Rationale and Design of the Efficacy of a Standardized Diuretic Protocol in Acute Heart Failure Study
Abstract
Aims
Although acute heart failure (AHF) with volume overload is treated with loop diuretics, their dosing and type of administration are mainly based upon expert opinion. A recent position paper from the Heart Failure Association (HFA) proposed a step-wise pharmacologic diuretic strategy to increase the diuretic response and to achieve rapid decongestion. However, no study has evaluated this protocol prospectively.
Methods and results
The Efficacy of a Standardized Diuretic Protocol in Acute Heart Failure (ENACT-HF) study is an international, multicentre, non-randomized, open-label, pragmatic study in AHF patients on chronic loop diuretic therapy, admitted to the hospital for intravenous loop diuretic therapy, aiming to enrol 500 patients. Inclusion criteria are as follows: at least one sign of volume overload (oedema, ascites, or pleural effusion), use ≥ 40 mg of furosemide or equivalent for >1 month, and a BNP > 250 ng/L or an N-terminal pro-B-type natriuretic peptide > 1000 pg/L. The study is designed in two sequential phases. During Phase 1, all centres will treat consecutive patients according to the local standard of care. In the Phase 2 of the study, all centres will implement a standardized diuretic protocol in the next cohort of consecutive patients. The protocol is based upon the recently published HFA algorithm on diuretic use and starts with intravenous administration of two times the oral home dose. It includes early assessment of diuretic response with a spot urinary sodium measurement after 2 h and urine output after 6 h. Diuretics will be tailored further based upon these measurements. The study is powered for its primary endpoint of natriuresis after 1 day and will be able to detect a 15% difference with 80% power. Secondary endpoints are natriuresis and diuresis after 2 days, change in congestion score, change in weight, in-hospital mortality, and length of hospitalization.
Conclusions
The ENACT-HF study will investigate whether a step-wise diuretic approach, based upon early assessment of urinary sodium and urine output as proposed by the HFA, is feasible and able to improve decongestion in AHF with volume overload.
Introduction
Volume overload is a hallmark symptom of heart failure (HF). Haemodynamic alterations and neurohormonal activation lead to increased renal sodium avidity resulting in salt and fluid retention.1 Ultimately, patients with acute heart failure (AHF) will present with signs and symptoms of congestion accompanied by fluid accumulation. Volume overload is not a mere symptom but can severely impair organ function and is an important treatment target.2 For more than 50 years, volume overload in AHF has been treated with loop diuretics. However, despite long-term experience, their dosing and type of administration are mainly based upon expert opinion. Current guidelines advise administering loop diuretics intravenously (IV) in AHF, using 20–40 mg of furosemide in loop diuretic naive patients and at least an equivalent to the chronic oral home dose in others (class I, level of evidence B).3 These recommendations are based on the Diuretic Strategies in Patients with Acute Decompensated Heart Failure (DOSE) trial's findings that high-dose (2.5 times the oral home dose) loop diuretics IV was not better in improving patients' global assessment of symptoms after 72 h compared with low-dose (equivalent dose to the oral home dose) IV.4 In addition, there has been conflicting evidence from registries regarding the association between high loop diuretics doses and increased mortality,5, 6 suggesting to use the lowest possible loop diuretic dose that has a significant effect. Of note, current guidelines give no specific recommendations on how the effect of loop diuretics should be evaluated, nor do they advise on treatment strategies if the diuretic response is insufficient. To overcome the current gaps in evidence, the Heart Failure Association (HFA) of the European Society of Cardiology, recently published a position paper to guide the use of diuretics in HF.7 The proposed strategy includes early IV loop diuretic administration, high dosing (one to two times the oral home dose), and early titration of the dose based upon evaluation of diuretic response using urinary sodium concentration and/or urine output as markers. However, this protocol has not been tested in clinical practice. The goal of the Efficacy of a Standardized Diuretic Protocol in Acute Heart Failure (ENACT-HF) study is to investigate whether the implementation of the HFA protocol can improve decongestion, assessed by natriuresis.
ENACT-HF study
Study design
The ENACT-HF study is an international, multicentre, non-randomized, open-label, pragmatic study in AHF patients with volume overload, designed according to a two-phased design. Objective is to enrol in at least four different continents to ensure feasibility of the protocol worldwide (Figure 1). In the first phase of the study, all participating centres treat a group of consecutive patients according to the institution's current standard of care, and the loop diuretic regime is left entirely at the discretion of the treating physician. Subsequently, the HFA protocol is implemented for the next group of consecutive patients in the second phase of the study. The study complies with the Declaration of Helsinki and has been approved by the local ethics committees of the participating centres.
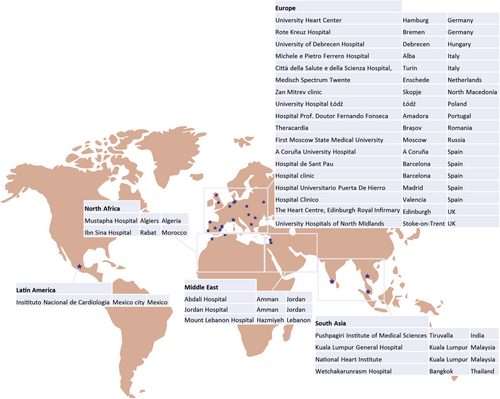
The ENACT-HF trial was powered for its primary endpoint: natriuresis after 1 day. A recent pilot trial in AHF patients on chronic loop diuretic therapy, similar to our study population, reported a 24 h natriuresis of 234 ± 133 mmol in patients receiving high-dose loop diuretics twice daily.9 For sample size calculation, a similar natriuresis after 1 day was considered in the standard of care group. As there are currently no data on the effect of a standardized diuretic protocol on natriuresis, an increase in natriuresis of 15% was deemed both achievable and clinically relevant. Considering a type I error rate α = 0.05 and a type II error rate β = 0.20 (statistical power = 80%), a sample size of 454 is defined based on a Student's t-test. Taking a drop-out or missing data of 10% into account, this renders a total study population of 500 patients.
Eligibility
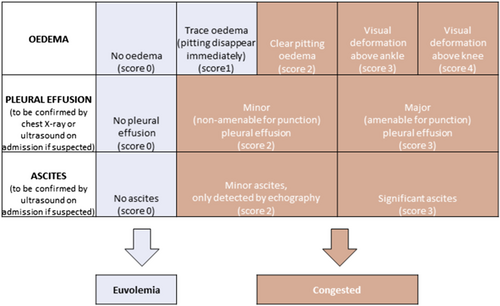
The study's inclusion and exclusion criteria are listed in Table 1. Patients are eligible for the study if they present with AHF and at least one sign of volume overload (oedema, ascites or pleural effusion) and have an N-terminal pro-B-type natriuretic peptide (NT-proBNP) > 1000 pg/mL or a BNP > 250 ng/mL. Importantly, volume status will be determined daily by the treating physician using a predefined congestion score allowing a quantitative standardization of congestion and decongestion (Figure 2). In addition, patients should be treated with loop diuretics chronically, defined at a dose of ≥ 40 mg of furosemide, ≥ 1 mg of bumetanide, or ≥ 20 mg of torsemide for ≥ 1 month prior to inclusion. Patients with cardiogenic shock, use of renal replacement therapy, or ultrafiltration or patients with (expected) use of inotropes (dopamine, dobutamine, levosimendan, milrinone, or adrenaline) are excluded. To enhance enrolment and in order to make urine collections more homogenous, it was decided that patients could only be included during working hours (8 AM to 4 PM). This ensures the presence of research personnel and allows for the collection of at least 16 h of urine during the first day.
| Inclusion criteria |
|---|
|
| Exclusion criteria |
|---|
|
- IV, intravenously; NT-proBNP, N-terminal pro-B-type natriuretic peptide.
Study intervention
The study flowchart is depicted in Figure 3. Every participating centre is assigned to include a fixed number of 10 patients in study Phase 1 (routine care) before including at least a similar number of patients in study Phase 2 (HFA protocol implementation). The set number of patients is determined for each centre specifically based upon a feasibility interview and ranges between 20 and 40 patients (10 patients in Phase 1 and 10–30 patients in Phase 2). To avoid that physicians would learn from the potential advantageous HFA protocol and change their standard of care accordingly, a centre has to finish Phase 1 first, before Phase 2 can be started.
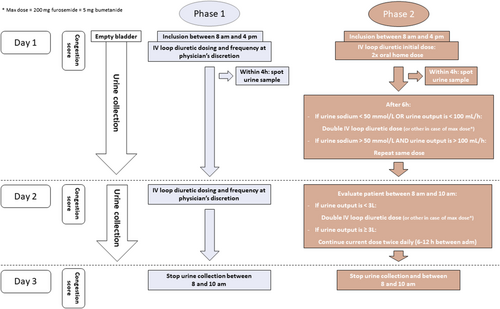
During Phase 1 of the study, the treatment with IV loop diuretics will be at the physician's discretion. All doses and frequencies are allowed, as well as the combination of different diuretic classes. In addition, continuous infusion or intermittent boluses can be used. In contrast, in study Phase 2, IV loop diuretic administration is performed according to the HFA protocol.7 Any values that are open for interpretation are specified in the amended protocol (e.g. one to two times oral home dose was defined as two times oral home dose). After inclusion, oral loop diuretics are stopped, and patients are administered two times the oral home dose IV as a bolus with a maximum of 200 mg of furosemide or equivalent (conversion factor 40 mg furosemide = 1 mg bumetanide = 20 mg torsemide). Patients need to empty their bladder before the diuretic is administered. A spot urine sample to assess urinary sodium concentration is taken within the first 4 h, preferably as closest to 2 h as possible. The diuretic response is reassessed after 6 h. The presence of both a urinary sodium > 50 mmol/L in the spot urine sample and a urine output > 100 mL/h during the first 6 h is defined as a good diuretic response and allows the physician to repeat the same loop diuretic dose IV, leading to a total of two bolus doses per day. If the diuretic response is insufficient, the loop diuretic dose should be doubled. In case the ceiling dose of loop diuretics was already reached, a thiazide should be added as the next step. On the morning of Day 2, diuretic response is again reassessed, using urine output solely. If the total urine output is ≥ 3L, diuretic response is deemed sufficient, and the same loop diuretic dose is repeated as an IV bolus twice daily. Otherwise, diuretic therapy should be escalated either by doubling the loop diuretic dose or addition of a thiazide in case the maximum loop diuretic dose has been reached. Again, the loop diuretic is administered as a bolus twice daily. If the patient is no longer volume overloaded at any time of the study treatment based upon the predefined congestion score (Figure 2), the protocol can be stopped. Physicians will be trained extensively on the details of the protocol and the congestion score. From Day 3 on, the further treatment is at the physician's discretion in both treatment arms. To standardize fluid input, sites are instructed to restrict daily oral intake of fluids and sodium to 1500 mL and 1.5 g, respectively.
Urine collection and sampling
Before administrating the first IV loop diuretic dose, patients will be asked to void empty. From then on, loop diuretics will be administered and the urine collection will start. A spot urine sample will be taken within the first 4 h either directly from the bladder catheter or from the first void if the patient does not have a bladder catheter. The first urine collection will be stopped on the morning of Day 2 between 8 and 10 AM and before the first loop diuretic administration on Day 2. A new 24 h urine collection will then be started until the morning of Day 3. Insertion of a bladder catheter is recommended but not mandatory to achieve an optimal urine collection. In case of urinary incontinence, placement is mandatory according to the study protocol. Special care should be taken to ensure that all urine is collected.
Objectives and endpoints
The primary objective of the ENACT-HF study is to test whether implementation of the HFA protocol can improve decongestion. Natriuresis after 1 day was set as the primary endpoint because (i) sodium is used as a metric for loop diuretic efficacy, (ii) the direct effect of loop diuretics is increasing renal sodium excretion, (iii) low natriuresis has been associated with poor outcomes, and (iv) it is an objective endpoint reflecting the goals of the study which is not open to bias.8 This will be assessed as the total renal sodium excretion from the first loop diuretic administration until the morning of Day 2. Secondary endpoints assessed after 2 days are changes in congestion score, cumulative natriuresis, cumulative diuresis, and change in weight (Table 2). Further, length of stay and in-hospital mortality will be additional secondary endpoints. Doubling of serum creatinine is set as a safety endpoint.
| Primary endpoint |
|---|
| Total natriuresis after 1 day |
| Secondary endpoints |
|---|
|
Change in congestion score after 2 days Cumulative natriuresis after 2 days Cumulative diuresis after 2 days Change in body weight after 2 days Length of hospital stay In-hospital mortality |
| Safety endpoint |
|---|
| Worsening renal function, defined as a doubling of serum creatinine after 2 days |
Statistical plan
All endpoints will be analysed according to the intention-to-treat principle. Given the non-randomized nature of the study, a linear mixed model with treatment arm and baseline factors influencing the endpoint as fixed effects and centre as a random effect will be used to compare the primary endpoint and all secondary endpoints that are on a continuous scale. Data will be transformed if model assumptions are not met (e.g. normality). In-hospital mortality will be evaluated with a generalized linear mixed model for a binary outcome, using treatment arm as a fixed effect and baseline differences as well as centre as a random effect. The heterogeneity of the primary endpoint will be tested on prespecified subgroups: (i) congestion score on admission > vs. < than the observed median, (ii) estimated glomerular filtration rate > vs. < than the observed median, (iii) chronic loop diuretic dose > vs. < than the observed median, (iv) sex, and (v) LVEF ≥ 50% vs. < 50%. A sensitivity analysis with multiple imputation of missing data will be performed for the primary endpoint. In a second sensitivity analysis, the primary endpoint will be assessed after normalization for the duration of the urine collection. All performed tests will be two-sided with a significance level set at P < 0.05.
Discussion
Although diuretics are the cornerstone treatment for volume overload in AHF, there has been little evidence to guide their use and how to assess treatment response. The DOSE trial specifically addressed the question whether high-dose loop diuretic therapy was beneficial compared with low-dose diuretic therapy in AHF, but also compared intermittent boluses with continuous infusion.4 There was no difference in the co-primary endpoint of patients' global assessment of symptoms and creatinine change after 72 h for either therapy, but high diuretic dosages led to more dyspnoea relief, more weight loss, and more net fluid loss. Although high-dose diuretics led to more worsening renal function, this was associated with improved outcomes.10 This finding is in concordance with other studies emphasizing that creatinine rises do not necessarily indicate injury nor do they impair outcomes in case of effective decongestion.11, 12 Importantly, only 15% of patients included in the DOSE trial were assessed to be dry by their treating physicians at the end of the treatment phase which likely explains why more than 50% of the patients were either readmitted or deceased after 3 months of follow-up.4
The Cardiorenal Rescue Study in Acute Decompensated Heart Failure trial investigated whether a step-wise pharmacologic diuretic strategy based upon the diuretic response resulted in better decongestion in comparison with ultrafiltration.13 In the diuretic treatment arm, patients were treated with a step-wise loop diuretic dosing protocol using continuous infusions adapted to the 24 h urine output with a of goal 3–5 L per day. In case the maximal loop diuretic dose was achieved, but response was still insufficient, a thiazide was added. After 96 h, protocolized diuretic treatment resulted in a similar weight loss as compared with ultrafiltration. In a post-hoc analysis, the step-wise diuretic treatment arm resulted in more weight loss, more net fluid loss, and slight improvement in renal function compared with standard diuretic therapy.14 Furthermore, a small single-centre study also suggested that a urine-output-guided diuretic protocol could improve decongestion and lower 30 day readmissions.15
The direct effect of loop diuretics is reducing sodium reabsorption at the loop of Henle and thus increasing natriuresis and diuresis. Weight loss and urine output are among the most used parameters to assess diuretic response. However, there is rather poor correlation between both measures,16 and neither weight loss nor fluid loss has been associated with improved outcomes.17, 18 In recent years, there has been increasing interest in urinary sodium as a new and more direct measure of diuretic response. Observational data indicate that a low urinary sodium concentration after loop diuretic administration is associated with poor outcomes.19-21 In addition, sodium loss is a better prognosticator than fluid loss.18
Based upon the aforementioned data, HFA recently designed a step-wise pharmacologic diuretic protocol,7 which will be tested in the ENACT-HF study. The pragmatic trial will prospectively test if early evaluation of the diuretic and natriuretic response is warranted and allows for the early intensification of diuretic therapy. It will determine if analysis of urinary spot sodium content within the first hours of diuretic administration is feasible, and if this will help the clinician to interpret diuretic response, thereby generating the opportunity for early intervention if sodium content is low. The strategy that will be tested in ENACT is important in several aspects. Firstly, persisting congestion further compromises organ function. Secondly, the plasma refill rate at which fluid is mobilized from the interstitial space into the plasma compartment might drop during decongestion. Thirdly, patients are often hospitalized in acute care units for the first days, where intensive adaptation of therapy is more likely to occur than in a regular ward. Last, faster decongestion might be especially valuable in health care systems where length of hospital stay needs to be short.
The ENACT-HF study is subject to certain limitations. Most importantly, it's non-randomized open-label design raise the possibility of investigator's altering their standard of care treatment to influence the endpoint. However, as stated before, the serial study design makes it impossible for investigators to compare their standard of care with the protocol, as they first have to finish Phase 1 (standard of care) before they can proceed to the standardized diuretic protocol. Investigators were also clearly instructed not to change their current practice. In addition, randomization could also create bias as investigators could apply methods they learned from the protocol in their own standard of care practice (e.g. using higher diuretic boluses). Second, as there is no blinding towards the urinary sodium measurements, it is possible that investigators could use this information to take therapeutic decisions during Phase 1. Last, because of lack of randomization, there is a possibility of unbalanced patient groups. This will be accounted for during statistical analysis but is never equivalent to randomization.
Conclusions
The ENACT-HF study will investigate whether a step-wise pharmacologic diuretic protocol as proposed by the HFA can increase natriuresis after 1 day in AHF patients on chronic loop diuretic therapy in a safe and efficacious manner across a wide spectrum of healthcare settings across the globe.
Conflict of interest
Marta Cobo-Marcos received honorary for advisory boards and lectures from Vifor, Novartis, Astra-Zeneca, and Boehringer-Ingelheim. Diane Barker received speaker fees from Astra Zeneca, Novartis, and Medtronic. Jeroen Dauw, Malgorzata Lelonek, Isabel Zegri-Reiriz, Cynthia P. Paredes-Paucar, Cornelia Zara, Varghese George, Dorit Knappe, Dmitry Shchekochikhin, Annop Lekhakul, Milka Klincheva, Simone Frea, Òscar Miró, Attila Borbély, Samer Nasr, Nawal Doghmi, Rafael de la Espriella, Jagdeep S. Singh, Virginia Bovolo, Inês Fialho, Noel T Ross, Mieke van den Heuvel, Riad Benkouar, Hajo Findeisen, Imad A. Alhaddad, Kais Al Balbissi, Gonzalo Barge-Caballero, Azmee M Ghazi, Liesbeth Bruckers, Pieter Martens, and Wilfried Mullens have nothing to disclose.
Funding
Jeroen Dauw and Wilfried Mullens are researchers for the Limburg Clinical Research Center (LCRC) UHasselt-ZOL-Jessa, supported by the foundation Limburg Sterk Merk (LSM), province of Limburg, Flemish government, Hasselt University, Ziekenhuis Oost-Limburg, and Jessa Hospital.




