Serum angiotensin-converting enzyme levels indicating early sarcoidosis diagnosis and immunosuppressive therapy efficacy
Abstract
Aims
This study aimed to determine the new cut-off value of serum angiotensin-converting enzyme (ACE) levels for detecting patients with sarcoidosis and to examine the change in ACE levels after the initiation of immunosuppressive therapy.
Methods and results
We retrospectively examined patients in whom serum ACE levels were measured for suspected sarcoidosis between 2009 and 2020 in our institution. For patients diagnosed with sarcoidosis, changes in ACE levels were also observed. Of the 3781 patients (51.1% men, 60.1 ± 17.0 years old), 477 were excluded for taking ACE inhibitors and/or immunosuppression agents or those with any diseases affecting serum ACE levels. In 3304 patients including 215 with sarcoidosis, serum ACE levels were 19.6 IU/L [interquartile range, 15.1–31.5] in patients with sarcoidosis and 10.7 [8.4–16.5] in those without sarcoidosis (P < 0.01), and the best cut-off value was 14.7 IU/L with 0.865 of the area under the curves. Compared with the current ACE cut-off of 21.4, the sensitivity improved from 42.3 to 78.1 at the new cut-off, although specificity slightly decreased from 98.6 to 81.7. The ACE level significantly decreased more in those with immunosuppression therapy than in those without it (P for interaction <0.01), although it decreased in both groups (P < 0.01).
Conclusions
Because the sensitivity for detecting sarcoidosis is comparatively low at the current standard value, further examinations are needed for patients suspected of sarcoidosis with relatively high ACE levels in the normal range. In patients with sarcoidosis, ACE levels decreased after the initiation of immunosuppression therapy.
Introduction
Sarcoidosis is a systemic disease associated with non-caseating granulomas.1-3 It can affect multiple organs; meanwhile, cardiac sarcoidosis (CS) can cause several complications, including fatal arrhythmias, congestive heart failure, and sudden cardiac death. Thus, early detection of CS is crucial for improving prognosis. Serum angiotensin-converting enzyme (ACE) is an acid glycoprotein that converts angiotensin I into angiotensin II. Since Lieberman reported elevated serum ACE levels in patients with sarcoidosis in 1975,4 it has been widely included in the screening for patients suspected of sarcoidosis. In the last few decades, the guidelines for sarcoidosis have changed. The field of diagnostic imaging has evolved greatly, and accurate diagnosis has been made.5 The current standard value of ACE has been determined based on healthy controls by taking the mean plus two standard deviations. However, in clinical practice, we frequently encounter patients with sarcoidosis showing relatively high ACE levels in the normal range. Thus, this study aimed to determine the new cut-off value of serum ACE for detecting patients with sarcoidosis and compare the change in ACE levels between patients having sarcoidosis with and without immunosuppression initiation after the initial ACE measurement.
Methods
Subjects
We retrospectively examined 3781 patients (≥20 years old) in whom ACE was measured for any reason including suspected sarcoidosis between 2009 and 2020 at Fujita Health University Hospital.
Data collection
The presence of hypertension, diabetes mellitus, dyslipidaemia, chronic kidney disease, and other co-existing diseases that can affect the ACE level were collected from the medical records. Hypertension was defined as blood pressure ≥140/90 mmHg or current use of antihypertensive medication. Dyslipidaemia was defined as a low-density lipoprotein cholesterol level ≥140 mg/dL, a high-density lipoprotein cholesterol level <40 mg/dL, a triglyceride level ≥150 mg/dL, or treatment with lipid-lowering medication. Diabetes mellitus was defined as a fasting blood glucose ≥126 mg/dL or haemoglobin A1c (National Glycohemoglobin Standardisation Programme) ≥ 6.5% or treatment with hypoglycaemic medication. Similarly, medical agents including ACE inhibitor (ACEi), steroids, or immunosuppressive agents were also examined. The diagnosis of sarcoidosis was determined by diagnostic standards and guidelines for sarcoidosis6, 7 and JCS Guideline on Diagnosis and Treatment of Cardiac Sarcoidosis.5 For patients diagnosed with sarcoidosis, ACE levels were measured after the initial diagnosis.
The study protocol was approved by the Institutional Review Board and ethics committees of Fujita Health University. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval by the institution's human research committee. We applied an opt-out method to obtain consent for this study on the website of our department.
Angiotensin-converting enzyme measurement
Peripheral blood samples for the measurements of biomarkers were collected at the time of clinical evaluation. Serum ACE levels were measured by a colorimetric method using p-hydroxyhip-puryl-l-histidyl-l-leucine as the substrate: Kasahara method (BML, Inc.).8 The normal range was ≤21.4 IU/L. In patients diagnosed with sarcoidosis, the lowest points of ACE levels after the initial diagnosis were also recorded to compare ACE levels between patients having sarcoidosis with and without immunosuppression initiation.6
Statistical analysis
The Shapiro–Wilk test was used to assess the normality of continuous data. Variables with a normal distribution are expressed as mean values ± standard deviation, and asymmetrically distributed data are given as the median and interquartile range. Categorical variables were presented as frequency (percentage). Differences between the two groups were evaluated by the Mann–Whitney test or Student's t-test for continuous variables and by the chi-squared test for categorical variables. Differences among the four groups were evaluated using the Kruskal–Wallis test for continuous variables in asymmetrically distributed data. A probability value of <0.05 was considered significant. Receiver operating characteristics (ROC) analysis was performed to calculate the sensitivity, specificity, area under the ROC curve, and optimal cut-off with 95% confidence limits. Two-way repeated-measures analysis of variance was used to compare the change in ACE levels between patients having sarcoidosis with and without immunosuppression initiation after ACE measurement. All statistical analyses were conducted using JMP version 13 (SAS Institute, Cary, NC, USA).
Results
Subject characteristics
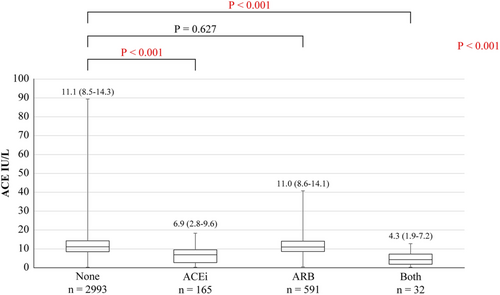
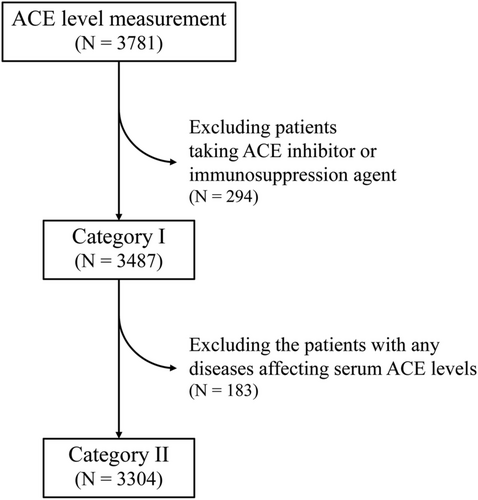
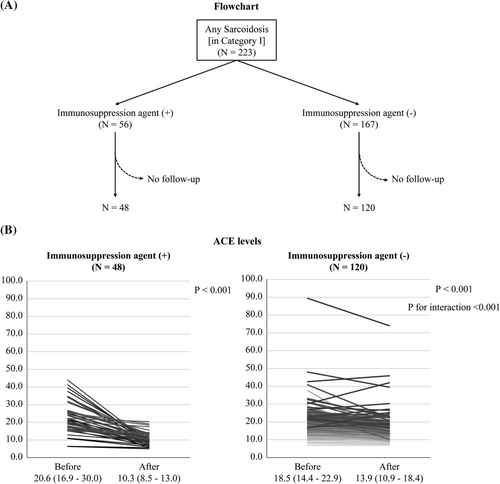
Of 3781 patients (51.1% men, 60.1 ± 17.0 years old), 293 were diagnosed with sarcoidosis. Of them, 197 (5.2%) and 109 (2.9%) patients were taking ACEi and steroid/immunosuppressive agents, respectively (Table 1). Of the 293 patients with sarcoidosis, 101, 212, 84, and 88 were diagnosed with sarcoidosis in the heart, lung, skin, and eyes, respectively (Table 2). First, we compared serum ACE levels among patients with ACEi, angiotensin II receptor blockers (ARB), both and none, because ACEi has been reported to lead to low ACE levels.9, 10 Serum ACE levels of patients taking ACEi or both were lower than those of patients with neither (6.9 vs. 11.1, P < 0.001, 4.3 vs. 11.1, P < 0.001), although that of patients taking ARB was similar (11.0 vs. 11.1, P = 0.627) (Figure 1). Therefore, we analysed 3487 patients (including 223 with sarcoidosis) after excluding 294 taking with ACEi and/or steroid/immunosuppressive agents (category I). Furthermore, 3304 patients (215 with sarcoidosis) after excluding 183 with other diseases that can affect serum ACE levels were analysed in category II (Figure 2).
| N = 3781 | % | |
|---|---|---|
| Age | 60.1 ± 17.0 | |
| Man | 1931 | 51.1 |
| Hypertension | 883 | 23.4 |
| Diabetes mellitus | 357 | 9.4 |
| Dyslipidaemia | 703 | 18.6 |
| Chronic kidney disease (eGFR <60) | 970 | 25.7 |
| Advanced chronic kidney disease (eGFR <30) | 205 | 5.4 |
| Lung cancer | 118 | 3.1 |
| Lung tuberculosis | 4 | 0.1 |
| Atypical mycobacterium infection | 2 | 0.1 |
| Chronic obstructive pulmonary disease | 2 | 0.1 |
| Cystic fibrosis | 1 | 0 |
| Multiple myeloma | 1 | 0 |
| Chronic leukaemia | 1 | 0 |
| Basedow disease | 12 | 0.3 |
| Cirrhosis | 53 | 1.4 |
| Sarcoidosis (overall) | 293 | 7.7 |
| Sarcoidosis (C+, eC+) | 80 | 2.1 |
| Sarcoidosis (C−, eC+) | 192 | 5.1 |
| iCS (C+, eC−) | 21 | 0.6 |
| iCS susp (C±, eC−) | 10 | 0.3 |
| Steroid/immunosuppressive agent | 109 | 2.9 |
| ACEi | 197 | 5.2 |
| ARB | 623 | 16.5 |
| Mineralocorticoid receptor antagonist | 66 | 1.7 |
| Calcium-channel blocker | 104 | 2.8 |
| Beta-blocker | 119 | 3.1 |
| Antidiabetic agent | 67 | 1.8 |
| Insulin use | 8 | 0.2 |
| Statin | 118 | 3.1 |
| Antiplatelet agent | 65 | 1.7 |
| Anticoagulant agent | 49 | 1.3 |
- Abbreviations: ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blockers; C, cardiac; CCB, calcium-channel blocker; eC, extra cardiac; eGFR, estimated glomerular filtration rate; iCS, isolated cardiac sarcoidosis.
| N = 293 | (%) | |
|---|---|---|
| Heart | 101 | (34.5) |
| Lung | 212 | (72.4) |
| Skin | 84 | (28.7) |
| Eye | 88 | (30.0) |
| Kidney | 13 | (4.4) |
| Muscle | 15 | (5.1) |
| Nerve | 5 | (1.7) |
| Hepatic | 6 | (2.0) |
| Splenic | 2 | (0.7) |
Angiotensin-converting enzyme levels among patients with each type of sarcoidosis and those without
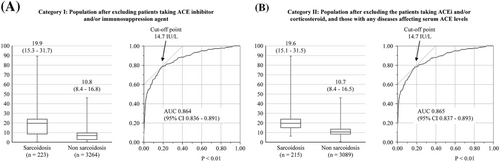
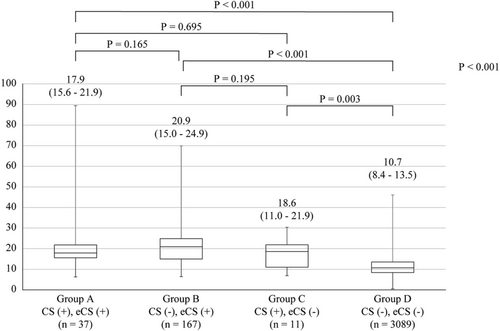
In category I, the serum ACE level was 19.9 [interquartile range (IQR), 15.3–31.7] IU/L in patients with sarcoidosis and 10.8 [8.4–16.8] in those without it (P < 0.01). In the ROC curve analysis of ACE for the diagnosis of sarcoidosis, the cut-off point associated with sarcoidosis was 14.7 IU/L. The area under the ROC curve was 0.864 [95% confidence interval (CI): 0.836–0.891, P < 0.001]. Similarly, in category II, the serum ACE levels were 19.6 [IQR, 15.1–31.5] IU/L in 215 patients with sarcoidosis and 10.7 [8.4–16.5] IU/L in 3089 patients without it (P < 0.01). The area under the ROC curve was 0.865 (95% CI: 0.837–0.893, P < 0.001) (Figure 3). In the current cut-off value (≥21.4), the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 42.3, 98.6, 68.4, 96.1, and 95.0, respectively. When we used the new cut-off value (≥14.7), the sensitivity, specificity, positive predictive value, negative predictive value, and accuracy were 78.1, 81.7, 23.0, 98.2 and 81.5%, respectively (Table 3). Finally, the 3304 patients were divided into four groups: group A, CS (+) extra CS (eCS) (+); group B, CS (−) eCS (+); group C, CS (+) eCS (−); and group D, CS (−) eCS (−)]. The ACE levels in groups A, B, C, and D were 17.9 (15.6–21.9), 20.9 (15.0–24.9), 18.6 (11.0–21.9), and 10.7 (8.4–13.5), respectively. Although the ACE levels in patients with isolated CS showed a decreasing trend, the difference was not significant. The ACE level in group D was significantly lower than that of groups A, B, or C (Figure 4).
| Sarcoidosis | Control | Sensitivity | Specificity | PPV | NPV | Accuracy | ||
|---|---|---|---|---|---|---|---|---|
| ACE ≥ 21.4 (IU/L) | 42.3 (35.6–49.2) | 98.6 (98.2–99.0) | 68.4 (59.8–76.2) | 96.1 (95.4–96.7) | 95.0 (94.2–95.7) | |||
| Positive | 91 | 42 | 133 | |||||
| Negative | 124 | 3047 | 3171 | |||||
| 215 | 3089 | 3304 | ||||||
| ACE ≥ 14.7 (IU/L) | 78.1 (72.0–83.5) | 81.7 (80.3–83.1) | 23.0 (20.0–26.2) | 98.2 (97.6–98.7) | 81.5 (80.1–82.8) | |||
| Positive | 168 | 564 | 732 | |||||
| Negative | 47 | 2525 | 2572 | |||||
| 215 | 3089 | 3304 |
- Abbreviations: ACE, angiotensin-converting enzyme; NPV, negative predictive value; PPV, positive predictive value.
Change in angiotensin-converting enzyme levels after the initiation of immunosuppressive agents or not for patients with sarcoidosis
In 223 patients with sarcoidosis in category I, an immunosuppressive therapy was initiated for 56 and not for 167; repeated ACE data were obtained in 48 of 56 and 120 of 167 patients, respectively. The pattern of change in ACE levels was different between the two groups (P for interaction <0.001). In both groups, the ACE levels decreased from 20.6 to 10.3 (P < 0.001) in patients with immunosuppression therapy and from 18.5 to 13.9 (P < 0.001) in those without; the reduction was more remarkable in those with immunosuppression therapy (P < 0.001) (Figure 5).
Discussion
We retrospectively examined the serum ACE levels in serial 3781 patients and performed ROC analysis for comparing patients with and without sarcoidosis. After excluding patients taking ACEi and/or corticosteroids and those with any diseases that affect serum ACE levels, the remaining population had ACE levels of 14.7 IU/L, which was the best cut-off value (area under the curve [AUC] 0.865) for detecting sarcoidosis. Compared with the current ACE cut-off of 21.4, the sensitivity improved from 42.3 to 78.1 at the new cut-off of 14.7, whereas specificity slightly decreased from 98.6 to 81.7. Despite the trend toward low ACE levels in patients with isolated CS, the difference was not significant. Diagnosing CS is a major challenge. The granuloma proportion and ACE level usually remain low at the beginning of CS, whereas ACE levels are often high in cases of pulmonary sarcoidosis. Identifying a threshold of ACE for detecting this damage is difficult. Once fibrosis is established, the granuloma load decreases, resulting in the reduction of the ACE level; this may explain the decrease in the ACE level without providing immunosuppression therapy. However, although the ACE levels decreased after diagnosis in both groups of patients with and without initiation of immunosuppressive therapy, the decrease in ACE levels was more apparent in those with immunosuppression therapy. In the current clinical setting, serum ACE measurement is used as the first-line screening method in patients suspected of sarcoidosis before specific imaging or biopsy. To avoid missing the diagnosis of sarcoidosis, the new cut-off value may be suitable. To the best of our knowledge, this is the first study in which more than 3000 serial subjects were examined on the basis of the current guideline including the definition of isolated cardiac sarcoidosis.
Previous studies
Approximately 30–80% of patients with sarcoidosis have increased ACE levels, and sensitivity ranges between 22% and 86% and specificity between 54% and 95%.11, 12 Given the variabilities in ACE levels, its value as a diagnostic tool remains controversial.
First, ACE levels are reported to be influenced by ACE gene polymorphisms. Allele I (presence of a nonsense DNA fragment) is associated with low ACE activity.13 Tomita et al. examined the distribution of genotypes in Japanese patients and the serum ACE levels of each genotype in both patients with sarcoidosis and normal controls.14 In normal controls, the average ACE levels of II, deletion/insertion (DI) and deletion/deletion (DD) genotype were 10.8 ± 3.1, 13.8 ± 4.3, and 17.2 ± 4.0 IU/L, respectively. On the contrary, the corresponding values in patients with sarcoidosis were 21.4 ± 7.9, 23.9 ± 7.2, and 27.3 ± 7.5 IU/L, respectively. The prevalence of DD was comparable in patients with sarcoidosis and normal controls (14.0% vs. 12.4%). Differences in ACE levels between Caucasians and Japanese may be attributed to gene polymorphisms. Compared with Caucasians, the prevalence of allele I is high in Japanese, resulting in a low ACE activity. Second, ACE levels correlate well with the estimated total bulk of granulomata in the body.15 Compared with systemic sarcoidosis, patients with only a single organ involved, such as isolated CS, may have lower ACE levels. Finally, the assay of serum ACE may be influenced; the variously reported sensitivities are related to the assay method.
Clinical implications
We examined the new cut-off value of serum ACE, which is useful for the diagnosis of sarcoidosis in the clinical setting, after excluding patients with diseases that can affect ACE levels or were taking ACEi or immunosuppressive agents. Serum ACE serves as a gatekeeper for detecting patients with sarcoidosis, and further examinations are needed for patients with ACE positive. If the sensitivity is low, some patients with ACE false negative may not undergo imaging or pathological examinations. Specifically, the presence of CS is associated with the prognosis of the patients. Immunosuppression therapy, usually with corticosteroids, is suggested for the treatment of clinically manifest CS. Compared with those with moderately reduced left ventricular ejection fraction (30–54%), immunosuppressive therapy does not improve the left ventricular function of patients with severely reduced left ventricular ejection fraction (≤30%) containing a large scar burden.16 The identification of patients at the early stage of CS is essential. ACE is expected to serve as a screening tool for the diagnosis of sarcoidosis.
Limitations
This study has several limitations. First, the study was retrospectively conducted in a single hospital, although many serial subjects were included. Second, in patients with sarcoidosis, all the involved organs might be not completely examined, although the diagnosis of sarcoidosis was confirmed. Finally, we did not examine the polymorphism of ACE in the patients. The study of ACE levels for each genotype is a great force in this study. The judgement based on the new normal range for each genotype improved the accuracy of diagnosing sarcoidosis.14
Conclusions
In the current standard value of serum ACE, the sensitivity for detecting sarcoidosis is comparatively low. Further examinations are needed for patients suspected with sarcoidosis and have relatively high ACE levels in the normal range (>14.7 IU/L). In patients with sarcoidosis, ACE levels decreased more in those with immunosuppression therapy than in those without it.
Conflict of interest
Hideo Izawa has received grant support through his institution from Bayer, Daiichi-Sankyo, Dainihon-Sumitomo, Kowa, Ono, Otsuka, Takeda and Fuji Film Toyama Kagaku, and honoraria for lectures from Boehringer Ingelheim, Daiichi-Sankyo, Novartis and Otsuka Corporation. The remaining authors have nothing to disclose.




