Coronary-subclavian steal syndrome causing myocardial infarction after arteriovenous fistula creation: a case report
Abstract
Coronary subclavian steal syndrome (CSSS) caused by left subclavian artery (LSA) stenosis is a rare cause of myocardial infarction in patients having coronary artery bypass grafting (CABG), and it has also been observed after an arteriovenous fistula (AVF) was made. A 79-year-old woman who had undergone CABG years earlier and an AVF creation 1 month before experienced a non-ST-elevation myocardial infarction (NSTEMI). While selective catheterization of the left internal thoracic artery graft was impossible, a computed tomography scanner showed patency of all bypasses and proximal subocclusive LSA stenosis, and the digital blood pressure measurements objectified a haemodialysis-induced distal ischaemia. LSA's angioplasty and covered stent placement were successfully performed, resulting in symptom remission. A CSSS-induced NSTEMI due to a LSA stenosis aggravated by a homolateral AVF several years after CABG has been documented only infrequently. If vascular access is required in the presence of CSSS risk factors, the contralateral upper limb should be preferred.
Introduction
Due to its superior patency rate and survival benefit when grafted to the left anterior descending artery, the left internal thoracic artery (ITA) is the conduit most frequently used for coronary artery bypass grafting (CABG).1 While the proximal end of the left ITA is kept connected to the left subclavian artery (LSA), the distal end is severed and anastomosed to the epicardial coronary artery. After a CABG procedure, the onset of a LSA stenosis just upstream of the ostium of the left ITA graft can lead to coronary subclavian steal syndrome (CSSS), a condition where the myocardial blood flow is reduced or reversed to maintain upper limb perfusion, with consequent myocardial blood steal.1 While 11.8% of patients with coronary artery disease (CAD) requiring CABG were found to have proximal LSA stenosis, CSSS prevalence has been estimated at 0.2% to 6.8% in patients with CABG using a left ITA graft.1 These estimations are likely underrated because of diagnostic difficulties and the frequency of asymptomatic cases, even in high-grade stenosis. Nevertheless, CSSS can cause unstable angina, myocardial infarction, ventricular arrhythmia, or congestive heart failure.1 Here, we describe an episode of concomitant non-ST-elevation myocardial infarction (NSTEMI) that happened 1 month after a left-arm proximal arteriovenous fistula (AVF) was made in an elderly woman with a history of triple-vessel CAD treated by CABG with a left ITA graft a few years ago. The myocardial infarction was attributed with certainty to the CSSS given the medical history, the presence of a severe LSA stenosis, and the patency of the bypass grafts. Clinical evaluation, an electrocardiogram, upper limb Doppler ultrasound (DUS), digital pressures (DPs), and angiography were used to support the diagnosis. Asymptomatic high-grade LSA stenosis suddenly becoming the cause of a life-threatening CSSS following the creation of an ipsilateral AVF in a patient with CABG has been rarely reported.
Case Report
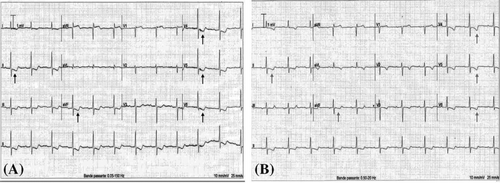
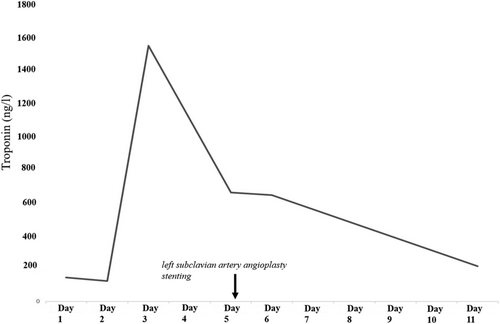
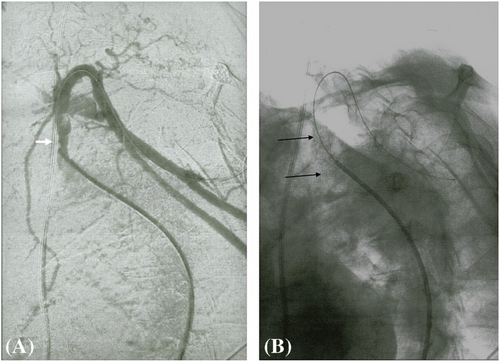
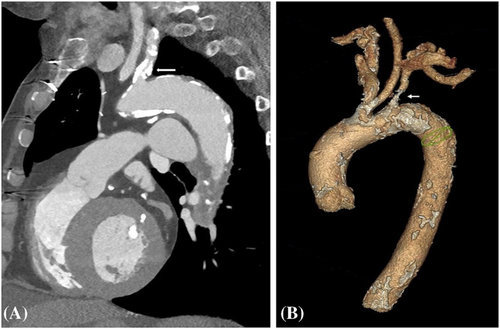
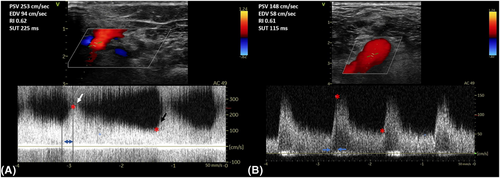
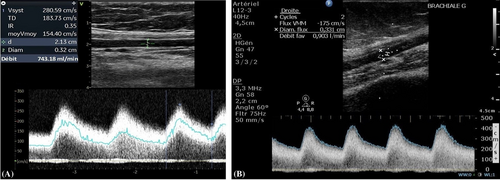
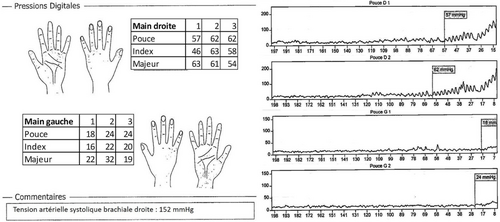
A 79-year-old right-handed woman was hospitalized in the cardiac intensive care unit after experiencing chest pain at rest for 5 days. The body mass index was 37.6 kg/m2 (weight: 94 kg), the systolic blood pressure (SBP) was 136 mmHg, the heart rate was 84 b.p.m., and the temperature was 36.5°C. She had no clinical signs of heart failure (New York Heart Association functional class I, Killip stage 1). The medical history included type II diabetes, hypertension, dyslipidaemia, grade 2 obesity, end-stage renal disease, low-grade left internal carotid stenosis, and moderate lower-limb peripheral artery disease. Additionally, a CABG surgery consisting of a pedicle left ITA to the left interventricular artery, a right ITA to the right coronary artery, and a long saphenous vein to the circumflex artery had been performed 6 years earlier, for which the pre-operative DUS revealed only a low-grade left internal carotid artery stenosis. Besides, a left-arm brachiocephalic AVF had been performed in preparation for haemodialysis 1 month prior to referral, and a few days after the AVF was fabricated, intermittent left-hand pain appeared without any physical signs of ischaemia. Three months before the AVF was made, radial pulses were felt and no upper limb arteries' obstruction was found on DUS. On admission, the electrocardiogram showed a horizontal ST depression ≥2 mm in leads DII, aVF, V4, V5, and V6, suggestive of inferior lateral ischaemia (Figure 1). A blood test revealed anaemia (haemoglobin: 10.40 g/dL), a creatinine level of 738 μmol/L, a hyperglycaemia of 1.51 g/L, a hypertriglyceridemia of 3.44 g/L, and normal liver function tests. N-terminal pro-B-type natriuretic peptides were 26 476 ng/L, and troponins were 145 ng/L (normal: <34 ng/L) (Figure 2). Transthoracic echocardiography showed a visually preserved left ventricular ejection fraction (LVEF) of 61% with normal overall and segmental kinetics and found a normal cardiac output. The global longitudinal strain was estimated at −14%. A coronary angiography was prompted and noted a severe triple-vessel native CAD and good circumflex saphenous bypass patency. The selective catheterization of the left and right ITA bypasses was impossible (Figure 3). A computed tomography scanner confirmed the patency of all bypasses and revealed a 50% calcified stenosis of the brachiocephalic artery trunk ostium and a subocclusive proximal LSA stenosis (Figure 4). DUS revealed high velocities and a prolonged systolic upstroke time (SUT) on LSA (Figure 5), while the AVF's flow volume was estimated to be around 740 mL/min (Figure 6). The DPs measured by laser Doppler flowmetry were <60 mmHg at all fingers for a right-arm SBP of 152 mmHg, confirming the haemodialysis access-induced distal ischaemia (HAIDI)2 (Figure 7). To avoid AVF compression, no left-arm SBP measurements were taken. Given the NSTEMI, the high-grade LSA stenosis, the recent creation of an AVF, and concomitant HAIDI, CSSS was diagnosed, so a percutaneous endovascular intervention in the LSA was prompted. After retrograde puncture of the right common femoral artery, a 65-cm-long 7F introducer was inserted, and the LSA was catheterized. A pre-dilatation of the LSA stenosis with an Armada balloon (7 × 20 mm) was performed, followed by a covered stent's implantation (Advanta V12, 8 × 38 mm) (Figure 3). The post-procedure angiographic result was optimal. In the following days, the chest pains subsided, the ST-segment depression on the electrocardiogram regressed (Figure 1), troponin levels dramatically decreased (Figure 2), the left-hand pain ceased, the SUT was shortened on the post-angioplasty DUS (Figure 5), and the post-interventional AVF's flow volume was estimated to be approximately 900 mL/min (Figure 6). Any signs of stress or rest angina were reported at the 1 month, 3 month, and 6 month follow-up consultations. Haemodialysis sessions were carried out three times a week using the left-arm brachiocephalic AVF.
Discussion
Whereas it is reasonable to assume that the LSA's lesion was present at the time of AVF creation, radial pulses were felt, and there was no evidence of LSA stenosis on DUS 3 months prior. The frequent asymptomatic nature of subclavian artery stenosis,1 the lack of a search for an inter-arm blood pressure difference prior to the AVF's creation, the difficulty in visualizing the origin of the retroclavicular LSA on DUS, and the frequent persistence of the triphasic waveform downstream of a high-grade subclavian artery stenosis3 may explain why it might have gone undetected in our patient. Moreover, 3 months had elapsed between the DUS and the AVF creation, giving the stenosis a chance to worsen. An AVF ipsilateral to an ITA graft in CABG patients may cause a life-threatening myocardial blood steal by creating a pressure gradient and diverting flow from the left ITA graft to the LSA.1, 4 However, in a recent retrospective cohort study of 111 patients with a history of CABG and an ITA graft who had benefited from the creation of an AVF within the previous 6 weeks, patients with an ipsilateral ITA graft did not have a significantly higher risk of major adverse cardiac events or late mortality.5 Low LVEF was found to be related with the occurrence of adverse cardiac events in a retrospective study of 25 patients in whom three cases of myocardial infarction occurred following AVF homolateral to a CABG graft.6 Among the few reported clinical observations of symptomatic CSSS caused by a left ITA graft, the recent creation of an AVF ipsilateral to the graft, and the presence of concomitant LSA stenosis,7-15 the clinical presentation was NSTEMI in two cases,14, 15 unstable angina in five cases,7, 9-11, 13 and pulmonary oedema in one case.8 A stress scintigraphy during a check-up enabled the diagnosis in one observation.12 Symptoms appeared during haemodialysis sessions in three cases,8, 9, 13 while they appeared after the AVF in four cases and were felt at rest or during exertion.7, 10, 11, 15 While in our report, DPs <60 mmHg and digital-brachial indexes (ratio of DPs to contralateral brachial SBP) <0.40 with compatible symptoms allowed the diagnosis of HAIDI2 and supported the steal phenomenon theory, symptoms suggestive of HAIDI had previously been reported only once and resolved when LSA patency was restored, but no objective haemodynamic measurements were performed at that time.9 In the case of symptomatic CSSS resulting from the concomitant presence of a LSA stenosis and a homolateral AVF, percutaneous endovascular intervention to raise the subclavian LSA obstruction is the first-line treatment for the NSTEMI, but also the HAIDI, and the AVF dysfunction.2, 16 In one observation, despite restoration of the LSA's patency, symptoms persisted and the AVF was ligated.11 In our patient, LSA angioplasty and stenting resulted in resolution of symptoms and preservation of the AVF.
CSSS is a diagnostic challenge that necessitates a thorough and multidisciplinary approach. DP measurements can be used to identify a concomitant HAIDI, hence supporting the steal phenomenon hypothesis. Prior to the creation of an AVF in a patient with CABG, an inter-arm blood pressure asymmetry should be investigated. Given the high prevalence of subclavian artery stenosis in this population and the possibility of percutaneous intervention, a subclavian artery stenosis ipsilateral to the graft must be considered in the case of myocardial infarction following the AVF's creation. When a CABG patient requires vascular access for haemodialysis, the contralateral upper limb should be preferred to maintain myocardial protection if CSSS risk factors exist (need for a proximal AVF, pre-operative low LVEF).
Conflict of interest
None declared.




