The impact of heart failure therapy in patients with mildly reduced ejection fraction: a network meta-analysis
The study was conducted at the Cardiology Department, Centro Hospitalar Vila Nova de Gaia/Espinho, Vila Nova de Gaia, Portugal.
Abstract
Background
Recent heart failure (HF) guidelines have re-classified HF patients with left ventricular ejection fraction (LVEF) between 41% and 49% as HF with mildly reduced ejection fraction (HFmrEF). HFmrEF treatment is often considered a grey zone as no randomized controlled trials (RCTs) were conducted exclusively on these patients.
Aims
A network meta-analysis (NMA) was performed to compare treatment effect of mineralocorticoid receptor antagonists (MRA), angiotensin receptor neprilysin inhibitor (ARNi), angiotensin receptor blockers (ARB), angiotensin-converting-enzyme inhibitors (ACEi), sodium-glucose cotransporter-2 inhibitors (SGLT2i), and beta-blockers (BB) in HFmrEF cardiovascular (CV) outcomes.
Methods and results
RCTs sub-analyses evaluating the efficacy of pharmacological treatment in HFmrEF patients were searched. Hazard ratios (HRs) and their variance were extracted from each RCT for (i) composite of CV death or HF hospitalizations, (ii) CV death, and (iii) HF hospitalizations. A random-effects NMA was performed to compare and assess the treatment efficiency. Six RCTs with subgroup analysis according to participants' ejection fraction, a patient-level pooled meta-analysis of two RCTs, and an individual patient-level analysis of eleven BB RCTs were included, totalling 7966 patients. To our primary endpoint, SGLT2i vs. placebo was the only comparison with significant results, with a 19% risk reduction in the composite of CV death or HF hospitalizations [HR 0.81, 95% confidence interval (CI) 0.67–0.98]. In HF hospitalizations, the impact of the pharmacological therapies was more notorious, and ARNi reduced in 40% the risk of HF hospitalizations (HR 0.60, 95% CI 0.39–0.92), SGLT2i in 26% (HR 0.74, 95% CI 0.59–0.93) and renin–angiotensin system inhibition (RASi) with ARB and ACEi in 28% (HR 0.72, 95% CI 0.53–0.98). Although BBs were globally less beneficial, they were the only class that supported a reduced risk of CV death (HR vs. placebo: 0.48, 95% CI 0.24–0.95). We did not observe a statistically significant difference in any comparison between active treatments. There was a sound reduction with ARNi on the primary endpoint (HR vs. BB: 0.81, 95% CI 0.47–1.41; HR vs. MRA 0.94, 95% CI 0.53–1.66) and on HF hospitalizations (HR vs. RASi 0.83, 95% CI 0.62–1.11; HR vs. SGLT2i 0.80, 95% CI 0.50–1.30).
Conclusions
In addition to SGLT2i, pharmacological treatment recommended for HF with reduced LVEF, namely, ARNi, MRA, and BB, can also be effective in HFmrEF. This NMA did not show significant superiority over any pharmacological class.
Introduction
Heart failure with mildly reduced ejection fraction (HFmrEF) is the new definition for heart failure (HF) with left ventricular ejection fraction (LVEF) between 41% and 49% in the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic HF.1
Several phenotypic characteristics can divide HF patients, namely, HF's aetiology, LVEF, specific types of cardiomyopathies or structural heart disease, or response to medical therapy.2 LVEF is the most frequent measure used to stratify HF, guide clinical practice and a frequent inclusion criterion in randomized control trials (RCTs).3 Routine assessment of LVEF in HF patients is important for prognostic value and to diagnose conditions warranting disease-specific therapy.3, 4
For a long time, HFmrEF was considered an intermediary stage in HF spectrum.5 This subgroup of HF is deeply heterogeneous and can include patients with recovered ejection fraction, patients with new-onset HFmrEF, or patients with declined LVEF.6, 7 In HF cohorts, 20–25% of the patients have LVEF between 41% and 49%, and HFmrEF is starting to be represented as a separate entity.8, 9
The recent definition of HFmrEF reinforces its similarity to HFrEF, not only in HF's aetiology (more likely to have coronary artery disease as primary cause) but also in pathophysiology mechanisms.10 A published analysis of the TIME-CHF trial comprising a population of 17% patients with established HFmrEF showed high N terminal pro brain natriuretic peptide (NT-proBNP) levels, serum creatinine, and troponin T in this subpopulation, similar to HFrEF patients.11 When looking at some cardiovascular (CV) risk factors, like the presence of atrial fibrillation, hypertension, and BMI, the incidence rates are not consensual between patient cohorts and seem to be in-between HFrEF and HF with preserved ejection fraction (HFpEF).6, 10, 11
More important than clinical resemblance with HFrEF is the potential similar response to pharmacological therapy. The benefit of HFrEF therapy is greater at the lower end of the LVEF spectrum, where the risk of CV events is higher and there is a greater neurohumoral activation.10, 12, 13 Although HF with improved EF (HFimpEF) can fit in HFmrEF definition, it can also be described as a subgroup of HF with reduced ejection fraction (HFrEF) given the importance of maintaining medical therapy in these patients.14 Nevertheless, NT-proBNP-guided therapy was proved to be beneficial in survival free of HF hospitalizations in HFmrEF patients, suggesting that HFmrEF is thought to be categorized and treated as HFrEF.11
The question arises if pharmacological treatment can improve CV outcomes in HFmrEF patients and which class has a greater effect.
Network meta-analysis (NMA) is a statistical technique that compares several interventions or treatments simultaneously in a single analysis by combining direct and indirect evidence across a network of RCTs studies.15
Our goal was to compare different pharmacological therapies' effects in patients with HF and LVEF between 40% and 49% (HFmrEF) using a NMA.
Methods
This NMA was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses for Network Meta-Analyses (PRISMA-NMA) recommendations.16
Eligibility criteria and search
We searched for RCTs, RCT's subgroup analyses, and RCTs meta-analysis that evaluated the efficacy of pharmacological treatment in patients with chronic HF and provided patients' data and major CV outcomes according to LVEF subgroups, specifically in LVEF between 40% and 49% (HFmrEF patients).
Studies that randomized patients for disease-modifying drugs indicated in patients with HFrEF such as mineralocorticoid receptor antagonists (MRA), angiotensin II receptor–neprilysin inhibitor (ARNi), angiotensin receptor blockers (ARB), angiotensin-converting-enzyme inhibitors (ACEi), sodium-glucose cotransporter-2 inhibitors (SGLT2i), and beta-blockers (BB), were included.
We excluded studies that selected patients exclusively for a specific HF aetiology, for medical comorbidities (viz. history of coronary disease, acute HF, or chronic kidney disease), or for old age.
Neither language nor year of publication was used as restriction/eligibility criteria.
A literature review was conducted in MEDLINE database through PubMed and clinicaltrials.gov for ongoing trials on treatments for HFmEF, in December 2021. Search terms were [heart failure] AND [mid-range ejection fraction OR mildly reduced ejection fraction OR ejection fraction spectrum] AND [treatment].
Records identified in each database were imported and managed through Microsoft Office Excel. Duplicates were automatically identified and manually confirmed. One single reviewer (M. L.) screened the titles and abstracts of all identified citations for checking eligibility. In addition, full texts of potentially eligible studies were evaluated by the same reviewer. Figure 1 represents the flow process chart of the studies' selection.
Data extraction and data items
One reviewer (M. L.) extracted data using a standardized form in Microsoft Office Excel. Clinical, and methodological data were gathered using data from texts, tables, figures, or supplemental material. For clinical outcomes, treatment effect and variance estimates, within HFmrEF subgroups, were collected [i.e. hazard ratio (HR) and 95% confidence intervals (CIs)].
Study period, country/region, number of participants, dosage, concomitant therapy, and clinical characterization, including age, sex, CV risk factors, and New York Heart Association (NYHA) classification class, were systematically collected.
Target population was HF patients as close as possible of the 40–49% LVEF interval. The most discrepant LVEF was in EMPEROR-subanalysis17 where patients with LVEF between 45% and 54% were more similar to the population in analysis, so we choose to collect data from this subgroup of HF patients.
Apart from BB where we only extracted data from patients in sinus rhythm, in general, we extracted global patients' data and results. In particular, TOPCAT18 data were not extracted by region. ARB and ACEi were lumped in the same pharmacological class in our NMA—renin–angiotensin system inhibitors (RASi).
The three main reported outcomes in the included RCTs, that is, (i) composite of CV death or HF hospitalization, (ii) CV death, and (iii) HF hospitalization, were the collected outcomes from each trial.
Transitivity, risk of bias, and quality assessment
Patients and study design features were analysed to check for transitivity. Mean age, sex distribution, HF aetiology, concomitant therapy, and CV comorbidities were pre-specified as relevant factors that could potentially affect the transitivity assumption.
The Cochrane Collaboration's tool assessed the risk of bias (low, medium, or high risk of bias).19
The GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) tool was used to access the quality of evidence of the studies selected (high, moderate, low, or very low).20
Network meta-analysis
Using R netmeta package (version 6.1-0), a random-effects NMA was performed. Random and fixed effects results were equal. HR and 95% CIs for indirect and direct comparisons between pharmacological treatments were calculated and represented in a ranking plot for each outcome. We deemed statistical significance at P < 0.05.
We intended to create and analyse indirect comparisons between pharmacological classes using direct comparisons tested in RCTs. Placebo was used as the comparator arm in five subanalyses.
The degree of inconsistency between indirect and direct comparison was not evaluated because there is only one direct comparison between pharmacological classes in our analysis (ARNi vs. RASi).
Statistical heterogeneity between trials was assessed using I2 statistic.21
Forest plots for the three analysed outcomes and network graphs that compare the six interventions (including placebo) were obtained, and they illustrate HRs and 95% CIs for direct and indirect comparisons.
Results
Study selection
Figure 1 displays the process of study selection.
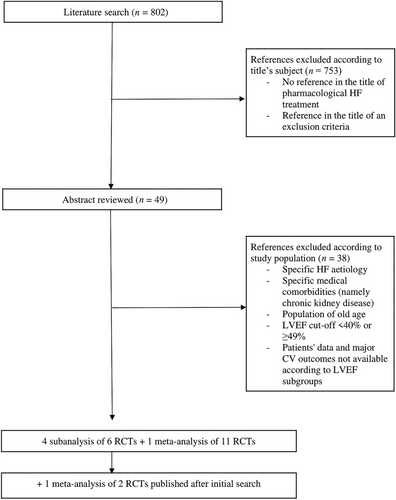
A total of 802 studies emerged from the literature search. According to the title, we excluded studies that did not directly evaluate the effect of pharmacological treatment in patients with HF on main CV outcomes. There were no RCTs conducted exclusively in HFmrEF patients. We selected those where the LVEF cut-off allowed the inclusion of patients with LVEF between 40% and 49% through screening the abstracts.
Then, we manually searched and found 4 subgroup analyses of RCTs with results separated accordingly to patients' LVEF—TOPCAT subanalysis18 for spironolactone (MRA); CHARM subanalysis22 for candesartan (RASi); PARADIGM-HF and PARAGON-HF combined subanalysis23 for sacubitril/valsartan (ARNi), enalapril and valsartan (RASi); EMPEROR-Preserved and EMPEROR-Reduced combined subanalysis17 for empagliflozin (SGLT2i); and one individual patient data meta-analysis of 11 BB RCTs, BB-meta-HF.24 In August of 2022, after our search was conducted, the publication of DELIVER trial25 and concomitant meta-analysis of DAPA-HF and DELIVER26 allowed the inclusion of dapagliflozin results to SGLT2i class in our NMA.
In BB-meta-HF24 an individual patient data meta-analysis was performed using 11 RCTs on BB in patients with chronic HF. As this study reported the pooled results from those trials across all LVEF ranges (not available for the individual published trials), we entered this information. In addition, data from sacubitril/valsartan, empagliflozin, and dapagliflozin were obtained from a pooled analysis that combined results of both preserved and reduced LVEF RCTs.17, 23, 26
A total of 7966 patients were included. The baseline characteristics of HFmrEF patients and the event rates are summarized in Tables 1 and 2. The data were harvested from the subanalysis and meta-analysis selected, and it is a description of the patients included. Transitivity can be clinically assumed because there are similar baseline characteristics and potential treatment effect modifiers.
| TOPCAT | CHARM-Preserved | BB-meta-HF | PARADIGM/PARAGON | EMPEROR | DAPA-HF/DELIVER | |
|---|---|---|---|---|---|---|
| Intervention | Spironolactone | Candesartan | Beta-blockers | LCZ696 | Empagliflozin | Dapagliflozin |
| Sacubitril/valsartan | ||||||
| Dose, mg/day | 15–45 | 32 | 100–200 | 10 | 10 | |
| 97/103 | ||||||
| n | 520 | 1322 | 575 | 1427 | 2260 | 1862 |
| LVEF, % | 45–50 | 40–49 | 40–49 | 42.5–52.5 | 45–54 | 44–51 |
| Age, years | 66±9 | 65±11 | 71 (61–75) | 71±9 | 71±9 | 70±10 |
| Women, n (%) | 190 (36.4) | 198 (34.4) | 564 (40) | 876 (39) | 667 (35.8) | |
| Black race, n (%) | 38 (7.3) | 43 (3.3) | 24 (2) | 101 (4) | 42 (2.3) | |
| BMI, kg/m2 | 31.5±7.2 | 27.8 (25.0–31.2) | 27 (25–30) | 30±4.9 | 29.8±5.7 | 30±6 |
| SBP, mmHg | 128±14 | 130 (120–145) | 131 (120–14) | 131±15 | 132±16 | 128±15 |
| Heart Rate, bpm | 69.98±10.17 | 76 (68–82) | 72±12 | 71±12 | 72±12 | |
| Diabetes Mellitus, n (%) | 149 (28.7) | 378 (28.6) | 135 (24.1) | 627 (44) | 1165 (52) | 844 (45.3) |
| Hypertension, n (%) | 450 (86.5) | 743 (56.2) | 1341 (94) | 1646 (88.4) | ||
| eGFR, mL/min/1.78 m2 | 69.6±19.9 | 66 (53–78) | 65 (20) | 62 (20) | 62±19 | |
| Creatinine, mg/dL | 1.16 ±0.43 | 103±31 | ||||
| History of HF hospitalization, n (%) | 374 (71.9) | 733 (51) | 540 (24) | 835 (44.8) | ||
| History of MI, n (%) | 230 (44.2) | 761 (57.6) | 412 (7.8) | 458 (32) | 635 (34.1) | |
| Ischaemic HF aetiology, n (%) | 885 (66.9) | 522 (90.8) | 980 (43) | |||
| Follow-up time, months | 40 | 33 | 17 | 27–35 | 21 | 22 (17–30) |
| Concomitant medication, n (%) | ||||||
| ACEi/ARB | 458 (88.1) | 359 (27.2) | 508 (90.6) | 1257 (88) | 1895 (84) | 1381 (74.2) |
| MRA | 151 (11.4) | 31 (5.8) | 430 (30) | 922 (41) | 853 (45.8) | |
| Beta-blockers | 407 (78.3) | 763 (57.7) | 1175 (82) | 2009 (89) | 1617 (86.8) | |
| Diuretics | 396 (76.2) | 984 (74.4) | 376 (65.4) | 1359 (95) | 1800 (80) | 1645 (88.3) |
- ACEi, angiotensin-converting enzyme inhibitors; ARB, angiotensin receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate; HF, heart failure; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MRA, mineralocorticoid receptor antagonists; SBP, systolic blood pressure.
- Values are n, number of patients; % percentage; median and interquartile range or average and standard deviation.
| TOPCAT | CHARM-Preserved | BB-meta-HF | Paradigm/PARAGON | EMPEROR | DAPA-HF/DELIVER | |
|---|---|---|---|---|---|---|
| Event rate per 100 patient-years median and interquartile range | number of events/number of patients (%) | |||||
| CV death + HF hospitalizations | 7.2 (6.0–8.7) | 305/1322 (23.1) | 164/566 (28.9) | 365/1427 (25.6) | 374/2260 (16.5) | 368/1862 (19.7) |
| HF hospitalizations | 3.8 (2.9–5.0) | 216/1322 (16.3) | 144/566 (25.4) | 262/1427 (18.4) | 201/2260 (8.9) | 238/1862 (12.7) |
| CV death | 4.1 (3.2–5.2) | 167/1322 (12.6) | 38/570 (6.7) | 163/1427 (11.4) | 229/2260 (10.1) | 205/1862 (11.0) |
- CV, cardiovascular; HF, heart failure.
Risk of bias, quality of evidence, and statistical heterogeneity
See Supporting information Table S1 for the risk of bias and quality of evidence of the RCTs included in this NMA.
The result of I2 statistic test, used to assess heterogeneity, was 0%.
- (i) Composite of cardiovascular death or heart failure hospitalizations
The main outcome analysed in HF RCTs and in our NMA was a composite of CV death and HF hospitalizations.
Compared with placebo, all pharmacological options had better results, but only SGLT2i reduced significantly in 19% the risk of CV death or HF hospitalization (HR 0.81, 95% CI 0.67–0.98), as shown in Figure 2.
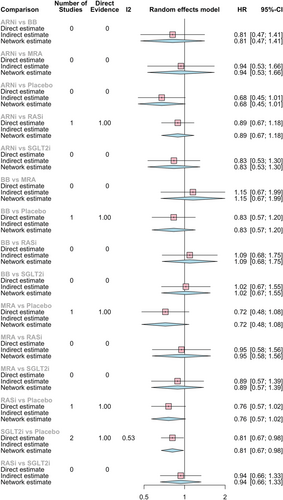
The most consistent reduction of the composite endpoint when compared with all the other pharmacological classes was observed with ARNi, although with no significant results (HR vs. BB: 0.81, 95% CI 0.47–1.41; HR vs. SGLT2i 0.83, 95% CI 0.53–1.30; HR vs. MRA 0.94, 95% CI 0.53–1.66). And, as expected from direct comparison, ARNi had a larger risk reduction than ACEi/ARB (HR vs. RASi 0.89, 95% CI 0.67–1.18).
MRA had the second larger absolute risk reduction (HR vs. BB: 0.86, 95% CI 0.54–1.38; HR vs. SGLT2i 0.89, 95% CI 0.57–1.39; HR vs. RASi 0.95, 95% CI 0.58–1.56).
None of these drug-to-drug comparisons had statistically significant results; there is only a trend towards the superiority of ARNi, MRA, and SGLT2i.
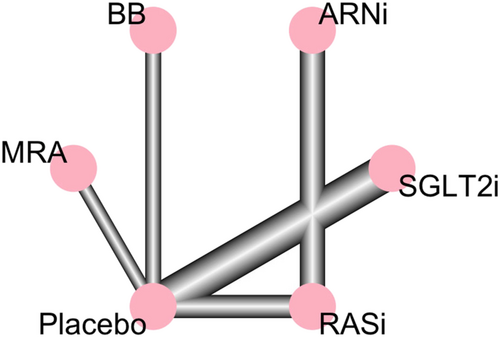
Network graph 1 represents the relation between pharmacological classes in our primary endpoint.
-
(ii) Heart failure hospitalizations
The results for HF hospitalizations endpoint are depicted in Figure 3 and Network graph 2.
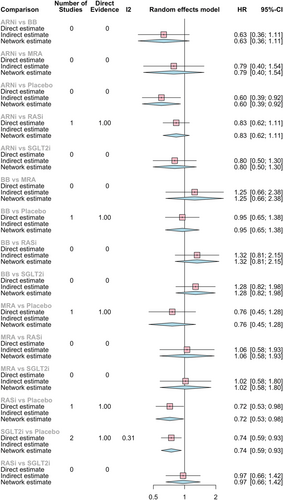
All pharmacological classes seemed to reduce HF hospitalization risk with significant results for ARNi reducing in 40% the risk of HF hospitalizations (HR 0.60, 95% CI 0.39–0.92), SGLT2i in 26% (HR 0.74, 95% CI 0.59–0.93), and ACEi/ARB in 28% (HR 0.72, 95% CI 0.53–0.98) vs. placebo.
In HF hospitalizations, like in the primary endpoint, ARNi appeared to have greater risk reduction effect compared with the other pharmacological classes (HR vs. BB: 0.63, 95% CI 0.36–1.11; HR vs. MRA: 0.79, 95% CI 0.40–1.54; HR vs. RASi 0.83, 95% CI 0.62–1.11; HR vs. SGLT2i 0.80, 95% CI 0.50–1.30).
-
(iii) Cardiovascular death
The results for CV death endpoint are represented in Figure 4 and Network graph 3.
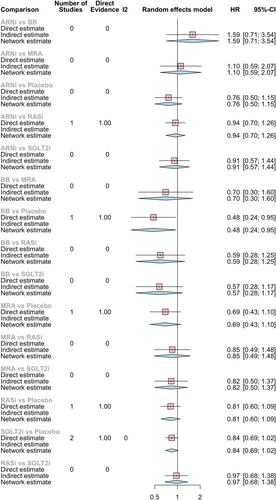
Most of the results for CV death were not statistically significant. Only BB reduced significantly the risk of CV death in 52% compared with placebo (HR 0.48, 95% CI 0.24–0.95). There are also results favouring BB in indirect comparisons (HR vs. ARNi 0.62, 95% CI 0.28–1.4; HR vs. SGLT2i 0.57, 95% CI 0.28–1.17; HR vs. MRA 0.70, 95% CI 0.30–1.60; HR vs. RASi 0.59, 95% CI 0.28–1.25).
MRA seemed to also have a superior effect in reducing CV death vs. placebo (HR 0.69, 95% CI 0.43–1.10), RASi (HR 0.85, 95% CI 0.49–1.48), ARNi (HR 0.90, 95% CI 0.48–1.69), and SGLT2i (HR 0.82, 95% CI 0.50–1.37).
Discussion
The positive effect of prognosis modifying therapy for HFpEF treatment was first observed with ARB in CHARM Study.27 CHARM analysed the effect of candesartan in patients with HF and LVEF > 40% using a separate arm from a larger cohort of HF patients across the LVEF spectrum. A post-hoc analysis emerged 14 years later, studying the interaction between LVEF and candesartan treatment effects.22 Candesartan significantly reduced the composite endpoint of CV death or HF hospitalization (HR 0.76, 95% CI 0.61–0.96; P = 0.02), first HF hospitalization (HR 0.72, 95% CI 0.55–0.95; P = 0.02), and HF hospitalization recurrence (HR 0.48, 95% CI 0.33–0.70; P < 0.001) in HFmrEF subgroup. This was not observed for CV and all-cause mortality.22
TOPCAT28 was designed to study the effect of spironolactone in HFpEF patients and included a reduced number of patients with LVEF between 45% and 50% (520 in 3444 patients). Even with no significant results in the LVEF subgroup analysis, there was a tendency for different treatment effects (for HF hospitalizations, with an interaction P value of 0.039), underlining the presumed relevance of the HFmrEF group and the potential benefit of spironolactone in these patients.18
The PEP-CHF29 studied the effects of perindopril in HF with LVEF >40%. The results were neutral, and the population selected was predominantly old. Also, I-PRESERVE30 was designed to evaluate the effect of irbesartan in HF patients with LVEF of at least 45%. To the best of our knowledge, these two RCTs do not have subgroup analyses according to LVEF published.
The use of BB showed a significant reduction in CV death for HF patients with LVEF between 40% and 49% in sinus rhythm (HR 0.48, 95% CI 0.24–0.97; P = 0.04). No significant results were observed for CV hospitalization (HR 0.95, 95% CI 0.68–1.32; P = 0.76) or composite endpoint CV death or hospitalization (HR 0.83, 95% CI 0.60–1.13; P = 0.23).24
PARADIGM-HF31 and PARAGON-HF32 trials were designed to compare ARNi - sacubitril/valsartan - with inhibitors of the renin–angiotensin system. When merging the two RCTs in one subgroup analysis, the composite endpoint of first HF hospitalization or CV death and first HF hospitalization were significantly lower with sacubitril/valsartan to an LVEF as high as 42.5% (HR 0.81, 95% CI 0.69–0.94; P = 0.005; and HR 0.77, 95% CI 0.63–0.94; P = 0.011, respectively). Regarding higher values of LVEF, the comparison was not statistically significant but showed a trend for better results with sacubitril/valsartan up to 62.5% of LVEF.23
Even so, only with EMPEROR-Preserved33 we begin to look at HF with LVEF ≥40% differently. Until recently, there was a lack of evidence-based pharmacological therapy in HFmrEF and HFpEF. SGLT2i was the first treatment that proved to be successful in reducing CV endpoints in HF with LVEF ≥40%. In EMPEROR-Preserved, the effects were attenuated at higher ejection fraction subgroups (LVEF >65%) but evident in HFmrEF subgroups, with significant and consistent risk reduction of the combine endpoint of HF hospitalizations or CV death.33, 34
In DELIVER trial, the effects of dapagliflozin among patients with HFmrEF and HFpEF resulted in a lower risk of the primary composite outcome (worsening HF or CV death), in fewer worsening HF events and CV deaths, and in a lower symptom burden, with no excess of adverse events.25 In contrast with EMPEROR-Preserved, there was no evidence that the effect of dapagliflozin differed according to LVEF pre-specified subgroups.26
The pharmacological treatment aforementioned and recommended in HFrEF may be considered in HFmrEF patients according to 2021 ESC HF guidelines.1 More recently published, AHA/ACC/HFSA HF guidelines recommend SGLT2i as Class IIA, Level B, for the treatment of HFmrEF and HFpEF.14
HFmrEF treatment is regarded as a grey zone as there are no RCTs conducted exclusively in these patients.5 The potential prognostic benefit of pharmacological treatment in HFmrEF is generally highlighted in observational studies considering that, in clinical practice, HFmrEF patients are frequently medicated with HF therapy due to concomitant indications, like medical comorbidities.7
For SGLT2i we already have RCTs proving its benefits in HF with LVEF ≥40%.25, 33 Our results support this knowledge with a significant risk reduction of 19% in the composite endpoint of CV death or HF hospitalization and 26% in HF hospitalization. SGLT2i appear to have direct cardioprotective and nephroprotective effects, which might be related to glycosuria, osmotic diuresis and natriuresis, energy homoeostasis, and mitigation of cellular stress, and decreased arterial stiffness.35
Besides SGLT2i, our results suggest that other pharmacological agents frequently used in HFrEF patients can also be effective in HFmrEF as they are superior to placebo in the composite endpoint although the results were not significant.
In HF hospitalizations endpoint, ARNi and RASi had a significant risk reduction compared with placebo, ARNi with 40% reduction, and 28% with ARB/ACEi. These results suggest that neurohumoral blockage has an important role in reducing CV events and hospitalizations in HFmrEF patients.
The results for CV death endpoint seem to be more neutral, and there is not yet a clear benefit of pharmacological therapy in reducing CV death in HFmrEF patients. Although BB were globally less beneficial in the composite endpoint and CV death, they seemed to be the only pharmacological class with some effect in preventing CV death. These results are probably in line with its effect in preventing ventricular tachycardia and ventricular fibrillation in ischaemic cardiomyopathy.24, 36 However, the large confidence interval gives a low magnitude to this effect.
Our NMA had with major goal to study which pharmacological classes were superior in HFmrEF patients. We did not observe a statistically significant difference in any comparisons between treatments (indirect comparisons), which suggests a similar magnitude of effect. Nevertheless, there was a sound reduction with ARNi concordant with its superior effect demonstrated in direct comparisons with RASi.23 The combined neprilysin inhibition and renin–angiotensin–aldosterone system blockade give this pharmacological class an already proven superior effect.
Limitations
Poorly connected networks depend extensively on indirect comparisons. Meta-analyses of such networks may be less reliable than those from networks where most treatments have been compared against each other.37 There are few direct comparisons between pharmacological classes in HF trials, and that probably would not be advantageous in patients' treatment. The goal is to add benefit with a new mechanism of action.
The main concomitant therapies are described in Table 1. Some of them were pharmacological classes tested in this NMA. They were similarly represented in the intervention and placebo groups, and they did not generate significant treatment effect modification. With the exception of PARADIGM/PARAGON,23 pharmacological interventions were compared with placebo and standard of care. Although there was not a standardized placebo group, we were allowed to use it as the common comparator.
Our goal was to compare the available pharmacological treatment in HF from a pharmacological class perspective. However, data from HFmrEF patients were scarce, and there are only one to two drugs from each class in these NMA. Nevertheless, our network diagrams exposed therapeutic options from a class perspective.
We included RCTs of all referred guideline pharmacological treatment for HFmrEF except ACEi because no results were published according to LVEF for ACEi RCTs. Therefore, enalapril (ACEi), candesartan, and valsartan (ARB) were included in the same group—RASi—to include data of PARADIGM/PARAGON subanalysis23 and with the assumption of similar treatment effects.
Heterogeneity between studies was examined and was found to be not significant. However, there is an inter-observer variability in the assessment of LVEF, and methods of LVEF estimation vary between RCTs.4 In addition, LVEF is not an objective variable and can change over time, like in response to therapy.
The benefit of BB in patients with HF and atrial fibrillation remains a controversial subject, and we chose to extract data from HF patients only in sinus rhythm.
Patients' region is a well-known modifier effect in the TOPCAT trial28 with more patients from Russia and the Republic of Georgia with LVEF <50% (62% patients from Russia and the Republic of Georgia vs. 38% from the Americas), although we did not make this distinction to our NMA.
All-cause death was also a frequent outcome but not present in all selected studies (like PARADIGM/PARAGON23 and EMPEROR17 subanalysis) and not included in our NMA.
One of the major limitations of our NMA is the small number of RCTs conducted to answer our clinical question. Our NMA was based on post-hoc subgroup analysis of HFpEF and HFrEF RCTs. HFmrEF patients are poorly represented in clinical research, with no exclusive RCTs and few pre-specified group results according to LVEF.
Conclusions
A trend to better results with ARNi, SGLT2i, and RASi in the composite endpoint CV death or HF hospitalizations and in HF hospitalizations, and with BB in CV death, was observed. However, overall there was not a significantly superior pharmacological class in HFmrEF treatment.
Evidence of new and effective therapies in HFmrEF is limited but rising. Our results are concordant with recent guideline recommendations for HFmrEF.
Funding
This work was supported by (i) national funds through FCT Fundação para a Ciência e Tecnologia, I.P., under the scope of the Cardiovascular R&D Center—UnIC (UIDB/00051/2020 and UIDP/00051/2020) and the (ii) European Regional Development Fund (ERDF), through the North Regional Operational Program in the framework of the project HEALTH-UNORTE: Setting-up biobanks and regenerative medicine strategies to boost research in cardiovascular, musculoskeletal, neurological, oncological, immunological and infectious diseases (NORTE-01-0145-FEDER-000039).




