Testosterone deficiency independently predicts mortality in women with HFrEF: insights from the T.O.S.CA. registry
Alberto M. Marra and Roberta D'Assante equally contributed to the work.
Funding information: The work was supported by unrestricted grant from Merck Serono Italy.
Abstract
Aims
Testosterone deficiency (TD) is associated with increased morbidity and mortality in heart failure with reduced ejection fraction (HFrEF). However, data in women are scanty. The aim of this study was to investigate the prognostic impact of TD on women with HFrEF.
Methods
Among 480 patients prospectively enrolled in the T.O.S.CA. (Terapia Ormonale Scompenso CArdiaco) registry, a prospective, multicentre, nationwide, observational study, 94 women were included in the current analysis. The TD was defined as serum testosterone levels lower than 25 ng/dl. Data regarding clinical status, echocardiography, exercise performance, cardiovascular hospitalization, and survival after an average follow-up of 36 months were analysed.
Results
Thirty patients (31.9%) displayed TD. TD was associated with lower tricuspid annular plane excursion (TAPSE) to pulmonary arterial systolic pressure PASP ratio (TAPSE/PASP) (P = 0.008), peak oxygen consumption (VO2 peak) (P = 0.03) and estimated glomerular filtration rate (P < 0.001). TD was an independent predictor of the combined endpoint of all-cause mortality/cardiovascular hospitalization (HR: 10.45; 95% CI: 3.54–17.01; P = 0.001), all-cause mortality (HR: 8.33; 95%: 5.36–15.11; P = 0.039), and cardiovascular hospitalization (HR: 2.41; 95% CI: 1.13–4.50; P = 0.02).
Conclusions
One-third of women with HFrEF displays TD that impacts remarkably on their morbidity and mortality. TD is associated with a worse clinical profile including exercise capacity, right ventricular-pulmonary arterial coupling, and renal function. These findings lend support to an accurate profiling of women with HF, a problem often overlooked in clinical trials.
1 Introduction
Despite improvements in therapy and management, chronic heart failure (CHF) is still one of the major health problems with a nowadays estimated 5 year mortality rate of 50%.1 In this regard, there is an urgent need of innovative therapeutic approaches potentially able to halt disease progression and increase survival.1 Notwithstanding, the overall magnitude of heart failure with reduced ejection fraction (HFrEF), a considerable lack of sex-specific data represent an unmet need of cardiovascular medicine.2 Taken all together, almost 30% of HFrEF patients are women3 and tend to be older and are burdened by higher number of co-morbidities than men.4 However, clinical outcomes of women with HFrEF do not differ from those of male counterpart.5 On the other hand, women with HFrEF represent a poorly characterized subgroup of patients, with little information available in the current literature regarding clinical presentation, natural history, and risk factors for worse outcomes. In this regard, among the whole spectrum of the HFrEF population, anabolic deficiencies play a relevant role, being associated with increased morbidity and mortality.6, 7 In unselected cohort of men with CHF, it has been demonstrated that reduced serum concentration of testosterone (T) was a powerful predictor of poor prognosis, independent of conventional risk predictors and of the underlying cause of CHF.7 Moreover, circulating level of T is directly and independently related to peak oxygen consumption and peak oxygen pulse in men with CHF.8, 9 However, data on testosterone deficiency (TD) in women with HFrEF are lacking. Recently published data from the Atherosclerosis Risk in Communities (ARIC) population study reported an increased risk of incident HF in men but not in post-menopausal women.10 In this light, it is still unclear whether TD also exerts a negative impact on morbidity and mortality in women suffering from HFrEF and if it shapes a cluster of patients burdened by a more advanced disease. Therefore, the aim of the current study was to investigate the prevalence and the clinical and prognostic impact of TD on women affected by HFrEF, particularly focusing on exercise capacity, left as well as right heart geometry and function.
2 Methods
2.1 Study population
Clinical data of female patients enrolled within the T.O.S.CA. (Terapia Ormonale Scompenso CArdiaco) were analysed. Study design and patient's baseline characteristics of the T.O.S.CA. study have been already described elsewhere.11, 12 Briefly, the T.O.S.CA. registry is a prospective and nation-wide observational study recruiting consecutive stable CHF patients with left ventricular ejection fraction (LVEF) ≤ 45%, in clinical stability during 6 months before enrolment (defined as no history of recent acute decompensation or acute coronary syndrome). Further exclusion criteria were (1) severe liver cirrhosis in Child–Turcotte–Pugh stage B or C; (2) clinically relevant kidney disease (creatinine level >2.5 mg/dl); (3) active malignancy; and (4) current hormonal treatment or overt endocrine diseases. Patients enrolled in the T.O.S.CA. were all on stable medications for at least 3 months before enrolment, including any beta-blocker which had to be started at least 6 months before entering the study.
2.2 Study design and procedures
The study protocol was approved by the Ethics Committees of all participating centres, and all patients gave written informed consent.6 Primary endpoint of the study was a composite of all-cause mortality and cardiovascular hospitalization. Information regarding clinical outcome was obtained directly from patients or their relatives and an independent endpoint committee adjudicated the outcome. Patients were subsequently followed up for 5 years, with a patient median follow-up of 36 months. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Medical history, medication use, and clinical data were recorded at the time of the enrolment as already described.6, 11 Briefly, patients underwent echocardiography (including two-dimensional, Doppler, Color, and tissue Doppler analysis) and symptom-limited exercise test and maximal oxygen uptake (VO2max) was measured by a breath-to-breath respiratory gas analysis. Blood samples were collected by venipuncture after overnight fast. To obtain serum and plasma, samples were centrifuged within 30 min, frozen, and stored at −80°C until assayed. Serum N-terminal prohormone brain natriuretic peptide (NT-proBNP) concentrations were measured by an electrochemiluminescence immunoassay (ECLIA by Roche diagnostics) using a Roche Elecsys analyser.
Serum hormones were analysed in a centralized core-lab (IRCCS SDN, Naples, Italy). Insulin and insulin growth factor-1 (IGF-1) were assayed by an enzyme-labelled chemiluminescent immunometric assay (IMMULITE 2000; IGF-1, interassay CV = 5.7%, Siemens Medical Solutions Diagnostics). DHEA-S was measured by a solid-phase, competitive chemiluminescent enzyme immunoassay. Total testosterone was measured with a DPC Coat-A-Count RIA kit. TD was defined according to current guidelines as serum testosterone levels lower than 25 ng/dl.13
2.3 Statistical analysis
Normally distributed continuous variables were expressed as mean ± standard deviation. Categorical variables were expressed as counts and percentages. Female HFrEF patients were compared according to the presence of TD or normal testosterone levels (TD−) with unpaired Student's t-test. Categorical variables were evaluated with χ2 test. For the multivariate analyses, two different models were used to test the primary endpoint: in the first model, established predictors of poor outcome in heart failure were employed as covariates [i.e. age, New York Heart Association (NYHA) class, LVEF, impaired estimated glomerular filtration rate (eGFR), and N terminal fraction of B-type natriuretic peptide (NTproBNP)], and in the second model, additional covariates were included such as haemoglobin levels, aetiology, HF duration, and peak VO2.
3 Results
3.1 Study population and prevalence of TD among women with HFrEF
Among 480 patients enrolled in the TOSCA registry with complete hormonal assessment, 94 (mean age 66.18 ± 10.26) were women. TD was present in 30 patients (31.9%) of them. Table 1 reports overall and TD-stratified clinical characteristics and biomarkers of the whole study populations and of the two groups based on the presence/absence of TD. The two study groups were homogeneous with regard to age, body surface, blood pressure, and New York Heart Association (NYHA) functional class. No differences were recorded with regard to therapies employed (Table 1).
| Total female population | TD | TD− | P-values | |
|---|---|---|---|---|
| (n = 94) | (n = 30) | (n = 64) | ||
| Mean ± SD | Mean ± SD | Mean ± SD | ||
| Age (years) | 66.18 ± 10.26 | 68.93 ± 9.745 | 64.89 ± 10.325 | 0.12 |
| NTproBNP (pg/ml) | 2597.05 ± 4093.04 | 2794.47 ± 3380.14 | 2504.52 ± 4409.55 | 0.72 |
| BSA (m2) | 1.70 ± 0.16 | 1.68 ± 0.1 | 1.71 ± 0.19 | 0.34 |
| SBP (mmHg) | 123.29 ± 17.82 | 121.32 ± 16.86 | 124.24 ± 18.37 | 0.53 |
| DBP (mmHg) | 75.46 ± 12.39 | 75.05 ± 9.51 | 75.65 ± 13.65 | 0.85 |
| eGFR (ml/min) | 68.61 ± 34.28 | 54.27 ± 23.02 | 75.55 ± 36.76 | 0.001 |
| Haemoglobin (g/dl) | 12.85 ± 1.53 | 12.50 ± 1.21 | 13.01 ± 1.65 | 0.13 |
| Albumin (%) | 56.8 ± 5.2 | 56.6 ± 7.7 | 56.9 ± 3.7 | 0.9 |
| Testosterone (ng/dl) | 48.80 ± 61.86 | 13.60 ± 6.63 | 62.55 ± 68.16 | < 0.001 |
| Ejection fraction (%) | 33.81 ± 6.46 | 33.20 ± 7.66 | 34.10 ± 5.85 | 0.53 |
| LAVi (ml/m2) | 49.46 ± 25.57 | 55.51 ± 30.24 | 46.63 ± 22.77 | 0.17 |
| e/E′ | 14.68 ± 9.96 | 13.04 ± 4.95 | 12.53 ± 6.40 | 0.16 |
| TAPSE (mm) | 18.82 ± 4.51 | 17.78 ± 4.26 | 19.31 ± 4.56 | 0.12 |
| PASP (mmHg) | 34.38 ± 13.25 | 37.69 ± 13.72 | 32.84 ± 12.84 | 0.98 |
| RVOT-AT (ms) | 103.27 ± 36.47 | 112.11 ± 34.74 | 97.15 ± 37.73 | 0.35 |
| TAPSE/PASP (mm/mmHg) | 0.63 ± 0.29 | 0.53 ± 0.22 | 0.68 ± 0.32 | 0.008 |
| Pericardial effusion | 14 (15.20%) | 7 (23.30%) | 7 (10.93%) | 0.11 |
| VO2 peak (ml*kg−1*min−1) (n = 67) | 20.61 ± 4.70 | 18.89 ± 4.92 | 21.31 ± 4.92 | 0.03 |
| NYHA class | ||||
| II | 66 (70.21%) | 21 (70.00%) | 45 (70.31%) | 0.98 |
| III | 23 (24.46%) | 7 (23.33%) | 16 (25.00%) | 0.87 |
| IV | 5 (5.33%) | 2 (6.67%) | 3 (4.69%) | 0.65 |
| Aetiology (ischaemic) | 27 (28.7%) | 17 (56.7%) | 10 (15.6%) | 0.01 |
| Year of disease | 5 [2–10.75] | 4 [1–9.75] | 7 [2.75–12] | 0.1 |
| AF | 11 (22.4%) | 4 (13.33%) | 7 (10.90%) | 0.73 |
| Beta-blockers | 66 (91.7%) | 24 (72.00%) | 42 (65.60%) | 0.15 |
| ACE-I | 31 (43.1%) | 12 (40.00%) | 19 (29.70%) | 0.22 |
| ARBs | 26 (36.1%) | 9 (30.00%) | 17 (26.60%) | 0.72 |
| MRA | 26 (36.1%) | 8 (26.70%) | 18 (28.12%) | 0.88 |
| ICD | 26 (36.6%) | 8 (26.66%) | 18 (28.10%) | 0.88 |
| CRT | 10 (14.1%) | 1 (3.33%) | 9 (14.10%) | 0.15 |
| IGF-1D | 50 (53.2%) | 16 (53.3%) | 34 (53.1%) | 0.98 |
| DHEA-S D | 73 (77.7%) | 29 (97.7%) | 44 (68.7%) | 0.01 |
| Type II diabetes | 26 (27.7%) | 6 (20%) | 20 (31.2) | 0.37 |
- Abbreviations: TD, testosterone deficiency; NTproBNP, N-terminal proB-type natriuretic peptide; BSA, body surface area; SBP, systolic blood pressure; DBP, diastolic blood pressure; eGFR, estimated glomelurar filtration rate; LAVi, left atrial volume index; e, early diastolic transmitral flow velocity; E′, early diastolic mitral annular velocity; TAPSE, tricuspid annular plane excursion; PASP, pulmonary arterial systolic pressure; RVOT-AT, right ventricular outflow tract acceleration time; VO2 peak, peak oxygen consumption; NYHA, New York Heart Association; AF, atrial fibrillation; ACE-I, angiotensin-converting-enzyme inhibitors; ARBs, angiotensin-receptor blockers; MRA, mineralocorticoid-receptor antagonist; ICD, implantable cardioverter-defibrillator; CRT, cardiac resynchronization therapy; IGF-1 D, IGF-1 deficiency; DHEA-S D, DHEA-S deficiency.
3.2 Clinical characteristics of TD women with HFrEF
Table 1 shows data regarding echocardiographic-evaluated right and left heart geometry, function and exercise performance of HFrEF women with TD and without (TD−). Female patients with TD showed similar left ventricular systolic and diastolic function, as compared with patients with normal T levels. Likewise, the two groups displayed similar estimated pulmonary pressures. However, female patients with TD showed lower level of tricuspid annular plane excursion (TAPSE) to pulmonary arterial systolic pressure PASP ratio (TAPSE/PASP) (0.53 ± 0.22 and 0.68 ± 0.32 mm/mmHg in TD and TD−, respectively, P = 0.008), which is a surrogate parameter of right ventricular-arterial uncoupling. Furthermore, HFrEF women with TD also displayed more impaired cardiopulmonary performance as shown by lower values of peak oxygen consumption (VO2 peak) than those without TD (18.89 ± 4.92 vs. 21.31 ± 4.92 ml*kg−1*min−1 P = 0.03). Women with TD also had worse renal function as shown by lower values of eGFR as compared with those TD− (54.27 ± 23.02 vs. 75.55 ± 36.76 ml/min, P < 0.001).
3.3 Clinical outcomes
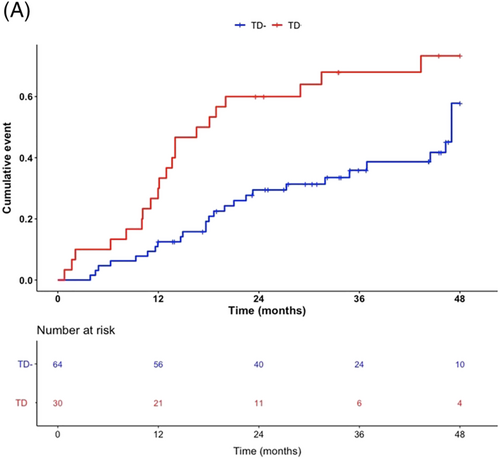
After a mean follow-up of 36 months, a total of 54 events were recorded, with 18 deaths and 36 cardiovascular hospitalizations. Nine women of the TD group (30%) and nine women of the TD− group (14%) died during follow-up. Fifteen women of the TD group (50%) underwent hospitalization due to cardiovascular reasons whereas 21 cardiovascular hospitalizations were recorded in the TD− group (32%). As depicted in Figure 1, TD was associated with a significant difference in the occurrence of the primary endpoint (unadjusted HR: 2.31, 95% CI 1.20–4.10, P = 0.004). Furthermore, TD was also associated with higher all-cause mortality (unadjusted HR: 2.87, 95% CI 1.11–7.40, P = 0 .03) (Figure S1A) and cardiovascular hospitalization (unadjusted HR: 2.37, 95% CI 1.20–7.40, P = 0 .01) (Figure S1B).
3.4 Multivariable analysis
Table 1 shows that TD resulted an independent predictor of the combined endpoint of all-cause mortality/cardiovascular hospitalization (adjusted, adj-HR: 10.45, 95% CI 3.54–17.01, P = 0.001). Same result was found considering the individual components of the composite endpoint (adj-HR: 8.33, 95% CI 5.36–15.11 P = 0.039 for all-cause mortality; adj-HR: 2.41, 95% CI 1.13–4.50; P = 0.02 for cardiovascular hospitalization) (Table S1). Similar results were obtained after excluding (n = 8) patients with LVEF between 40 and 45% (Table 2).
| Combined endpoint | HR | 95% CI | P-value |
|---|---|---|---|
| TD | 10.45 | 3.54–17.01 | 0.001 |
| Age (years) | 1.01 | 0.96–1.05 | 0.47 |
| NTproBNP (pg/ml) | 18.77 | 4.73–22.62 | 0.001 |
| Ejection fraction (%) | 1.02 | 0.96–1.44 | 0.53 |
| eGFR <60 ml/min | 1.69 | 0.89–3.17 | 0.09 |
- Note: Adjusted hazard ratios for combined endpoint according to the presence of testosterone deficiency.
- Abbreviations: CV, cardiovascular; eGFR, estimated glomerular filtration rate; TD, testosterone deficiency.
4 Discussion
The main findings of the current study are that (1) TD is highly prevalent among women with HFrEF, around one-third; (2) when present, TD shapes a cluster characterized by reduced exercise capacity, abnormal right ventricular-arterial coupling and impaired renal function; (3) TD has a significant impact on morbidity and mortality of female patients affected by HFrEF being an independent predictor of all-cause mortality and cardiovascular hospitalization. To the best to our knowledge, this report is the first addressing the impact of TD in women affected by HFrEF.
4.1 Impact of testosterone deficiency on clinical status, morbidity, and mortality of female HFrEF
The evidence of a positive effect played by testosterone on cardiovascular system may be gathered from case–control studies examining subjects affected by endocrinological disorders marked out by chronic androgens deprivation, such as Klinefelter's syndrome14-16 or congenital adrenal hyperplasia.17 Indeed, endogenous testosterone is endowed with a wide range of beneficial effects on the cardiovascular system such as improved endothelial function, alongside with myocardial contractility, increase in skeletal muscle strength and cardiopulmonary performance, amelioration of metabolic profile, and reduction of inflammatory activation.18 In the general population, TD is associated with worse cardiovascular mortality in post-menopausal women.19 Taken together, extant data may represent the pathophysiological underpinning of the reduced peak oxygen consumption in TD HFrEF women, considering that the main component of VO2 peak are cardiac contractility and skeletal muscle oxygen extraction.20 VO2 peak is a powerful predictor of mortality in the whole heart failure spectrum and is also the key examination to select candidates for heart transplant.21 An effect of testosterone and other sexual hormones on cardiopulmonary performance of women has been already hypothesized22 and is rooted on the aforementioned positive effect of testosterone on cardiac contractility and muscle strength. Accordingly, right ventricular-arterial uncoupling might contribute to this finding. Right ventricular-pulmonary arterial uncoupling is defined by the capability of the right ventricle to increase its contractility in response to increased afterload.23 TAPSE/PASP is indeed a validated marker of right ventricular-pulmonary arterial uncoupling24 and is also itself a strong and independent predictor of mortality in HFrEF.25, 26 A study performed by Ventetuolo et al. elegantly demonstrated how menopause and waning testosterone levels have a dramatic impact on lung haemodynamics and right ventricular function.27 Interestingly, when combined, both VO2 peak as well as TAPSE/PASP are able to predict poor clinical outcomes in patients with HFrEF.28 Another finding from our study is that HFrEF for women with TD also display worse renal function which is a further independent predictor of mortality in HF patients.29 Mounting evidence suggest a strong impact of testosterone on renal function.30 Indeed, testosterone replacement has been also proposed as potential therapy in renal transplant recipients in order to preserve kidney function.31
4.2 Testosterone deficiency in the context of multiple hormonal anabolic deficiency in heart failure
Despite the potential association between low testosterone and cardiovascular mortality having been largely debated,18 data on its role in women with HF are particularly scanty. Recently published data from the ARIC population study reported an increased risk of incident HF in men but not in post-menopausal women.10
According to a landmark study published in 2006 by Jankowska et al., TD (together with other hormones) independently predicted all-cause mortality in a sample of 208 men affected by HFrEF.7 The same group also reported that circulating estradiol levels independently predict mortality in a cohort of 501 with HFrEF.32 Furthermore, according to the recently published T.O.S.CA. registry TD was significantly associated with two-fold increase of morbidity and mortality in the whole population.6 This puts TD in a larger context of multiple hormonal deficiency syndrome in HF, together with the somatotropic axis [including growth hormone (GH) and its tissue effector insulin-like growth factor-1 (IGF-1), anabolic steroids (testosterone and DHEA-S), and thyroid hormones].
The current post hoc analysis puts forward the concept that TD is likely to impact outcomes strongly in women.
4.3 Is testosterone deficiency a potential pharmacological target for HFrEF patients?
The importance of TD within the context of HFrEF in women should prompt attention for a possible hormonal replacement therapy to improve long-term clinical outcomes. Despite the lack of a randomized controlled clinical trial of testosterone replacement therapy in patients with HFrEF and TD, encouraging results regarding testosterone administration in unselected HFrEF have been reported so far. Caminiti et al. reported positive effects of testosterone on exercise capacity and muscle strength on 70 men affected by HFrEF.33 In this work, testosterone was administered regardless the presence of TD and brought an improvement of almost 20% (P < 0.05) in peak VO2 in patients under testosterone therapy whereas no changes in the same endpoint was recorded in the placebo group.33 Similarly, muscle strength, insulin resistance, and baroreflex sensitivity improved in the active treatment group without any concomitant changes in the placebo one.33
The same group also reported subsequently the results of a small pilot trial of low-dose testosterone administration in unselected population of women with HFrEF.34 Positive effects were recorded in the groups of patients receiving testosterone on exercise capacity (+30%, P < 0.05) and muscle strength (almost +90%, P < 0.05) without any remarkable changes in the placebo group. Although very interesting, both studies did not consider testosterone status before enrolment. In this regard, our group recently reported a small case series of concomitant replacement therapy of HFrEF patients presenting simultaneously GH and TD. Specifically, after 1 year of GH replacement therapy, one further year of testosterone replacement (still combined with ongoing GH replacement) led to a remarkable improvement of VO2 peak (+27.7%, final delta change +52.44%, P < 0.01) and muscular strength assessed by handgrip dynamometry (+17.5%, final delta change +25.8%, P < 0.01).35 However, to date, no clinical trials were conducted on the correction of TD in HFrEF women, and our data might represent the bedrock for the implementation of such an investigation.
5 Study limitations
Our study owes several limitations. First, the small sample size. Second, we defined TD only based on total testosterone without analysing the whole androgens panel. Moreover, several confounders could be missed due to the observational design of the study, such as various degrees of co-morbidities. Moreover, only Caucasian white patients have been enrolled; therefore, our results might not be applicable to other ethnic groups.
6 Conclusions
In conclusion, testosterone deficiency impacts remarkably on exercise capacity, right ventricular-pulmonary arterial coupling, renal function, and clinical outcomes of women affected by HFrEF. These findings lend support to an accurate profiling of women with HF, a problem often overlooked in clinical trials. Future studies will address whether TRT may convey beneficial cardiovascular actions in this specific patient subgroup.
Acknowledgements
We are especially grateful to patients and nurses who have participated in this study. Dr Salzano receives research grant support from Cardiopath, Department of Advanced Biomedical Sciences, Federico II University of Naples, Naples, Italy, and UniNa and Compagnia di San Paolo, in the frame of the Programme STAR. Dr. D'Agostino was supported by an institutional grant from Italian Healthcare Ministry (Ricerca Finalizzata for young researchers) project GR-2016-02364727. The authors are grateful to Sekisui Medical Co. for provision of RapidPIA™ BNP kits. The T.O.S.CA. registry is an Investigator initiated trial with external funding being provided mainly by unrestricted grants from Merck Serono Italy.
Conflicts of interest
The authors declare that they have no conflicts of interest.




