Heart failure and the risk of left atrial thrombus formation in patients with atrial fibrillation or atrial flutter
Abstract
Aims
The aim of the study was to evaluate the prevalence of left atrial thrombus (LAT) on transoesophageal echocardiography (TOE) in patients with atrial fibrillation or atrial flutter (AF/AFl) with reference to the presence of heart failure (HF) and its subtypes.
Methods and results
The research is a sub-study of the multicentre, prospective, observational Left Atrial Thrombus on Transoesophageal Echocardiography (LATTEE) registry, which comprised 3109 consecutive patients with AF/AFl undergoing TOE prior to direct current cardioversion or catheter ablation. TOE parameters, including presence of LAT, were compared between patients with and without HF and across different subtypes of HF, including HF with preserved (HFpEF), mid-range (HFmrEF), and reduced ejection fraction (HFrEF). HF was diagnosed in 1336 patients (43%). HF patients had higher prevalence of LAT than non-HF patients (12.8% vs. 4.4%; P < 0.001). LAT presence increased with more advanced type of systolic dysfunction (HFpEF vs. HFmrEF vs. HFrEF: 7.4% vs. 10.5% vs. 20.3%; P < 0.001). Univariate analysis revealed that HFrEF (odds ratio [OR] 4.13; 95% confidence interval [95% CI]: 3.13–5.46), but not HFmrEF or HFpEF, was associated with the presence of LAT. Multivariable logistic regression indicated that lower left ventricular ejection fraction (OR per 1%: 0.94; 95% CI 0.93–0.95) was an independent predictor of LAT formation. Receiver operator characteristic analysis showed LVEF ≤48% adequately predicted increased risk of LAT presence (area under the curve [AUC] 0.74; P < 0.0001).
Conclusion
The diagnosis of HFrEF, but neither HFmrEF nor HFpEF, confers a considerable risk of LAT presence despite widespread utilization of adequate anticoagulation.
Introduction
The steadily growing coexistence of heart failure (HF) and atrial fibrillation (AF) is associated with significantly increased in-hospital mortality.1 The clinical significance of HF in AF patients consists in the more pronounced left atrial (LA) remodelling translating into impaired success rate of rhythm control strategy,2 as well as higher risk of ischaemic stroke, systemic embolism, and death.3 In turn, AF may facilitate clinical progression of HF leading to symptomatic deterioration and increased mortality.4, 5 Accordingly, accumulating evidence suggests that early rhythm control strategy in patients with HF, particularly with reduced ejection fraction (HFrEF) may show benefit in terms of improved outcome.6, 7
Impaired systolic and diastolic function of the left ventricle is an established risk factor of thrombosis; however, its exact effect on the risk of left atrial/left atrial appendage thrombus (LAT) formation is unknown. Although congestive HF was incorporated into the routinely used CHA2DS2-VASc score recommended for stratification of ischaemic stroke and systemic embolism risk,8 the definition of HF at the times of CHA2DS2-VASc score creation pertained mainly to patients with HFrEF. Although HF has been deemed a risk factor of stroke in AF population, the threshold of left ventricular ejection fraction (LVEF) is yet to be established.9 As AF is even more prevalent among HFpEF than HFrEF patients,1, 9 the question remains, whether HFpEF modulates the risk of LAT formation and resultant ischaemic stroke to the same extent as HFrEF.10 LAT formation may also be affected by higher rate of co-morbidities in HFpEF patients in contrast to HFrEF population.11, 12 A meta-analysis by Kotecha et al. demonstrated that patients with HFpEF share similar risk of ischaemic stroke to patients with HFrEF,13 while another report indicated a universally increased risk of stroke among all subtypes of HF.9 Conversely, large body of evidence suggests that inclusive HF diagnosis, including HFpEF, may not be an independent predictor of stroke on top of other risk factors incorporated in the CHA2DS2-VASc score.14, 15
Although formation of thrombus within left atrial appendage (LAA) is the dominant but not exclusive mechanism of ischaemic stroke in AF/atrial flutter (AFl), one should state that not all cases of LAT are complicated by ischaemic stroke. The current European guidelines on AF recommend that electrical cardioversion can be pursued without transoesophageal echocardiography (TOE) on condition that it is preceded by at least 3 week adequate anticoagulation therapy or the episode unequivocally lasts shorter than 48 h.16 Yet a recent high-volume meta-analysis delivered evidence that LAT on TOE can be found in 5.5% of patients referred for electrical cardioversion and in 1.8% of patients scheduled for catheter ablation despite adequate anticoagulation.17 Of note, broadly defined HF remained the strongest independent predictor of LAT (odds ratio [OR] 4.3; 95% confidence interval [95% CI] 2.7–6.8).14 In the formerly published results of the real-world LATTEE registry, LAT was present in 8.0% of patients referred for electrical cardioversion or catheter ablation.18 Thus, it is unknown if all subtypes of HF lead to similar risk of thrombus development within LAA. Given the heterogeneity of the results of hitherto reports, the objective of the current sub-study was to evaluate the prevalence and predictors of LAT by means of pre-procedural TOE in patients with AF or AFl and different subtypes of HF.
Methods
The article represents a sub-analysis of the real-world Left Atrial Thrombus on Transoesophageal Echocardiography (LATTEE) registry (NCT03591627), which evaluated the prevalence and determinants of left atrial thrombus depending on the presence of different forms and stages of HF in patients with AF/AFl referred for electrical cardioversion or catheter ablation. The rationale and design of the registry were described in the former manuscript,19 while the primary data concerning the rate of thrombus depending on the mode of anticoagulation were further precisely described.18 In brief, the registry constituted a prospective observational study covering patients with AF or AFl referred for urgent or elective electrical cardioversion or percutaneous catheter ablation who underwent TOE prior to the procedure. Patients were enrolled in 13 cardiology departments (11 academic centres and 2 territorial departments) in Poland from November 2018 to May 2020.
The study involved acquisition of baseline demographic and clinical parameters, including data on type and duration of anticoagulation (transient preprocedural vs. chronic), the diagnosis of HF and its subtype and symptomatic New York Heart Association class. Mandatory laboratory test involved complete blood count, serum creatinine concentration with estimated glomerular filtration rate, alanine and aspartate aminotrasferases concentration, international normalized ratio (INR) and activated partial thromboplastin time (APTT). In all patients enrolled in registry the mandatory TOE parameters were presence and location of LAT, presence of spontaneous echocardiographic contrast within LA and LAA (SEC) and dense SEC (DSEC consistent with grades 3–4 by Fatkin et al. classification20), as well as LAA outflow velocity (LAAV). The optional TOE parameter was the specification of LAA morphology (windsock, chicken wing, cactus or cauliflower). Though optional, transthoracic echocardiography (TTE) was performed in vast majority of patients and involved measurement of LVEF in apical 4-chamber and 2-chamber view using Simpson's method, evaluation of LA diameter in parasternal long axis view (PLAX), measurement of LA area in apical 4-chamber view and LA volume index in 4-chamber and 2-chamber view, evaluation of the presence of valvular heart disease.19 Both TTE and TOE parameters were analysed and interpreted locally.
The AF/AFl diagnosis was consistent with 2016 European Society of Cardiology Guidelines on the management of AF,21 while the diagnosis of HF was made in accordance with the 2016 Guidelines for the treatment of acute and chronic heart failure.22 Patients with HF were further categorized based on left ventricular ejection fraction (LVEF) into HF with preserved ejection fraction (HFpEF), HF with mid-range ejection fraction (HFmrEF) and HF with reduced ejection fraction (HFrEF).18 The diagnosis of HFpEF required presence of symptoms, structural abnormalities, and presence of elevated natriuretic peptides.18 Symptoms of heart failure were classified using the New York Heart Association (NYHA) classification. Different variables as well as the prevalence of LAT were compared across the specified subgroups. Thorough study definitions and list of variables were described previously.18, 19
- 1. The presence of LAT was assessed by the subtype of HF in patients with fulfilled applicable HF criteria: HFpEF vs. HFmrEF vs. HFrEF
- 2. The range of LVEF irrespective of symptoms and HF diagnosis (including patients without HF): LVEF ≥50%, 40–49%, and <40%
Statistical analysis
The distribution of continuous variables was verified using the Shapiro–Wilk's test. Continuous variables were expressed as arithmetic mean ± standard deviation (SD) or median and 1–3 quartile boundary, whereas categorical variables were shown as absolute counts with percentages (%). In case of continuous variables, Mann–Whitney test or Kruskal–Wallis test were applied, while in case of qualitative parameters, χ2 test was utilized. In multiple group comparisons of quantitative parameters, post hoc Dunn's test was applied. Univariable ORs with 95% CIs were calculated for the prediction of presence of LAT. Subsequently, all the parameters with P < 0.1 were incorporated into the stepwise multivariable logistic regression model in order to establish the independent predictors of LAT. A receiver operating characteristic (ROC) curve analysis was performed so as to assess the predictive role of LVEF. A two-sided P-value of 0.05 was considered statistically significant.
Results
Overall population characteristics
The characteristics of study population were described in the former report.15 The registry incorporated 3109 patients in total, who were qualified either for direct current cardioversion (n = 1595; 52%) or catheter ablation (n = 1468; 48%).18 AF was diagnosed in 2733 patients (88%) and AFl in 472 (15%).18 Chronic anticoagulation was used in 88% of patients, transient in 1.5%, while 10% of study participants received no anticoagulation prior to the procedure.18 The predominance of patients on chronic anticoagulation therapy received non-vitamin K antagonist oral anticoagulants (NOACs; 82%), while the rest were treated with vitamin K antagonists (VKAs; 18%).18
Heart failure population characteristics
The criteria for HF diagnosis were met in 1336 patients (43% of the study population). Among the HF population, 503 patients were diagnosed with HFpEF (37.6%), 361 with HFmrEF (27.0%), and 472 with HFrEF (35.3%). Among patients with HF, 959 patients (71.8%) experienced symptoms consistent with class I-II of NYHA classification, 322 patients (24.1%) in NYHA class III, while 35 patients (2.6%) in NYHA class IV.
The precise comparison of HF vs. non-HF population was delineated in Table 1. The comparison of different HF subtypes in terms of demographic and clinical and echocardiographic parameters was presented in Table 2. The analysis of different clinical variables depending on baseline LVEF was denoted in Table 3. In brief, patients diagnosed with HF were older and had greater prevalence of co-morbidities than patients without HF (Table 1). Patients with HF more frequently had persistent AF/AFl (67.7% vs. 36.6%; P < 0.001) and less frequently were on chronic anticoagulation therapy (86.6% vs. 90.1%; P = 0.003). Patients with HF were more likely to be treated with VKAs than non-HF patients (22.8% vs. 14.8%; P < 0.001) and less likely with NOACs (77.8% vs. 85.2%; P < 0.001; Table 2). If treated with NOACs, the utilization of the reduced doses was more prevalent in HF population (25.7% vs. 11.7%; P < 0.001). The proportion of patients with AFl to AF was higher in HF vs. non-HF population and increased along with the progression of HF (Table 2).
| Variable | No HF + AF/AFl (N = 1764) | HF + AF/AFl (N = 1336) | P |
|---|---|---|---|
| N (valid %) or mean ± SD or median (1, 3 quartile) | N (valid %) or mean ± SD or median (1, 3 quartile) | ||
| Age (years) | 64.07 ± 11.57 | 68.08 ± 11.53 | <0.001* |
| Female sex | 678 (38.5%) | 461 (34.5%) | 0.024** |
| BMI (kg/m2) | 29.49 ± 4.71 | 30.16 ± 5.56 | 0.012* |
| AF | 1591 (90.2%) | 1133 (84.8%) | <0.001** |
| AFl | 240 (13.6%) | 231 (17.3%) | 0.005** |
| Paroxysmal AF/AFl | 984 (56.0%) | 289 (21.7%) | <0.001** |
| Persistent AF/AFl | 643 (36.6%) | 903 (67.7%) | <0.001** |
| Long-standing persistent AF | 129 (7.4%) | 142 (10.6%) | 0.001** |
| Time from first AF diagnosis (years) | 3 (1; 5) | 3 (1; 5) | 0.884* |
| PVI – index hospitalization | 1103 (63.8%) | 358 (27.0%) | 0.001** |
| DCC – index hospitalization | 627 (36.2%) | 966 (73.0%) | <0.001** |
| Successful DCC of all patients submitted | 471 (72.1%) | 708 (77.2%) | 0.022** |
| EHRA | |||
| 1 | 249 (14.2%) | 361 (27.1%) | <0.001** |
| 2a | 400 (22.9%) | 212 (16.1%) | <0.001** |
| 2b | 562 (32.2%) | 314 (23.9%) | <0.001** |
| 3 | 477 (27.1%) | 347 (26.0%) | 0.497** |
| 4 | 59 (3.4%) | 81 (6.1%) | <0.001** |
| INR 2.0–3.0 prior to admission | 155 (9.6%) | 178 (14.6%) | <0.001** |
| Chronic OAC | 1588 (90.1%) | 1156 (86.6%) | 0.003** |
| Transient OAC | 25 (1.4%) | 22 (1.7%) | 0.604** |
| VKA | 239 (14.8%) | 261 (22.2%) | <0.001** |
| NOAC | 1374 (85.2%) | 917 (77.8%) | <0.001** |
| Rivaroxaban | 651 (36.9%) | 416 (31.2%) | 0.001** |
| Dabigatran | 509 (28.9%) | 309 (23.2%) | <0.001** |
| Apixaban | 214 (12.1%) | 192 (14.4%) | 0.067** |
| NOAC at reduced dose | 160 (11.7%) | 235 (25.7%) | <0.001** |
| CHA2DS2-VASc score | 2 (1; 3) | 4 (3; 5) | <0.001* |
| CHADS2 | 1 (1; 2) | 3 (2; 3) | <0.001* |
| Amiodarone therapy | 234 (14.1%) | 264 (20.0%) | <0.001** |
| History of SSE | 127 (7.2%) | 186 (13.9%) | <0.001** |
| Ischaemic stroke | 91 (5.2%) | 109 (8.2%) | 0.001** |
| MR moderate - severe | 131 (7.4%) | 392 (29.3%) | <0.001* |
| History of mitral valve replacement | 21 (1.2%) | 40 (3.0%) | <0.001** |
| ICD/CRT implantation | 12 (0.7%) | 137 (10.3%) | <0.001** |
| Arterial hypertension | 1272 (72.2%) | 1088 (81.5%) | <0.001** |
| Diabetes mellitus | 378 (21.4%) | 393 (29.4%) | <0.001** |
| CAD | 303 (17.2%) | 598 (44.8%) | <0.001** |
| PAD | 53 (3.0%) | 122 (9.1%) | <0.001** |
| Myocardial infarction | 121 (6.9%) | 308 (23.1%) | <0.001** |
| COPD | 60 (3.4%) | 100 (7.5)% | <0.001** |
| Hyperthyroidism | 97 (5.5%) | 72 (5.4%) | 0.899** |
| Smoking | 504 (30.0%) | 505 (39.1%) | <0.001** |
| Neoplastic disease | 51 (2.9%) | 56 (4.2%) | 0.049** |
| Haemoglobin (g/dL) | 14.2 ± 1.7 | 13.9 ± 1.8 | <0.001* |
| Platelet count (×1000/mm3) | 219.6 ± 57.12 | 217.9 ± 70.16 | 0.026* |
| White blood cells (×1000/mm3) | 7.24 ± 2.03 | 7.69 ± 2.24 | <0.001* |
| eGFR (mL/min/1.73 m2)*** | 89.2 ± 36.8 | 80.3 ± 32.9 | <0.001* |
| Chronic kidney disease | 155 (8.8%) | 340 (25.5%) | <0.001** |
| LVEF (%) | 56.9 ± 6.4 | 44.3 ± 13.1 | <0.001* |
| Left atrial diameter (mm) | 43.7 ± 5.9 | 47.6 ± 6.2 | <0.001* |
| Thrombus on TOE | 77 (4.4%) | 171 (12.8%) | <0.001** |
| LAA thrombus | 73 (94.8%) | 165 (96.5%) | 0.532** |
| LA thrombus | 4 (5.2%) | 6 (3.5%) | |
| SEC on TEE | 346 (19.9%) | 460 (34.7%) | <0.001** |
| DSEC on TEE | 79 (4.6%) | 199 (15.2%) | <0.001** |
| LAAV on TEE (cm/s) | 49.12 ± 23.83 | 34.52 ± 17.31 | <0.001* |
- AF, atrial fibrillation; AFl, atrial flutter; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DCC, direct current cardioversion; DSEC, dense spontaneous echocardiographic contrast; eGFR, estimated glomerular filtration rate; EHRA, European Heart Rhythm Association; HF, heart failure; ICD/CRT, implantable cardioverter-defibrillator/cardiac resynchronization therapy; INR, international normalized ratio; LA, left atrium; LAA, left atrial appendage; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; NOAC, non-vitamin K antagonists oral anticoagulants; NYHA, New York Heart Association; SD, standard deviation; SEC, spontaneous echocardiographic contrast; TOE, transoesophageal echocardiography; LAAV, left atrial appendage outflow velocity; PVI, pulmonary vein isolation; SSE, stroke or systemic embolism; TIA, transient ischaemic attack; VKA- vitamin K antagonists.
- * Mann–Whitney U-test.
- ** χ2 test.
- *** Calculated using Modification of Diet in Renal Disease (MDRD) formula.
| Variable | No HF (N = 1763) | HFpEF (N = 503) | HFmrEF (N = 361) | HFrEF (N = 472) | P* (1–4) | P** (1 vs. 2) | P** (1 vs. 3) | P** (1 vs. 4) | P** (2 vs. 3) | P** (2 vs. 4) | P** (3 vs. 4) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | (4) | ||||||||
| N (valid %) or mean ± SD or median (1; 3 quartile) | |||||||||||
| Age (years) | 64.07 ± 11.57 | 70.73 ± 10.35 | 67.44 ± 11.92 | 65.73 ± 11.87 | <0.001 | <0.001 | <0.001 | 0.004 | <0.001 | <0.001 | 0.069 |
| Female sex | 678 (38.5%) | 253 (50.3%) | 109 (30.2%) | 99 (21.0%) | <0.001 | <0.001 | 0.003 | <0.001 | <0.001 | <0.001 | 0.002 |
| AF | 1591 (90.2%) | 437 (86.9%) | 314 (87.0%) | 382 (80.9%) | <0.001 | 0.033 | 0.068 | <0.001 | 0.965 | 0.011 | 0.020 |
| AFl | 240 (13.6%) | 72 (14.3%) | 59 (16.3%) | 100 (21.2%) | 0.001 | 0.684 | 0.173 | <0.001 | 0.412 | 0.005 | 0.078 |
| Paroxysmal AF/AFl | 984 (56.0%) | 123 (24.5%) | 88 (24.4%) | 78 (16.6%) | <0.001 | <0.001 | <0.001 | <0.001 | 0.998 | 0.002 | 0.005 |
| Persistent AF/AFl | 643 (36.6) | 330 (65.6%) | 227 (63.1%) | 346 (73.5%) | <0.001 | <0.001 | <0.001 | <0.001 | 0.440 | 0.008 | 0.001 |
| Long-standing persistent AF | 129 (7.4%) | 50 (9.9%) | 45 (12.5%) | 47 (10.0%) | 0.006 | 0.058 | 0.001 | 0.060 | 0.236 | 0.984 | 0.251 |
| Time from first AF diagnosis (years) | 2 (1; 5) | 3 (1; 5) | 2 (1; 5) | 2 (1; 5) | 0.015 | 0.122 | 0.263 | 0.030 | 0.909 | 0.003 | 0.013 |
| PVI – index hospitalization | 1103 (63.8%) | 128 (25.6%) | 109 (30.5%) | 121 (26.0%) | <0.001 | <0.001 | <0.001 | <0.001 | 0.108 | 0.882 | 0.148 |
| DCC – index hospitalization | 627 (36.2%) | 373 (74.5%) | 248 (69.5%) | 345 (74.0%) | <0.001 | <0.001 | <0.001 | <0.001 | 0.108 | 0.882 | 0.148 |
| INR 2.0–3.0 prior to admission | 155 (9.6%) | 66 (14.1%) | 47 (14.6%) | 65 (15.0%) | 0.001 | 0.005 | 0.007 | 0.001 | 0.832 | 0.710 | 0.898 |
| Chronic OAC | 1588 (90.1%) | 444 (88.3%) | 321 (88.9%) | 391 (83.0%) | <0.001 | 0.241 | 0.508 | <0.001 | 0.768 | 0.019 | 0.016 |
| Transient OAC | 25 (1.4%) | 9 (1.8%) | 5 (1.4%) | 8 (1.7%) | 0.916 | 0.546 | 0.961 | 0.654 | 0.643 | 0.914 | 0.718 |
| VKA | 239 (14.8%) | 93 (20.5%) | 64 (19.6%) | 104 (26.1%) | <0.001 | 0.003 | 0.029 | <0.001 | 0.758 | 0.056 | 0.041 |
| NOAC | 1374 (85.2%) | 360 (79.5%) | 262 (80.4%) | 295 (73.9%) | <0.001 | 0.003 | 0.029 | <0.001 | 0.758 | 0.056 | 0.041 |
| CHA2DS2-VASc score | 2 (1; 3) | 5 (4; 6) | 4 (3; 5) | 4 (3; 5) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.191 |
| SSE in anamnesis | 127 (7.2%) | 79 (15.7%) | 46 (12.8%) | 61 (13.0%) | <0.001 | <0.001 | <0.001 | <0.001 | 0.228 | 0.221 | 0.941 |
| Arterial hypertension | 1272 (72.2%) | 435 (86.5%) | 292 (80.9%) | 361 (76.7%) | <0.001 | <0.001 | 0.001 | 0.051 | 0.026 | <0.001 | 0.140 |
| Diabetes mellitus | 378 (21.4%) | 143 (28.4%) | 93 (25.8%) | 157 (33.3%) | <0.001 | 0.001 | 0.072 | <0.001 | 0.385 | 0.098 | 0.018 |
| CAD | 303 (17.19%) | 219 (43.5%) | 154 (42.7%) | 225 (47.8%) | <0.001 | <0.001 | <0.001 | <0.001 | 0.797 | 0.185 | 0.142 |
| PAD | 53 (3.0%) | 34 (6.8%) | 43 (11.9%) | 45 (9.6%) | <0.001 | <0.001 | <0.001 | <0.001 | 0.009 | 0.110 | 0.273 |
| Myocardial infarction | 121 (6.9%) | 76 (15.1%) | 82 (22.7%) | 150 (31.9%) | <0.001 | <0.001 | <0.001 | <0.001 | 0.004 | <0.001 | 0.004 |
| COPD | 60 (3.40%) | 31 (6.16%) | 21 (5.82%) | 48 (10.19%) | <0.001 | 0.005 | 0.029 | <0.001 | 0.833 | 0.021 | 0.023 |
| History of hyperthyroidism | 97 (5.5%) | 32 (6.4%) | 11 (3.1%) | 29 (6.2%) | 0.149 | 0.457 | 0.053 | 0.584 | 0.027 | 0.889 | 0.038 |
| Chronic kidney disease | 155 (8.8%) | 122 (24.3%) | 85 (23.6%) | 133 (28.2%) | <0.001 | <0.001 | <0.001 | <0.001 | 0.810 | 0.158 | 0.127 |
| MR moderate - severe | 131 (7.4%) | 89 (17.7%) | 97 (26.9%) | 206 (43.6%) | <0.001 | <0.001 | <0.001 | <0.001 | 0.001 | <0.001 | <0.001 |
| LVEF (%) | 56.87 ± 6.39 | 57.05 ± 6.22 | 44.83 ± 4.64 | 30.82 ± 8.49 | <0.001 | 0.808 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Left atrial diameter (mm) | 43.72 ± 5.86 | 46.52 ± 6.14 | 46.96 ± 5.88 | 49.18 ± 6.05 | <0.001 | <0.001 | <0.001 | <0.001 | 0.223 | <0.001 | <0.001 |
| Thrombus on TOE | 77 (4.4%) | 37 (7.4%) | 38 (10.5%) | 96 (20.3%) | <0.001 | 0.007 | <0.001 | <0.001 | 0.103 | <0.001 | <0.001 |
| SEC on TOE | 346 (19.9%) | 131 (26.2%) | 126 (35.5%) | 203 (43.3%) | <0.001 | 0.002 | <0.001 | <0.001 | 0.003 | <0.001 | 0.024 |
| DSEC on TOE | 79 (4.6%) | 46 (9.4%) | 59 (16.8%) | 94 (20.1%) | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.227 |
| LAAV on TOE (cm/s) | 49.12 ± 23.83 | 37.09 ± 17.49 | 35.37 ± 16.44 | 31.25 ± 17.27 | <0.001 | <0.001 | <0.001 | <0.001 | 0.167 | <0.001 | <0.001 |
- DSEC, dense spontaneous echocardiographic contrast; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFmrEF, heart failure with mid-range ejection fraction; HFrEF, heart failure with reduced ejection fraction; LA, left atrium; LAA, left atrial appendage outflow velocity; SEC, spontaneous echocardiographic contrast; TOE, transoesophageal echocardiography.
- * Kruskal–Wallis test or χ2 test for multiple variables.
- ** Post hoc Dunn's test or post hoc χ2 test with Bonferroni adjustment.
| Variable | LVEF ≥50% (N = 1724) | LVEF 40–49% (N = 416) | LVEF <40% (N = 429) | P* (1–3) | P** (1 vs. 2) | P** (1 vs. 3) | P** (2 vs. 3) |
|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | |||||
| N (valid %) or mean ± SD or median (1; 3 quartile) | |||||||
| Age (years) | 65.54 ± 11.63 | 67.14 ± 11.85 | 65.72 ± 11.69 | 0.046 | 0.014 | 0.651 | 0.651 |
| Female sex | 714 (41.4%) | 138 (33.2%) | 83 (19.4%) | <0.001 | 0.002 | <0.001 | <0.001 |
| BMI (kg/m2) | 29.99 ± 4.94 | 29.87 ± 5.37 | 29.60 ± 5.55 | 0.117 | 0.581 | 0.038 | 0.038 |
| AF | 1541 (89.3%) | 356 (85.6%) | 346 (80.7%) | <0.001 | 0.030 | <0.001 | 0.056 |
| AFl | 228 (13.2%) | 74 (17.8%) | 94 (21.9%) | <0.001 | 0.016 | <0.001 | 0.133 |
| Paroxysmal AF/AFl | 815 (47.2%) | 120 (29.0%) | 67 (15.6%) | <0.001 | <0.001 | <0.001 | <0.001 |
| Persistent AF/AFl | 748 (43.6%) | 245 (59.2%) | 313 (73.0%) | <0.001 | <0.001 | <0.001 | <0.001 |
| Time from first AF diagnosis (years) | 3 (1; 5) | 2 (1; 5) | 2 (1; 4) | <0.001 | 0.267 | <0.001 | <0.001 |
| Long-standing persistent AF | 154 (9.0%) | 49 (11.8%) | 49 (11.4%) | 0.104 | 0.075 | 0.120 | 0.851 |
|
PVI – index hospitalization DCC – index hospitalization |
918 (54.2%) | 130 (31.9%) | 108 (25.6%) | <0.001 | <0.001 | <0.001 | 0.046 |
| 776 (45.8%) | 278 (68.1%) | 314 (74.4%) | <0.001 | <0.001 | <0.001 | 0.046 | |
| Successful DCC of all patients submitted | 618 (77.1%) | 212 (76.5%) | 206 (71.5%) | 0.162 | 0.859 | 0.061 | 0.175 |
| EHRA | |||||||
| 1 | 351 (20.4%) | 75 (18.1%) | 66 (15.4%) | 0.052 | 0.293 | 0.019 | 0.295 |
| 2a | 334 (19.5%) | 70 (17.0%) | 67 (16.0%) | 0.161 | 0.232 | 0.094 | 0.698 |
| 2b | 487 (28.5%) | 144 (34.9%) | 103 (24.5%) | 0.004 | 0.011 | 0.106 | 0.001 |
| 3 | 480 (27.9%) | 106 (25.5%) | 143 (33.3%) | 0.029 | 0.342 | 0.025 | 0.013 |
| 4 | 57 (3.3%) | 18 (4.3%) | 41 (9.6%) | <0.001 | 0.306 | <0.001 | 0.003 |
| NYHA | |||||||
| 1–2 | 428 (25.0%) | 264 (65.2%) | 193 (45.7%) | <0.001 | <0.001 | <0.001 | <0.001 |
| 3 | 59 (3.4%) | 57 (14.1%) | 189 (44.8%) | <0.001 | <0.001 | <0.001 | <0.001 |
| 4 | 3 (0.2%) | 3 (0.7%) | 26 (6.2%) | <0.001 | 0.054 | <0.001 | <0.001 |
| INR 2.0–3.0 prior to admission | 179 (10.8%) | 51 (13.5%) | 61 (15.2%) | 0.032 | 0.137 | 0.014 | 0.504 |
| Chronic OAC | 1567 (90.9%) | 365 (87.7%) | 351 (82.0%) | <0.001 | 0.051 | <0.001 | 0.020 |
| Transient OAC | 24 (1.4%) | 4 (1.0%) | 8 (1.9%) | 0.532 | 0.488 | 0.465 | 0.265 |
| VKA | 256 (16.1%) | 75 (20.3%) | 100 (27.9%) | <0.001 | 0.050 | <0.001 | 0.017 |
| NOAC | 1335 (83.9%) | 294 (79.7%) | 259 (72.1%) | <0.001 | 0.050 | <0.001 | 0.017 |
| Rivaroxaban | 649 (37.7%) | 138 (33.2%) | 102 (23.8%) | <0.001 | 0.090 | <0.001 | 0.003 |
| Dabigatran | 475 (27.6%) | 96 (23.1%) | 95 (22.2%) | 0.027 | 0.064 | 0.025 | 0.760 |
| Apixaban | 211 (12.2%) | 60 (14.4%) | 62 (14.5%) | 0.291 | 0.229 | 0.211 | 0.979 |
| NOAC at reduced dose | 200 (15.0%) | 73 (24.9%) | 72 (27.9%) | <0.001 | <0.001 | <0.001 | 0.426 |
| CHA2DS2-VASc score | 3 (2; 4) | 4 (2; 5) | 4 (2; 5) | <0.001 | <0.001 | <0.001 | <0.001 |
| CHADS2 | 1 (1; 2) | 2 (2; 3) | 2 (2; 3) | <0.001 | <0.001 | <0.001 | <0.001 |
| Amiodarone therapy | 279 (16.4%) | 80 (19.6%) | 94 (22.3%) | 0.012 | 0.130 | 0.005 | 0.336 |
| History of SSE | 169 (9.8%) | 54 (13.0%) | 54 (12.6%) | 0.071 | 0.056 | 0.090 | 0.864 |
| Ischaemic stroke | 107 (6.2%) | 29 (7.0%) | 35 (8.2%) | 0.333 | 0.208 | 0.144 | 0.514 |
| MR moderate – severe | 194 (11.3%) | 99 (23.8%) | 197 (45.9%) | 0.223 | <0.001 | <0.001 | <0.001 |
| History of mitral valve replacement | 32 (1.9%) | 13 (3.1%) | 9 (2.1%) | 0.269 | 0.105 | 0.742 | 0.349 |
| ICD/CRT implantation | 23 (1.3%) | 13 (3.1%) | 100 (23.4%) | <0.001 | 0.011 | <0.001 | <0.001 |
| Arterial hypertension | 1357 (78.7%) | 334 (80.3%) | 315 (73.6%) | 0.038 | 0.466 | 0.024 | 0.021 |
| Diabetes mellitus | 409 (23.7%) | 109 (26.2%) | 135 (31.5%) | 0.004 | 0.287 | 0.001 | 0.087 |
| CAD | 428 (24.8%) | 162 (38.9%) | 200 (46.7%) | <0.001 | <0.001 | <0.001 | 0.022 |
| Myocardial infarction | 141 (8.2%) | 98 (23.6%) | 138 (32.2%) | <0.001 | <0.001 | <0.001 | 0.005 |
| COPD | 74 (4.3%) | 23 (5.5%) | 44 (10.3%) | <0.001 | 0.275 | <0.001 | 0.011 |
| History of hyperthyroidism | 96 (5.6%) | 21 (5.1%) | 20 (4.7%) | 0.731 | 0.675 | 0.463 | 0.800 |
| Smoking | 503 (30.5%) | 147 (36.8%) | 203 (48.3%) | <0.001 | 0.016 | <0.001 | 0.001 |
| Malignant neoplasm | 52 (3.0%) | 18 (4.3%) | 14 (3.3%) | 0.401 | 0.177 | 0.783 | 0.422 |
| Haemoglobin (g/dL) | 14.04 ± 1.63 | 14.04 ± 1.80 | 13.95 ± 1.89 | 0.733 | 0.819 | 0.481 | 0.481 |
| Platelet count (×1000/mm3) | 221.82 ± 60.40 | 212.39 ± 62.75 | 212.90 ± 76.02 | <0.001 | 0.002 | <0.001 | <0.001 |
| White blood cells (×1000/mm3) | 7.26 ± 2.02 | 7.49 ± 2.17 | 8.06 ± 2.34 | <0.001 | 0.040 | <0.001 | <0.001 |
| eGFR (mL/min/1.73 m2) | 87.70 ± 37.44 | 84.41 ± 33.33 | 77.12 ± 33.67 | <0.001 | 0.075 | <0.001 | <0.001 |
| Chronic kidney disease | 245 (14.2%) | 83 (20.0%) | 119 (27.8%) | <0.001 | 0.003 | <0.001 | 0.008 |
| LVEF (%) | 57.80 ± 4.91 | 44.08 ± 2.89 | 29.23 ± 6.76 | <0.001 | <0.001 | <0.001 | <0.001 |
| Left atrial diameter (mm) | 44.43 ± 5.99 | 46.80 ± 5.77 | 49.05 ± 6.54 | <0.001 | <0.001 | <0.001 | <0.001 |
| Thrombus on TOE | 74 (4.3%) | 40 (9.6%) | 96 (22.4%) | <0.001 | <0.001 | <0.001 | <0.001 |
| LAA thrombus | 2 (2.7%) | 2 (5.0%) | 4 (4.2%) | 0.804 | 0.525 | 0.608 | 0.829 |
| LA thrombus | 72 (97.3%) | 38 (95.0%) | 92 (95.8%) | ||||
| SEC on TOE | 334 (19.6%) | 128 (31.1%) | 190 (44.5%) | <0.001 | <0.001 | <0.001 | <0.001 |
| DSEC on TOE | 105 (6.2%) | 51 (12.5%) | 94 (22.1%) | <0.001 | <0.001 | <0.001 | <0.001 |
| LAAV on TOE (cm/s) | 45.86 ± 22.57 | 35.85 ± 17.86 | 30.37 ± 16.42 | <0.001 | <0.001 | <0.001 | <0.001 |
- AF, atrial fibrillation; AFl, atrial flutter; BMI, body mass index; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; DCC, direct current cardioversion; DSEC, dense spontaneous echocardiographic contrast; eGFR, estimated glomerular filtration rate; EHRA, European Heart Rhythm Association; HF, heart failure; ICD/CRT, implantable cardioverter-defibrillator/cardiac resynchronization therapy; INR, international normalized ratio; LA, left atrium; LAA, left atrial appendage; LAAV, left atrial appendage outflow velocity; LVEF, left ventricular ejection fraction; MR- mitral regurgitation; NOAC, non-vitamin K antagonists oral anticoagulants; NYHA, New York Heart Association; PVI, pulmonary vein isolation; SD, standard deviation; SEC, spontaneous echocardiographic contrast; SSE, stroke or systemic embolism; TIA, transient ischaemic attack; TOE, transoesophageal echocardiography; VKA, vitamin K antagonists.
- * Kruskal–Wallis test or χ2 test for multiple variables.
- ** Post hoc Dunn's test or post hoc χ2 test with Bonferroni adjustment.
Also, patients with HF had greater median CHA2DS2-VASc score (4 vs. 2 points; P < 0.001) than those without HF and higher rate of stroke or systemic embolism in anamnesis (13.9% vs. 7.2%; P < 0.001). Additionally, HF patients had a greater LA diameter (47.6 vs. 43.7 mm; P < 0.001) and higher rate of moderate-to-severe mitral valve insufficiency (29.3% vs. 7.4%; P < 0.001), which further increased at more advanced stages of HF (Table 2).
Heart failure and the presence of left atrial thrombus
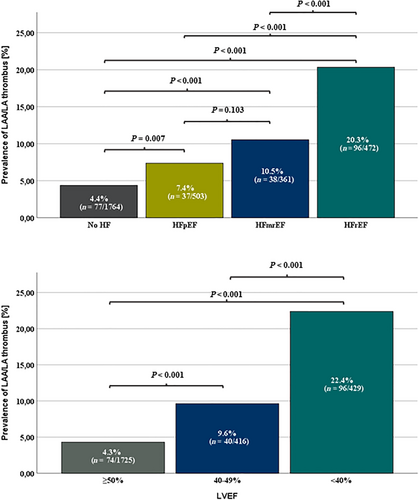
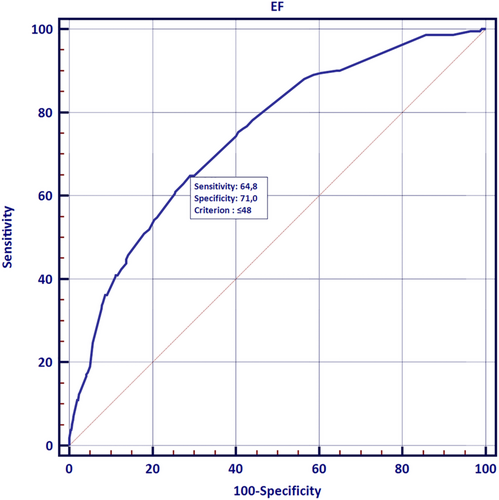
HF patients had higher prevalence of LAT (12.8% vs. 4.4%; P < 0.001), SEC (34.7 vs. 19.9%; P < 0.001), and dense SEC (15.2% vs. 4.6%; P < 0.001) (Table 1). The presence of HF did not interfere with the predilection of thrombus to LAA localization (96.5 vs. 94.8%; P = 0.532). The more advanced the LV dysfunction was, the greater the prevalence of LA thrombus (HFrEF vs. HFmrEF vs. HFpEF: 20.3% vs. 10.5% vs. 7.4%; P < 0.001; Figure 1) and the higher the rate of SEC and dense SEC (Table 2) were documented. The prevalence of thrombus also increased with the gradually decreasing global systolic function (LVEF <40% vs. LVEF 40–49% vs. LVEF ≥50%: 22.4% vs. 9.6% vs. 4.3%, P < 0.001; Figure 1).Patients with HF had decreased LAAV in comparison with patients without HF (34.5 vs. 49.1 cm/s; P < 0.001). Also, patients with HFrEF had lower LAAV than patients with HFmrEF and HFpEF (37.1 vs. 35.4 vs. 31.3 cm/s; P < 0.001).The ROC curve analysis revealed that LVEF was an accurate predictor of the presence of LAT on TOE (AUC 0.74; 95% CI 0.71–0.78; P < 0.0001; Figure 2). For the threshold ≤48% LVEF had 65% sensitivity and 71% specificity for prediction of LAT presence.
Univariable and multivariable regression analysis
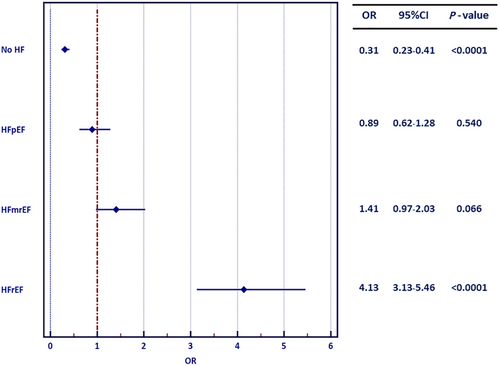
The univariable analysis showed that the diagnosis of HF was linked to increased risk of LAT presence (OR 3.22; 95% CI 2.43–4.25; P < 0.0001). The detailed analysis revealed that HFrEF (OR 4.13; 95% CI 3.13–5.46; P < 0.0001), but neither HFmrEF (OR 1.41; 95% CI 0.97–2.03; P = 0.0656) nor HFpEF (OR 0.89; 95% CI 0.62–1.28; P = 0.5397), predicted the presence of LAT on TOE (Figure 3).
Symptoms in NYHA class I-II (OR 1.46; 95% CI: 1.12–1.92; P = 0.0054) and NYHA class III (OR 3.79; 95% CI: 2.78–5.17; P < 0.0001) were significantly associated with the presence of LAT, unlike NYHA class IV symptoms (OR 2.42; 95% CI 0.99–5.89; P = 0.0514).
Also, univariable analysis indicated that LA diameter >45 mm (OR 2.38; 95% CI: 1.7552 to 3.2164; P < 0.0001) and left atrial volume index >43 mL/m2 (OR 2.64, 95% CI: 1.78–3.92; P < 0.0001) were associated with the presence of LAT.
The stepwise multivariable regression analysis revealed that older age (OR for a 1 year increase 1.03, 95% CI 1.02–1.05), persistent vs. paroxysmal AF/AFl (OR 2.1; 95% CI 1.46–3.02), presence of chronic oral anticoagulation (OR 0.55; 95% CI 0.37–0.83), history of mitral valve replacement (OR 2.45; 95% CI 1.08–5.57), and LVEF (OR for a 1% higher 0.94; 95% CI 0.93–0.95) were independently associated with the detection of LAT by means of TOE (AUC for the model 0.78; 95% CI 0.76–0.79; Hosmer–Lemeshow P = 0.63).
Discussion
The present analysis delivered evidence that the diagnosis of HF is associated with markedly higher prevalence of LAT in comparison to patients without HF (OR 3.22). While all subtypes of HF, including HFpEF, are characterized by higher rate of LAT detected on TOE than patients without HF, only HFrEF remained a significant predictor of LAT presence (Figures 1 and 3). The multivariable regression analysis denoted that lower LVEF, but not the HF itself, was the independent predictor of LAT presence on TOE. The results of the study underscore the notion that depressed LVEF rather than symptoms of HF itself herald the presence of thrombi within LAA. A cut-off value of LVEF ≤48% was established, which accurately predicted increased risk of LAT presence. One more notable finding was that presence of HF does not alter the proportion of LA to LAA thrombus with the vast majority of thrombi located in the latter.
Importantly, our results are consistent with the largest to date meta-analysis by Noubiap et al.17 This meta-analysis covering 56 660 AF patients found that even among adequately anticoagulated patients the prevalence of LAT reached 1.3% in case of catheter ablation and 4.9% in case of direct current cardioversion.17 The general diagnosis of HF was linked to increased risk of LAT both in patients referred for cardioversion (OR 2.8; 95% CI 1.3–6.2) and catheter ablation (OR 4.3; 95% CI: 2.7–6.8), which is in line with the results of the present trial. Although LVEF was included in study variables, it remained a predictor of LAT only in univariate analysis.17 Still, due to heterogenous definition of HF and lack of differentiation between its subtypes, the results of this meta-analysis cannot be an adequate benchmark for the current results.17
Similar results were provided by Habara et al., who performed TOE in a cohort of 925 vastly non-anticoagulated patients with AF in the pre-CHA2DS2-VASc era.23 LAT was found in nearly 9% of patients and was independently predicted by the presence of HF defined as symptoms in NYHA class II-IV (OR 3.1, 95% CI: 1.77–5.50, P < 0.0001).22 Of note, in this study, the definition of HF did not involve LVEF measurement and was based solely on symptoms.23
The link between more advanced LV dysfunction and presence of LAT was provided by Yarmohammadi et al. based on a cohort 2369 patients subject to TOE prior to electrical cardioversion, as LVEF ≤20% was the best predictor of LAT in multivariable model (OR 2.99, P < 0.001).24
Former report by Uziębło-Życzkowska et al. on 768 patients referred for cardioversion and/or catheter ablation indicated LVEF represents and independent predictor of LAT presence, along with bleeding and treatment with VKA.25
In a different case–control study by Wysokiński et al., patients with LAT were found to more frequently have HF defined as existence of symptoms and signs of HF with or without left ventricular systolic dysfunction within preceding 3 months (HR 5.78, P < 0.001).26
In a more recent retrospective study on 401 patients with AF (87% of patients with adequate anticoagulation) undergoing catheter ablation by Al Rawahi and coworkers, the total incidence of LAT reached 11.2%.27 Patients with thrombus on TOE had greater prevalence of HF (62.8% vs. 32.0%; P < 0.001) and significantly lower LVEF (50% vs. 58%; P = 0.013).27 HF remained an independent predictor of LAT presence (OR 2.21; 95% CI 1.04–4.71; P = 0.04), along with prior stroke and treatment with NOAC.27
Our study provides data on the prevalence of LAT in contemporary subtypes of HF, including HFrEF, HFmrEF, and HFpEF. It should be, however, noted that LAT presence constitutes only a surrogate endpoint, but it is still the main mechanism underlying systemic embolism in AF/AFl population.28 Both systolic and diastolic dysfunction of the left ventricle increase the AF-related stasis of blood in left atrium, trigger endothelial dysfunction and confer the risk of coagulation abnormalities.28 This was reflected by the decreasing LAAV with the progression of systolic dysfunction in the present study (Table 2). In case of HFpEF, the risk of thrombus formation may be increased by highly prevalent multimorbidity.11, 12 Also, the highest rate of LAT in HFrEF patients might have been caused by significantly lower rate of chronic anticoagulation in this subset of patients in comparison to HFmrEF and HFpEF, while the prevalence of transient preprocedural anticoagulation was comparable between HF subtypes (Table 2). The explanation may be partially related with higher rate of tachycardia-induced cardiomyopathy justifying more acute rhythm control strategy without pre-emptive 3 week anticoagulation.29 The key message is that both the diagnosis of HFrEF and LVEF < 40% identify patients who are fraught with increased risk of LAT despite high prevalence of adequate anticoagulation. Yet the current study concentrated on surrogate endpoint of LAT and did not evaluate the risk of stroke or systolic embolism in patients with HFrEF, which precludes routine use of TOE in this subset of considering current guidelines.16 It should be stressed that depressed LVEF seems to one of the crucial non-classical determinants of LAA thrombus presence, along with renal function and AF subtypes,30 that are not directly covered in CHA2DS2-VASc score.
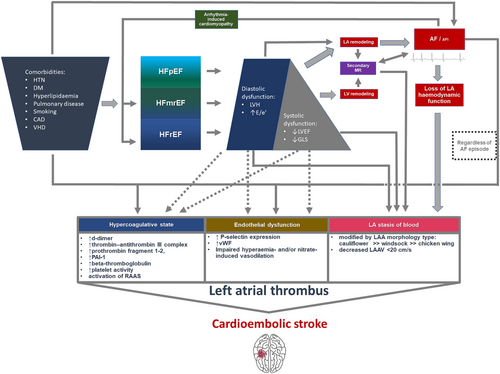
One should note, however, that all subtypes of HF are characterized by increased rate of LAT in comparison to patients without HF (Figure 1). This finding underscores the importance of the use of adequate anticoagulation regimen in AF/AFl patients with HF diagnosis regardless of LVEF according to the CHA2DS2-VASc scale.8 Yet a doubt was cast on the role of HF diagnosis as a risk factor of systemic embolism in the study by Friberg and Lund, who found that inclusive HF diagnosis did not additionally modulate the risk of stroke on top of other covariates included in CHA2DS2-VASc scale (HR 1.01, 95% CI 0.96–1.05).14 This problem is particularly valid for HFpEF population, as high prevalence of co-morbidities may account for higher risk of LAT development triggered by this conditions and more prevalent symptoms mimicking HF.11 In our study, the population of patients with HFpEF had higher CHA2DS2-VASc score than HFmrEF and HFrEF population (Table 2). Reassuring data was provided by a high-volume study by Sartipy et al.,9 which covered 41 446 patients enrolled in Swedish Heart Failure Registry. The authors documented higher prevalence of AF in HFpEF than HFrEF population (65% vs. 53%) and provided evidence for universally increased risk of stroke or transient ischaemic attack between HF strata in contrast to patients without HF (HFpEF: adjusted HR 1.15; 95% CI 1.07–1.25; HFrEF: adjusted HR 1.19, 95% CI 1.14–1.).9 In the light of conflicting data from registries, the results of our prospective analysis underscore the predictive significance of HF diagnosis in estimation of the risk of LAT, and indirectly, highlight the increased risk of stroke and justify chronic anticoagulation in this subset of patients with AF/AFl. The proposed pathophysiology of LAT formation in patients with AF/AFl combined with HF was highlighted in Figure 4.31, 32
The limitations of the present study were thoroughly described in the previous manuscript.18 The study constituted a registry and is subject to the limitation of its design. Although the study was prospective and gathered data regarding both the diagnosis of HF, subtype of HF, main echocardiographic parameters, the study variables did not include the concentrations of natriuretic peptides. Additionally, the study did not investigate into the rate of ischaemic stroke on follow-up, but only the presence of LAT. Furthermore, TOE was performed routinely in the majority of centres prior to direct current cardioversion and catheter ablation; however, six participating centres performed TOE only in subjects with suboptimal anticoagulation before the procedure. Because the study was designed in 2018,19 HF subtypes were defined based on the criteria present in the 2016 ESC Guidelines22 and do not comply with the current definition set forth in the 2021 ESC HF Guidelines.33
In conclusion, the presence of HF significantly augments the risk of LAT presence. Taking into consideration the subtype of HF, only HFrEF is associated with LAT formation despite high prevalence of adequate anticoagulation therapy. It is unknown whether the presence of LAT in patients on adequate anticoagulation translates into increased risk of stroke/systolic embolism, which merits evaluation in future randomized controlled trials.
Acknowledgements
The registry was performed using the Scientific Platform of Club 30 of Polish Cardiac Society. The authors would like to thank Dr. Elżbieta Wabich, Dr. Jan Budzianowski, Dr. Konrad Pieszko, Dr. Bogdan Musielak, Dr. Agnieszka Woronowicz-Chróściel, and Prof. Mirosław Dłużniewski for contributing to the current research.
Conflict of interest
AKC received honoraria for lectures from Bayer, Boehringer Ingelheim, Pfizer, outside the submitted work. All the other authors declared no conflict of interest.
Funding
The study received no external funding.




