Churg–Strauss syndrome-associated heart failure and left ventricular thrombosis
Abstract
We present a case of a 47-year-old woman with a history of asthma and mononeuritis who presented with shortness of breath and fatigue. Heart failure was diagnosed and echocardiography revealed large floating thrombi attached to the left ventricular walls. Cardiac magnetic resonance imaging showed evidence of myocarditis and angiitis. Blood count revealed eosinophilia. She was diagnosed with eosinophilic granulomatosis with polyangiitis or Churg–Strauss syndrome (CSS) according to recently updated criteria. Medical management with specific aetiology (anticoagulation or immunosuppression) and heart failure treatment resulted in clinical improvement. We further discuss the diagnostic approach of CSS with cardiovascular complications and therapeutic management.
Introduction
Churg–Strauss syndrome (CSS), recently renamed eosinophilic granulomatosis with polyangiitis (EGPA), is defined as necrotizing granulomatous inflammation with eosinophil infiltration and necrotizing vasculitis that affects small-to-medium-sized vessels. It was first described by Churg and Strauss in 1951.1 In a recent meta-analysis, the global pooled prevalence of antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis was 198.0 per million persons whereas EGPA pooled prevalence per million persons was 1.7.2
EGPA is a systemic disease and its diagnosis may be challenging. Clinical evidence of cardiac involvement in EGPA includes heart failure, intracardiac thrombi, myocardial ischaemia, arrhythmias, and pericarditis.3 The presence of heart failure as well as renal, gastrointestinal, and central nervous system involvement and proteinuria (>1 g/day) are risk factors for increased mortality.1
Case report
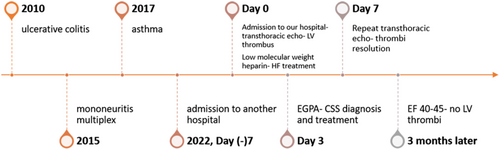
We present a 47-year-old Caucasian woman, height 160 mm, weight 52 kg, who was admitted to our hospital because of fatigue and shortness of breath of several weeks. She had a recent admission in another hospital but was self-discharged because of high level of anxiety.
Her past medical history included ulcerative colitis (on mesalazine) diagnosed 12 years ago, mononeuritis multiplex (right ‘drop foot’) 7 years ago, and asthma (on inhaled corticosteroids) 5 years ago. There were no traditional risk factors for cardiovascular disease.
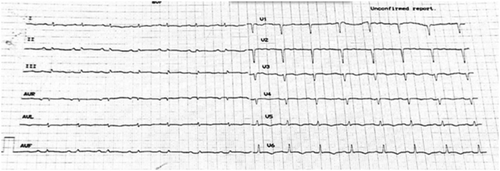
Upon presentation, she was in New York Heart Association (NYHA) class II–III. Vital signs were heart rate of 95 beats/min, blood pressure of 100/70 mmHg, peripheral oxygen saturation of 98%, and normal temperature. On clinical examination, there was no jugular vein distention. Heart sounds were audible with no murmurs or additional sounds. Lung auscultation revealed attenuation of respiratory murmur at both lower lobes. There was nothing noticeable from the abdomen, no peripheral oedema, while she had a right dropped foot. Her electrocardiogram (Figure 1) revealed sinus rhythm, 90/min, and QS in leads V1–V4. No significant arrhythmias were recorded in 24 h telemetry during the hospitalization.
Her basic biochemistry revealed normal renal function and electrolytes, increased aspartate aminotransferase (184 IU/mL) and alanine aminotransferase (137 IU/mL), increased high sensitive troponin I (803 pg/mL), and increased N-terminal natriuretic propeptide (NT-proBNP, 5085 pg/mL). Her white blood count was normal (8.820 K/μL), but there was marked eosinophilia (2140 μL, 24% of total white blood count). C-reactive protein was 7 mg/L.
She underwent transthoracic echocardiography (Figure 2), which showed normal left ventricular (LV) and left atrium dimensions but significantly impaired global LV systolic function with an estimated ejection fraction (EF) of 30%. Right ventricular systolic pressure was estimated at 40 mmHg. There was also a small pericardial effusion. Of note, there were multiple floating thrombi attached to the LV walls, with the larger being 15 mm × 36 mm.
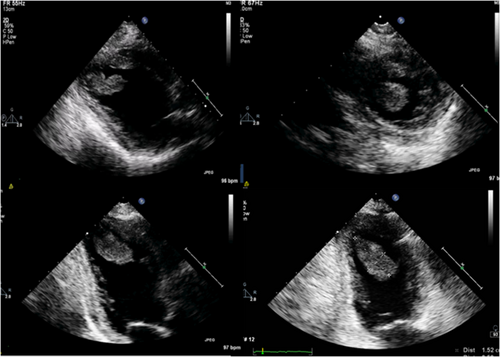
She was immediately started on low-molecular weight heparin according to her weight and anti-Xa factor level was monitored, with an aim of more than 1 IU/mL. She was also started on oral medical treatment for heart failure, namely, metoprolol, eplerenone, empagliflozin, furosemide intravenously, and ramipril 2.5 mg, because her blood pressure was borderline.
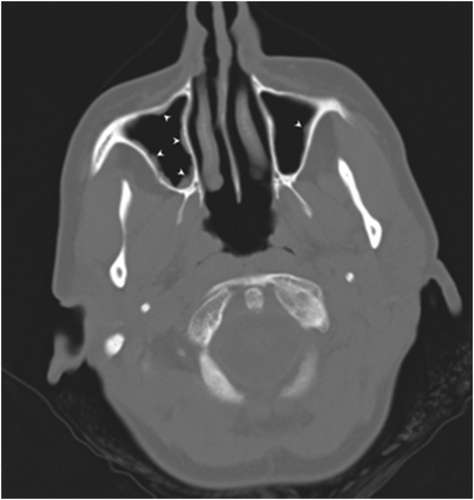
She underwent coronary angiogram, which revealed patent coronary arteries; it was decided not to perform cardiac biopsy as there was a significant risk of haemorrhage due to anticoagulation. The patient underwent daily transcranial doppler embolus detection for up to an hour. No microembolic signals were identified. Brain and head computed tomography (CT) was negative for stroke, but there was thickening of the maxillary sinuses (Figure 3), consistent with EGPA. Chest CT revealed ground glass appearance of the lungs and bilateral pleuritic effusion as well as lung consolidation (Figure 4), whereas abdomen CT was positive for splenic infarcts. Immunological tests, including ANCA-PR3, ANCA-MPO, anti-cyclic citrullinated peptide, rheumatoid factor, and antinuclear antibodies, were negative whereas C3 and C4 were normal. Immunoglobulin E value was increased (188 IU/mL).
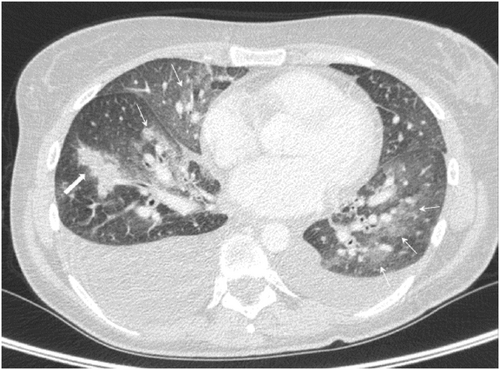
Following rheumatology consultation and the exclusion of other systemic diseases, the diagnosis of EGPA-CSS was made based on eosinophilia, abnormality of the maxillary sinuses, and history of asthma and mononeuritis multiplex. It was decided not to proceed with peripheral tissue biopsy, as she was under anticoagulation and diagnosis was already made according to the new EGPA classification criteria. She was started on high-dose corticosteroids and cyclophosphamide.
On the seventh day post admission, a repeat transthoracic echocardiogram was consistent with thrombi resolution.
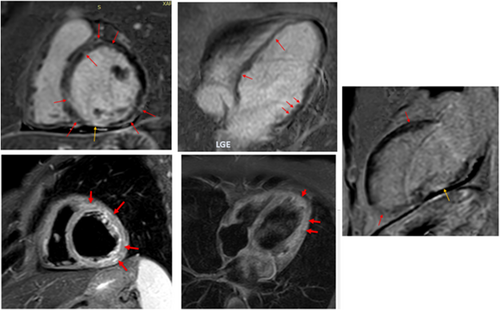
Cardiac magnetic resonance performed on the ninth day post admission (Figure 5) revealed an EF of 33%, with diffuse hypokinesia. Late gadolinium enhancement (LGE) showed midmyocardial and epicardial patterns consistent with myocarditis, coexisting with patchy subendocardial LGE not corresponding to coronary vessel distribution, consistent with angiitis-induced ischaemia, and transmural LGE with thinning of the inferior wall corresponding to ischaemia/scar due to an epicardial branch vessel occlusion, and of the pericardium. It was also demonstrated oedema (on Short TI Inversion Recovery sequences). In addition, the flow in the LV apex was sluggish, with a remaining small thrombus < 5 mm.
There was significant clinical improvement following the aforementioned interventions. Her discharge EF was not significantly changed since admission. Her NYHA class was II before discharge, whereas phychiatric examination was negative for major disorders. She was discharged on daily acenocoumarol, metoprolol 150 mg divided in 3 doses, eplerenone 100 mg, empagliflozin 10 mg, ramipril 2.5 mg, furosemide 20 mg, and tapering corticosteroids along with monthly treatment with intravenous cyclophosphamide. She was treated with budesonide and ipratropium for asthma. On discharge, NT-proBNP was 3311 pg/mL and eosinophil count was 130/μL, 1.5% of total white blood cells. As she was improved on medical treatment, and no arrhythmias were recorded, implantable cardioverter defibrillator insertion was not an option.
Follow-up at 3 months confirmed clinical stability; repeat echocardiogram revealed an EF of 40–45% while there were no LV thrombi. As there was no clear what the coagulation duration should be, in this case that EF is not completely recovered, and the course of EGPA is still unclear, patient will be reassessed in 3 months, with the question of continuing oral anticoagulation.
Discussion
EGPA is a rare multi-system disease that may affect skin, eyes, ear–nose–throat, kidneys, intestine, lungs, nerves, and the heart. Symptomatic cardiovascular involvement occurs in 27–47% of Churg–Strauss cases, being one of the most serious manifestations.4
It is characterized by necrotizing vasculitis with granulomatous inflammation and eosinophils infiltration, predominantly affecting small to medium vessels, in association with asthma and eosinophilia.5 EGPA's clinical phenotypes are usually classified according to ANCA status, as the major eosinophil-driven complications are most frequently found in the ANCA-negative form of EGPA, namely, lung infiltrates, myocardiopathy, and gastrointestinal manifestations. In contrast, ANCA-MPO-positive patients present a more ‘vasculitic phenotype’, which comprises palpable purpura, peripheral neuropathy, rapidly progressive glomerulonephritis, and rarely alveolar haemorrhage.6
In vitro evidence suggests that cardiotoxicity is mainly mediated by eosinophilic cationic protein (ECP) by altering the membrane sodium permeability of cardiomyocytes and inhibiting mitochondrial respiration.6
Typical pathohistological presentations of EGPA are different among the affected organs.1 Cardiac involvement shows a mixture of eosinophilic infiltration of the myocardium and endocardium causing myocarditis and endocarditis, and small-vessel vasculitis; pericarditis may also be present.7 Heart failure may develop as a consequence of the above lesions. The pathogenesis of EGPA remains largely unknown. Like many other autoimmune diseases, both genetic and environmental factors appear to contribute to its development.8
An emerging aspect in eosinophils pathophysiology is their ability to induce a prothrombotic microenvironment on the endothelium, via several mechanisms. This clinically relates to an increased risk of arterial and venous thrombosis, which can be observed in EGPA.6
EGPA diagnosis used to be made based on the 1990 American College of Rheumatology classification criteria for CSS, which include asthma, eosinophilia > 10% of the white blood cells, mono- or poly-neuropathy, pulmonary infiltrates, non-fixed paranasal sinus abnormalities, and extravascular eosinophils6 whereby four of the six criteria were required.9
Since March 2022, new EGPA classification criteria are published. Diagnosis is based on a score that includes clinical and laboratory-biopsy criteria. A score > 7 is required for EGPA classification.10
Differential diagnosis includes other autoimmune diseases, hypereosinophilic syndrome (HES), eosinophilic myocarditis, and Loeffler's endomyocardial fibrosis.2 HES is characterized by eosinophilia (>1500 eosinophils/mm3), absence of a primary cause of eosinophilia (such as parasitic or allergic disease), and evidence of eosinophil-mediated end-organ damage.2 There is sustained production of eosinophils, which impair tissues and organs through infiltration and release of toxins by degranulation. There is significant overlap between HES and EGPA. Eosinophilic myocarditis does not involve other organs and may be preceded by allergic disease,11 whereas Loeffler's endomyocardial fibrosis is the cardiac manifestation of the HES.12
Our patient met diagnostic criteria for EGPA with a score of 9 points (+3 asthma, +5 eosinophilia, and +1 mononeuritis).
Timely diagnosis and treatment of the underlying autoimmune disease, as well as of complications, result to a favourable clinical outcome as in our patient (Figure 6).
In our case, there was almost complete resolution of the gigantic thrombus in 7 days without any major embolism, especially in the brain, resulted from the low-molecular weight heparin regimen and underlines the importance of anti-Xa level close monitoring in high-risk cases, as well as the significance of monitoring for peripheral emboli. As the patient was on intense anticoagulation because of LV thrombi, after extensive discussion with the rheumatologist, it was felt that immediate medical treatment with corticosteroids and cyclophosphamide should be started as she was considered high risk according to current recommendations8 because of life-threatening cardiac involvement. The response to immunosuppressive therapy was satisfactory and the patient improved clinically, and improvement was sustained for 3 months. Heart failure treatment started in admission as per guidelines.
Untreated EGPA carries a significant morbidity and mortality burden. Studies identified higher age at disease onset and heart failure as risk factors for poor prognosis.13 It is of great importance to be aware of rheumatologic disorders that may affect the heart, as timely treatment may prevent mortality or severe permanent myocardial damage. Another question to be answered is in what extent myocardial recovery may occur. It can be assumed that if there is disease remission, under immunosuppressive therapy, the not yet fibrotic LV tissue would be expected to recover under heart failure treatment.
In conclusion, EGPA diagnosis remains challenging and the development of predictive biomarkers would greatly improve the ability to determine the clinical course of the disease.
As stressed by European Society of Cardiology Heart Failure Guidelines recommendations,14 it is important to investigate for specific causes of heart failure as aetiological treatment of the underlying disease is of paramount importance along with guidelines-guided medical treatment for patients' clinical condition and prognosis.
Acknowledgement
We would like to thank Professor Gerasimos Filippatos, Head of Second Cardiology Department, Attikon University Hospital, Athens University, for his input and support.
Conflict of interest
None declared.




