blockers and heart rate (P = 0.216) (Table 3, Table S1). Beta-blocker therapy was still associated with improved survival in the sub-group restricted to pacemaker rhythm (Table S2, HR 0.62 [0.49–0.79], P < 0.001) and when further restricted to subjects without an ICD (Table S2, HR 0.60 [0.46–0.79], P < 0.001). We found similar results in the subgroup restricted to EF ≤ 40% (N = 6154, HR 0.79 [0.71–0.88], P < 0.001). In the subgroup with an EF > 40%, beta-blocker therapy was not significantly associated with survival (N = 635, HR 0.76 [0.54–1.06], P = 0.102). In the subgroup of participants with a history of atrial fibrillation, beta-blocker therapy was associated with improved survival (N = 1299, HR 0.82 [0.67–1.00], P = 0.048). In a Cox model using the full cohort, beta-blocker therapy was significantly associated with the combined endpoint of cardiovascular mortality and cardiovascular hospitalization (HR 0.88 [0.82–0.94], P < 0.001).
| N = 6975 subjects | Cox Model 0 | Cox Model 1 | Cox Model 2 | Cox Model 3 | Cox Model 4 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Beta-blocker use | 0.60 (0.54–0.65) | <0.001 | 0.79 (0.72–0.87) | <0.001 | 0.81 (0.73–0.90) | <0.001 | 0.58 (0.35–0.96) | 0.033 | 0.69 (0.40–1.18) | 0.180 |
| Pacemaker rhythm*Beta-blocker | - | - | - | - | 0.88 (0.69–1.11) | 0.275 | - | - | 0.25 (0.05–1.15) | 0.075 |
| Heart rate*Beta-blocker use | - | - | - | - | - | - | 1.00 (1.00–1.01) | 0.216 | 1.00 (1.00–1.01) | 0.561 |
| Heart rate*Pacemaker rhythm | - | - | - | - | - | - | - | - | 0.99 (0.98–1.01) | 0.240 |
| Heart rate*Pacemaker rhythm*Beta-blocker use | - | - | - | - | - | - | - | - | 1.02 (1.00–1.04) | 0.101 |
- Model 0 is unadjusted for co-variates without interaction terms. Model 1 is adjusted for 27 co-variates without interaction terms. Model 2 includes interaction term between pacemaker rhythm and beta-blocker use. Model 3 includes interaction term between heart rate and beta-blocker use. Model 4 includes interactions between pacemaker rhythm, beta-blocker use, and heart rate. Co-variates included in adjusted analyses include age, gender, BMI, systolic blood pressure, heart rate, NYHA class, ischaemic heart disease, history of diabetes, history of bypass surgery, history of percutaneous coronary intervention, history of ICD, history of pacemaker, history of stroke, history of peripheral arterial disease, history of atrial fibrillation, history of COPD, history of cancer, ejection fraction, QRS duration, haemoglobin, creatinine, sodium, ACE/ARB use, MRA use, loop diuretic use, thiazide diuretic use and allopurinol use.
Discussion
In this post hoc analysis of the GISSI-HF trial, we have demonstrated the favourable association between beta-blocker use and all-cause mortality in patients with chronic heart failure and a pacemaker rhythm. This effect remained significant after adjustment for baseline heart rate. We did not detect an interaction between beta-blockers and pacemaker use or beta-blockers and heart rate.
Beta-blockers have been a cornerstone of heart failure therapy since they first demonstrated improved symptoms in the Metoprolol in Dilated Cardiomyopathy trial in 1993.1 Beta-blockers were subsequently approved for treatment of heart failure in 1997.5 Of the major clinical trials demonstrating improvements in mortality, the inclusion of patients with pacemakers is unclear. The MERIT-HF trial excluded patients with ‘atrioventricular block of the second and third degree, unless the patient had an implanted pacemaker and a spontaneous heart rate of 68 b.p.m. or more’.6 The CIBIS-II trial excluded patients if they had ‘atrioventricular block greater than first degree without a chronically implanted pacemaker’, or a ‘resting heart rate of <60 b.p.m.’.7 Neither the COPERNICUS trial nor the US Carvedilol trial specifically stated how atrioventricular block or pacemaker patients were to be included or excluded.8, 9
Contemporary studies have further refined the population of heart failure patients that derives the most benefit from beta-blocker therapy. Consistent with our study, patients with heart failure with preserved ejection fraction do not appear to benefit from beta-blocker therapy and in fact may be harmful.10, 11 A patient level meta-analysis, including >14 000 participants in randomized clinical trials of beta-blockers in heart failure, found no association between randomization to beta-blockers and survival in the subgroup with an EF > 50%.12 However, the Kyoto Congestive Heart Failure registry reported an association between beta-blocker therapy use at admission and lower in-hospital mortality among patients admitted with acute decompensated heart failure, which effect was not modified by EF or the presence of atrial fibrillation.13 Observational studies from Korea suggest that a lower global longitudinal strain (<10%) may identify patients who benefit from beta-blockers, including those with heart failure with preserved ejection fraction.14, 15 Three meta-analyses reported that beta-blocker therapy was not associated with improved survival or decreased heart failure hospitalizations among patients with HFrEF and atrial fibrillation.12, 16, 17 A post hoc analysis of the BEST trial suggests that reduced right ventricular ejection fraction (<35%) may identify patients who do not benefit from beta-blockers.18 Despite extensive evaluation of clinical sub-groups, the utility of beta-blocker therapy in patients with an atrial or ventricular pacemaker was previously unanswered.
A question central to the relation between beta-blockers and a paced rhythm is whether the beneficial effects of beta-blockers are mediated purely through heart rate reduction. An individual level meta-analysis of 11 randomized trials of beta-blockers evaluated the relationship between baseline heart rate and mortality.19 Regardless of pretreatment heart rate, beta-blocker therapy improved mortality. A lower heart rate was associated with lower mortality, but survival was similar between subjects who achieved a heart rate <70 b.p.m. versus heart rate <60 b.p.m. These data indicate that beta-blocker use may be beneficial in all patients, but increased doses may not have a mortality benefit when a heart rate <70 b.p.m. has been achieved. Unfortunately, this study excluded all subjects in a paced rhythm. Our study further supports that beta-blocker therapy is beneficial in patients with a paced rhythm. We attempted to study whether beta-blocker therapy is associated with mortality benefit in patients with a low heart rate and a pacemaker rhythm. While our study did not identify an interaction between beta-blocker therapy and heart rate, beta-blocker and pacemaker, or beta-blocker and heart rate and pacemaker, our study may have been underpowered to detect such an interaction.
In the current era with cardiac resynchronization therapy (CRT), patients with severe HFrEF (EF ≤ 35%) are recommended to have CRT if the QRS duration is ≥150 ms, or ≥120 ms with a left bundle branch block.20 Additionally, if the patient is expected to have >40% ventricular pacing, CRT is recommended. Beta-blocker therapy is beneficial and indicated in patients receiving CRT.21 GISSI-HF did not collect information on CRT or the mode of pacing. We believe the use of CRT is minimal in the study population since the GISSI-HF trial enrolled between 2002 and 2005. The US Food and Drug Administration first approved CRT for use in 2001 and the first trial to report improvement in mortality from CRT was published in 2004, so it is likely that very few patients in this cohort would have undergone that procedure given its investigational status at the time of study enrolment.22 The InSync Italian Registry reported 1181 CRT implantations between 1995 and 2005, which may not reflect the entire country but indicates overall low use during the time frame our data was collected.23 The few patients who may have a CRT device in this cohort would most likely have a CRT-D device, and after excluding patients with ICDs, a favourable association with mortality was still seen with beta-blocker therapy (Table S2).
A clinical question that occasionally arises is whether to implant a pacemaker device in order to initiate or increase beta-blocker therapy. The 2018 ACC/AHA/HRS Guidelines on management of bradycardia give a Class I recommendation (Level of Evidence C: Expert Opinion) for pacemaker implant in patients who develop symptomatic atrioventricular block in response to guideline-directed therapy.24 The 2022 ACC/AHA/HFSA and the 2021 European Society of Cardiology guidelines for the management of heart failure provide no guidance in this clinical scenario.20, 25 One study attempted to simulate this strategy using patient data from the US Carvedilol trial, and their simulation results support this strategy.26 Despite the simulated evidence, we feel this strategy would benefit from testing in a randomized control trial to demonstrate efficacy.
In the modern heart failure clinic, our data is most applicable to patients who already have a non-CRT or CRT pacemaker device implanted, those who suffer from sinus node dysfunction or those who develop atrioventricular block or symptomatic bradycardia in response to beta-blocker therapy. While our data demonstrates the benefit of beta-blockers in patients with pacemakers, our data does not directly address the strategy of implanting pacemaker to facilitate initiation or titration of beta-blocker therapy or when slowing the atrial rate might result in an increase in the frequency of ventricular pacing.
Limitations
A main limitation to this dataset is the inability to distinguish the type of pacemaker device, including the presence of CRT. We believe the prevalence of CRT devices to be relatively low given the arguments presented above. Regarding atrial pacing versus ventricular pacing, we are unfortunately unable to reliably distinguish between these populations and are unable to test for differences in response to beta-blocker therapy. We are similarly unable to analyse for differences based on proportion of paced beats. As a post hoc analysis, we are limited to describing associations and the observed effects may be artifactual, and there may be critical reasons for participants not being prescribed beta-blockers that are not capture in this dataset (i.e. intolerance to beta-blocker therapy). Limitations to the generalizability include the low proportion of women enrolled and limited ethnic diversity of participants. With advances in pharmacological therapy for patients with heart failure since the GISSI-HF trial (e.g. sodium-glucose cotransporter-2 inhibitor [SGLT2i] and angioensin receptor/neprilysin inhibitor [ARNI]), similar results may not be seen in a contemporary cohort.
Conclusions
Beta-blocker therapy is associated with better survival among patients with heart failure and a pacemaker rhythm on ECG, primarily among patients with HFrEF. Further studies are necessary to analyse differences between atrial and ventricular pacemakers.
Conflict of interest
A.S.P., None. A.P.M. personal fees from Bayer, Novartis, AstraZeneca for the participation in study committees. L.T. personal fees from Servier and CVIE Therapeutics as trial committee member. W.C.L. Clinical Endpoint Committee – SOLVE-CRT (EBR Systems), GUIDE-HF (CardioMems, Abbott), NT-proBNP diagnostic evaluation (Siemens, Beckman Coulter), Anthem-HF (Liva Nova); Steering Committee – Respicardia, Cardiac Dimensions; Consultant – Impulse Dynamics, Medtronic Inc. Research Funding – Medtronic.




