HeartMate 3 biventricular support exceeding 4.5 years
Drs. Gasparovic and Milicic contributed equally to this work.
Abstract
A 47-year old male with ischaemic cardiomyopathy was referred to us for durable left ventricular assist device placement. He was found to have prohibitively elevated pulmonary vascular resistance for heart transplantation. He underwent HeartMate 3 left ventricular assist device implantation, with additional temporary right ventricular assist device (RVAD) placement. Following a 2-week period of unweanable temporary right ventricular support, the patient was switched to durable biventricular support with two Heartmate 3 pumps. The patient was placed on a transplant waiting list but was not offered a heart for over 4 years. While on Heartmate 3 biventricular support (BiVAD), he returned to full activity and enjoyed an excellent quality of life. He underwent laparoscopic cholecystectomy 7 months after the BIVAD implant. After 52 months of uneventful BiVAD support, he presented with a combination of adverse events that occurred over a short period. These included subarachnoidal haemorrhage and a new motor deficit, followed by RVAD infection and RVAD low-flow alarms. After over 4 years of unimpeded RVAD flows, new imaging revealed an outflow graft twist with subsequent flow reduction. The patient underwent heart transplantation after a total of 1655 days of Heartmate 3 BiVAD support and continues to do well on latest follow-up.
Introduction
Subject to varying definitions, right ventricular (RV) failure occurs in 9–42% of left ventricular assist device (LVAD) recipients.1, 2 It remains a significant contributor to post-LVAD morbidity and mortality. Options for biventricular mechanical support are currently limited. No continuous-flow device has been designed to match the anatomical and physiological characteristics of the RV. HeartMate 3 BiVAD (Abbott, Chicago, IL) is a viable, but off-label, option for this clinical scenario. Patient selection is paramount in planning VAD configurations, as is the sequence in which they should be placed.
Case report
We present a 47-year-old male with extended continuous flow biventricular support. The underlying aetiology was ischaemic cardiomyopathy, notable for past percutaneous coronary interventions. He was found to have fixed and elevated pulmonary vascular resistance, prohibitive for heart transplantation. We opted for an implantable LVAD as a bridge to transplantation. His right ventricle (RV) was dilated, with both his radial and longitudinal RV functions moderately depressed. Despite the high systolic pulmonary artery (PA) pressures the RV was capable of generating, it was clear that RV dysfunction was present because both the PA pulsatility indices and CVP/PCWP ratios were unfavourable.
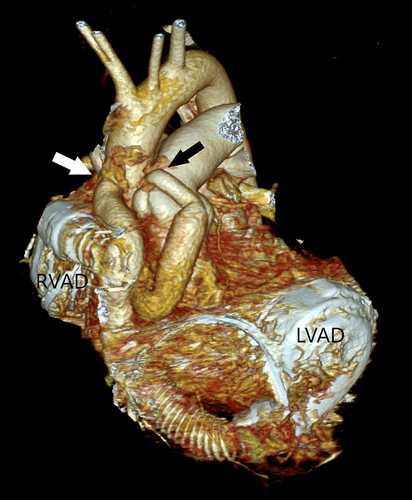
For reasons stated above, we maintained a low threshold for initiating intraoperative (temporary) RV support at the time of, an otherwise unremarkable, HeartMate 3 LVAD placement. Over the course of the following 2 weeks, multiple RVAD weaning trials had failed, which prompted an exchange of the temporary RVAD for a durable device. The HeartMate 3 RVAD was placed in the right atrium with multiple felt strips placed underneath the sewing ring to reduce the intraluminal segment of the inflow canula (Figure 1). Notwithstanding the long-term challenges of dealing with two sets of peripherals, our patient returned to full activity and was placed on a transplant waiting list. After 7 months on BIVAD support, he underwent an uneventful laparoscopic cholecystectomy, for which his anticoagulation regimen consisting of ASA 100 mg and warfarin was bridged with low molecular weight heparin.
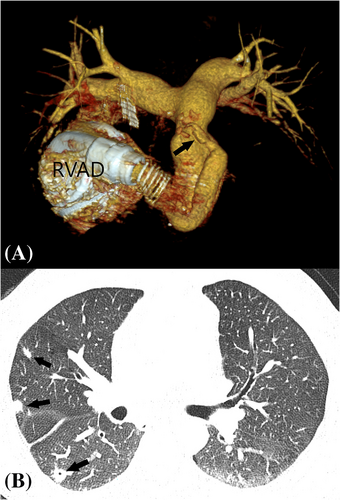
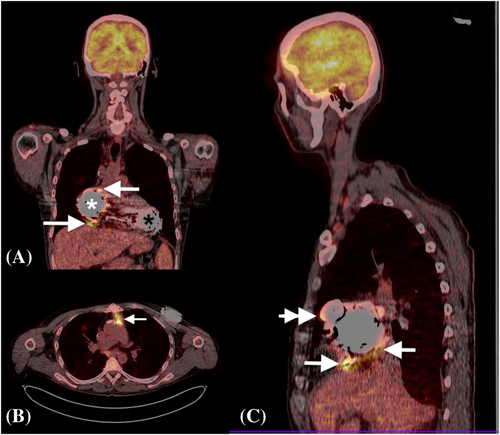
Fifty-two months after BIVAD placement, the patient was admitted to our emergency department with new facial nerve and hand paresis, as well as motor dysphasia. He was found to have subarachnoidal haemorrhage related to accidental over-anticoagulation (INR 4.0). This prompted withdrawal of both anticoagulation and antiplatelet treatment. The patient received 10 mg of intravenous vitamin K in the emergency department. Gradual reinstitution of warfarin was started 2 weeks after the index event, following careful deliberation in a multidisciplinary team. This was performed in concert with serial brain imaging, in order to continuously monitor the evolution of intracranial pathology. Soon thereafter, the patient started experiencing low-flow RVAD alarms. Of note, he had adequate RVAD flows for over 4 years. Computed tomographic imaging revealed an RVAD outflow graft twist (Figure 2). Additionally, thrombotic and possibly endocarditic masses were described within the outflow graft, and evidence of multiple pulmonary emboli were noted (Figure 2). During the same hospitalization, the patient developed signs of systemic infection. A parallel workup of the systemic infection included a positron emission tomography CT scan, which showed infection of the RVAD components (Figure 3). The blood cultures were repetitively sterile. His neurologic status recovered, with only mild residual right hand paresis. This was not felt to be a contraindication for heart transplantation. A combination of device-related infection and underlying mechanical dysfunction of the RVAD prompted the patient to be placed on ‘high-urgent’ transplant status. A heart was allocated for our patient after a total of 1655 days on biventricular HeartMate 3 support. The actual heart transplantation was uneventful, albeit technically more challenging due to two intersecting outflow grafts and two intrapericardial pumps. Following heart transplantation, the patient's pulmonary vascular resistance was found to vary between 180 and 320 dynes/sec/cm−5.
Discussion
Feasibility of biventricular continuous-flow therapy has been repeatedly demonstrated in the past.3 In a recent meta-analysis of continuous flow BiVAD support, the median time on support was 237 days.4 Survival data for BiVAD support are universally inferior to those for isolated LVADs,4, 5 underscoring the greater morbidity burden of these patients. Croatia is among the highest ranked countries in the numbers of heart transplantations per capita in the Eurotransplant organization. It was, therefore, unusual for our patient not to have been offered a suitable organ for 4.5 years. Our patient was supported with HeartMate 3 BiVAD for 1655 days. To the best of our knowledge, this constitutes the longest reported HeartMate 3 BiVAD support to date. We opted for staged VAD placement, first testing temporary RVAD support in addition to the HeartMate 3 LVAD. Contemporaneous placement of a durable RVAD at the time of LVAD implantation avoids the detrimental effects of temporary RV support, but risks placing a second device in a patient whose RV could have recovered. In hindsight, we feel that we should have opted for simultaneous implantation of HeartMate 3 devices as predictors of post-LVAD RV failure were identified preoperatively. Our case illustrates that, whereas the quality of life may be excellent for extended periods of support, abrupt and profound clinical deteriorations are always possible. Heightened vigilance should be maintained continuously in this population of patients, regardless of the duration of previously unremarkable mechanical support.




