Relation of left atrial function with exercise capacity and muscle endurance in patients with heart failure
Abstract
Aims
Both left atrial strain (LAS) and skeletal muscle endurance demonstrate a linear relationship to peak VO2. Less is known about the relationship between central (cardiac) and peripheral (muscle endurance) limitations of exercise capacity in patients with heart failure (HF). We investigated this relationship using novel cardiac markers such as LAS and left atrial emptying fraction (LAEF).
Methods and results
We analysed echocardiographic measurements, cardiopulmonary exercise testing (CPET), and isokinetic muscle function in 55 subjects with HF and controls [17 heart failure with preserved ejection fraction (HFpEF), 18 heart failure with reduced ejection fraction (HFrEF), and 20 healthy controls]. Patients with reduced LAEF showed reduced peak VO2: 14.3 ± 3.5 vs. 18.5 ± 3.5 mL/min/kg, P = 0.003, and reduced muscle endurance (RME): 64.3 ± 23.9 vs. 88.5 ± 32.3 Nm/kg, P = 0.028. Patients with reduced LAS showed similar results. Neither left ventricular global longitudinal strain (LVGLS) nor left atrial volume index (LAVI) was associated with RME. The area under the curve of LAS and LAEF in patients with HF in association with RME were (0.76 vs. 0.80) with 95% confidence interval (CI) (0.59–0.96, P = 0.012 vs. 0.63–0.98, P = 0.006, respectively). In a multiple linear regression, LAEF and working load measured during CPET (watt) were independent factors for RME after adjusting for age, LVGLS, and 6 min walk test (6MWT) [LAEF (B: 0.09, 95% CI: 1.01; 1.18, P = 0.024), working load (B: 0.05, 95% CI: 1.01; 1.08, P = 0.006)]. Peak torque of the left leg was associated with E/LAS (E: early diastolic) in patients with HFpEF (r = −0.6, P = 0.020). Endurance of the left leg was associated with LAEF (r = 0.79, P = 0.001) in patients with HFrEF.
Conclusions
LAS/LAEF are potential cardiac markers in demonstrating the link between cardiac and peripheral limitations of exercise capacity. Thus, integrating LAS/LAEF in the evaluation of exercise intolerance in patients with HF could be useful.
Introduction
Patients with heart failure with preserved (HFpEF) and reduced (HFrEF) ejection fraction present mainly with dyspnoea and reduced exercise capacity.1 These manifestations could be explained with central (cardiac) or peripheral (skeletal muscle) factors. One of the suggested mechanisms is elevated left ventricular (LV) filling pressure.2 Several studies showed that left atrial strain (LAS) measured using two-dimensional speckle tracking echocardiography (2D-STE) is a surrogate of elevated LV filling pressure.3-5 Recently, left atrial (LA) function has gained attention due to the pivotal role of the left atrium in the resting and exercising cardiovascular system. Studies found a linear relationship between LA function and maximal oxygen uptake (peak VO2) during cardiopulmonary exercise testing (CPET) in different disease states such as HFpEF, diabetes mellitus, ischaemic, and dilated cardiomyopathies.6-10
Similarly, reduced exercise capacity measured as reduced peak VO2 has been shown in several studies to be linked to peripheral factors such as skeletal muscle dysfunction both in patients with HFpEF and HFrEF.11-13 In fact, about 20% of patients with HF suffer from skeletal muscle wasting, which is associated with reduced exercise and functional capacity (peak VO2).11, 12 We found recently that molecular, mitochondrial, and metabolic abnormalities in skeletal muscle in patients with HFpEF and HFrEF were associated clinically with reduced exercise capacity and reduced muscle function.14
As mentioned earlier, reduced peak VO2 is associated with central and peripheral limitations. However, no direct relationship between central and peripheral factors could be proven yet. On the contrary, exercise training was shown to improve exercise tolerance in patients with HF independent of improving cardiac function measured with left ventricular ejection fraction (LVEF).15
One of the possible explanations for the failure in demonstrating a link between central and peripheral limitations of exercise capacity is likely not using more sensitive and novel cardiac measurement such as left atrial emptying fraction (LAEF) and LAS.
We hypothesized, as the result to the multi-organ involvement in HF syndrome, an association between central and peripheral factors involved in the reduced exercise capacity in HF and searched for a sensitive cardiac parameter to demonstrate this relationship. Therefore, we investigated the association between LAS, LAEF, and left ventricular global longitudinal strain (LVGLS), on one hand, and muscle endurance as surrogate of skeletal muscle function in patients with HFpEF, HFrEF, and healthy controls (HC), on the other hand. We hypothesized that central novel parameters (LAEF and LAS) are capable to detect the peripheral limiting factors (reduced skeletal muscle function).
Methods
Study population
Heart failure patients were recruited from the HF outpatient clinic at the University Hospital Jena from September 2016 until June 2017. Age-matched HC were recruited from the general healthy population in Jena, Germany.
Heart failure inclusion criteria
Clinically stable men and women's outpatients with age > 55 years both with HFpEF and HFrEF (LVEF < 40%) with New York Heart Association (NYHA) class II or III were recruited. Definitions of HFpEF and HFrEF were according the European Society of Cardiology-HF (ESC-HF) 2016. LVEF cut-off for HFpEF was 50%, BNP > 35 pg/mL. In addition, one of the following criteria was met: relevant structural heart disease (LV hypertrophy and/or LA enlargement) or diastolic dysfunction as defined in the ESC-HF 2016.16 Patients were on standard and stable HF medication for the last 3 months.
Heart failure exclusion criteria
Patients with major cardiovascular event or procedure in the last 6 weeks, or patients with HF secondary to severe uncorrected valvular disease as well as patients with uncontrolled diabetes mellitus, progressive renal dysfunction (glomerular filtration rate < 60 mL/min), and those with primary muscle disorder, for example, muscular dystrophies, were excluded.
Definition of heart failure comorbidities
Arterial hypertension was defined as blood pressure > 140/90 mmHg and/or receiving antihypertensive medication. Diabetes mellitus was defined as HbA1C ≥ 6.5% and/or taking oral hypoglycaemic agents and/or receiving insulin injection. Atrial fibrillation was diagnosed based on electrocardiogram (ECG) or on the patient's medical records. Acute myocardial infarction was defined by a history of an acute presentation with acute chest discomfort described as pain, pressure, tightness, and burning or with chest pain-equivalent symptoms such as dyspnoea, epigastric pain, and pain in the left arm and either with ST-segment elevation in ECG or non-ST-segment elevation but with increase or decrease in sensitive troponin.17
Control subjects
Healthy controls with a history of cardiovascular disease or other diseases except arterial hypertension and diabetes mellitus were excluded.
Study protocol
All subjects (HFpEF, HFrEF, and HC) underwent a standardized series of assessments over two visits.
Dual-energy X-ray absorptiometry scan
We performed a whole-body dual-energy X-ray absorptiometry (DEXA) scan in order to characterize the different compartments of soft tissue in the body. DEXA scan uses a low radiation dosage and is an established method to characterize the body composition in patients with advanced HF.18 We used the lean mass of the extremities.
Muscle strength and endurance
- Maximum muscle strength
- Muscle strength endurance
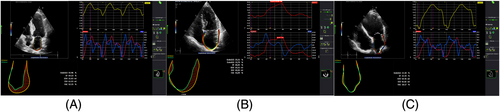
Analysis of left atrial function and strain using two-dimensional speckle tracking
Left atrial strain, LAEF, and left atrial fractional area change (LAFAC) were measured in all patients and HC both in four-chamber and two-chamber dedicated views by a blinded reviewer. LVGLS was presented as the average of the measurements in two-chamber, three-chamber, and four-chamber views. Image quality in six patients was reduced. Therefore, we did not include these patients in the final analysis. LAS was analysed using as zero point the R wave of the ECG. The recordings were processed using an acoustic-tracking dedicated software (Image-Arena™ Version 4.6; TomTec Imaging Systems, Unterschleissheim, Germany), which allowed for an off-line semi-automated analysis of speckle-based strain. All data were analysed by one observer (S. I.).
Left atrial strain represented the average from the peak positive longitudinal strain curve from all LA segments in four-chamber and two-chamber views. We performed our measurement as recommended from the European Association of Cardiovascular Imaging (EACVI).21 Normal values of LAS and LAEF were measured in a large European cohort and were published recently.22 Accordingly, an abnormal LAS was defined as LAS < 26% and an abnormal LAEF was defined as LAEF < 48%. To show the profile of patients with reduced LAEF (< mean value of the cohort = 34.5%), we divided the cohort of patients into two groups according to the mean value of LAEF.
Cardiopulmonary exercise testing
The non-invasive measurement of ventilatory gas exchange during exercise is the main principle of CPET. This involves the acquisition of expired ventilation and concentrations of oxygen (O2) and carbon dioxide (CO2) during exercise. Exercise testing in association with air–gas exchange using breath-by-breath method of analysis is considered to be an optimal gauge of functional capacity. We performed CPET in all participants using incremental biking exercise on an electronically braked cycle ergometer as described elsewhere.23, 24 CPET was performed on a bicycle ergometer. Ramp protocol (15 W/min) was used. The results were read by an experienced physician in CPET.
Six-minute walk test
The 6 min walk test (6MWT) is a commonly used clinical tool, which allows testing of the functional capacity in HF patients in a ‘real-life’ setting. Using standard methodology,25 patients were asked to walk as fast as possible on a 25 m course for 6 min. The test was scored in rounded metres walked in 6 min.
All subjects provided written informed consent at enrolment, and the protocol was approved by the responsible ethical review boards and fulfilled all principles of the Declaration of Helsinki. The study was funded by a grant from the interdisciplinary centre for clinical research at the University Hospital in Jena.
Statistical analysis
All data and statistics are reported as mean ± standard deviation (n ± SD) for continuous data. Categorical data were summarized by percentages. The χ2 test was used to compare categorical variables, and the Kruskal–Wallis test was applied for not normally distributed data. Analysis of variance (ANOVA) was used as appropriate. Variables perceived as clinically important and those with P < 0.2 in univariate analyses were included in a multivariable regression model. Final model selection was based on stepwise regression. A two-tailed P-value < 0.05 indicates statistical significance. The Statistical Package for Social Sciences software (SPSS 26, IBM, Armonk, USA) was used for statistical analysis.
Results
We recruited 62 patients and HC. Altogether, 55 subjects fulfilled our inclusion and exclusion criteria and were included in this study: 17 HFpEF patients, 18 HFrEF patients, and 20 HC. Seven patients, who did not fulfil the criteria for HFpEF, were excluded.
Basic characteristics of HF patients and HC are summarized in Table 1. 2D echocardiographic data are shown in Table 2. Patients with HFpEF and HFrEF showed reduced LAEF compared with HC (LAEF: 42.9 ± 14.0 vs. 28.0 ± 11.4 vs. 52.2 ± 10.7%, P < 0.001) (Table 2 and Figure 1).
| HFpEF N = 17 | HFrEF N = 18 | HC N = 20 | P-value | |
|---|---|---|---|---|
| Age (years) | 71 ± 6 | 68 ± 9 | 66 ± 7 | 0.17 |
| Sex (m/f) f% | 8/9 (53%)§ | 15/3 (17%)# | 7/13 (65%) | 0.009 |
| BMI (kg/m2) | 28.7 ± 4.6 | 27.9 ± 5.3 | 26.4 ± 4.2 | 0.18 |
| NYHA (II/III) % | (76.5/23.5)* | (83.3/16.7)# | (0/0) | <0.001 |
| GFR (mL/min) | 71.8 ± 13.9 | 72.2 ± 22.6 | 82.4 ± 14.1 | 0.11 |
| BNP (pg/mL) | 168 ± 167*,§ | 374 ± 290# | 45.7 ± 35.5 | <0.001 |
| AMI % | 5 (29%)* | 6 (33%)# | 0 (0%) | 0.019 |
| Hypertension % | 15 (88%)* | 15 (83%)# | 10 (50%) | 0.016 |
| Diabetes mellitus % | 6 (35%) | 7 (39%)# | 2 (10%) | 0.091 |
| Atrial fibrillation % | 9 (53%)* | 5 (28%) | 1 (5%) | 0.005 |
| ASS % | 7 (29%)* | 5 (39%)# | 2 (10%) | 0.004 |
| Oral anticoagulation% | 9 (53%)* | 7 (39%) | 2 (10%) | 0.017 |
| Beta-blocker % | 13 (77%)* | 17 (94%)# | 4 (20%) | <0.001 |
| ACEI/ARB/neprilysin inhibitor % | 12 (71%)*,§ | 18 (100%)# | 8 (40%) | <0.001 |
| Aldosterone antagonist % | 3 (18%)§ | 12 (67%)# | 0 (0%) | <0.001 |
| Diuretics % | 9 (53%)§ | 16 (89%)# | 6 (30%) | <0.001 |
| Statins % | 11 (65%)* | 13 (72%)# | 2 (10%) | <0.001 |
| Oral antidiabetic therapy % | 5 (29%)* | 4 (22%) | 0 (0%) | 0.039 |
- ACEI/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; AMI, acute myocardial infarction; ASS, aspirin; BMI, body mass index; BNP, brain natriuretic peptide; GFR, glomerular filtration rate; HC, healthy controls; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; NYHA, New York Heart Association.
- * P < 0.05 in comparison between HFpEF and HC.
- # P < 0.05 in comparison between HFrEF and HC.
- § P < 0.05 in comparison between HFpEF and HFrEF.
| HFpEF N = 17 | HFrEF N = 13 | HC N = 19 | P-value | |
|---|---|---|---|---|
| IVSD (mm) | 13.8 ± 1.7*,§ | 11.2 ± 2.9 | 11.3 ± 2.1 | 0.001 |
| PWD (mm) | 12.0 ± 1.7* | 11.5 ± 2.3# | 9.9 ± 1.6 | 0.003 |
| LVED (mm) | 47.7 ± 6.5*,§ | 61.7 ± 8.5# | 42.7 ± 4.2 | <0.001 |
| LVEDVI (mL/m2) | 24.9 ± 3.2§ | 28.6 ± 7.0# | 23.3 ± 2.6 | 0.004 |
| LVESVI (mL/m2) | 16.8 ± 3.6§ | 25.8 ± 4.8# | 14.6 ± 2.9 | <0.001 |
| LV mass (g) | 295 ± 79.5* | 341 ± 126# | 179 ± 46.7 | <0.001 |
| LVMI (g/m2) | 152 ± 30.8* | 165 ± 53.2# | 97.3 ± 22.7 | <0.001 |
| LVEF % | 59.7 ± 10.2§ | 28.4 ± 5.9# | 61.9 ± 6.0 | <0.001 |
| LVGLS (average) % | −18.9 ± 5.5§ | −6.8 ± 2.9# | −19.8 ± 3.3 | <0.001 |
| TAPSE (mm) | 21.3 ± 4.5 | 18.3 ± 4.6# | 23.2 ± 3.2 | <0.01 |
| E/A | 1.4 ± 0.7 | 1.1 ± 0.7 | 1.0 ± 0.2 | 0.3 |
| E′ (average) (cm) | 0.06 ± 0.01*,§ | 0.05 ± 0.02# | 0.08 ± 0.02 | <0.001 |
| E/E′ | 12.8 ± 3.2§ | 18.1 ± 7.5# | 10.0 ± 3.1 | 0.001 |
| LAVI (mL/m2) | 34.1 ± 7.1*,§ | 44.9 ± 19.0# | 17.2 ± 8.3 | <0.001 |
| LAS (average) % | 26.0 ± 19.1§ | 11.8 ± 5.8# | 31.3 ± 12.0 | 0.001 |
| LAEF (average) % | 42.9 ± 14.0*,§ | 28.0 ± 11.4# | 52.2 ± 10.7 | <0.001 |
| LAFAC (average) | 31.6 ± 11.2*,§ | 19.7 ± 8.1# | 39.9 ± 9.1 | <0.001 |
| LAEDV (average) | 70.4 ± 84.7* | 89.5 ± 41.6# | 25.2 ± 11.9 | 0.005 |
| LAESV (average) | 87.6 ± 25.2*,§ | 123 ± 46.2# | 51.6 ± 18.3 | <0.001 |
- HC, healthy controls; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IVSD, interventricular septum thickness at end-diastole; LAEDV, left atrial end-diastolic volume; LAEF, left atrial emptying fraction; LAESV, left atrial end-systolic volume; LAFAC, left atrial fractional area change; LAS, left atrial strain; LAVI, left atrial volume index; LVED, left ventricular diameter at end-diastole; LVEDVI, left ventricular volume index at end-diastole; LVEF, left ventricular ejection fraction; LVESVI, left ventricular volume index at end-systole; LVGLS, left ventricular global longitudinal strain; LVMI, left ventricular mass index; PWD, posterior wall thickness at end-diastole; TAPSE, tricuspid annular plane systolic excursion.
- * P < 0.05 in comparison between HFpEF and HC.
- # P < 0.05 in comparison between HFrEF and HC.
- § P < 0.05 in comparison between HFpEF and HFrEF.
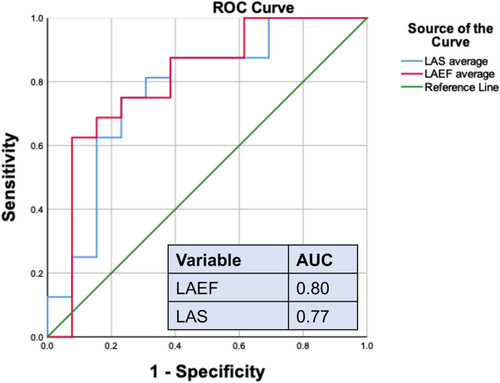
We analysed the area under the curve (AUC) of LAS and LAEF in patients with HF in association with reduced muscle endurance (RME) and found AUC (0.76 vs. 0.80) with 95% confidence interval (CI) (0.59–0.96, P = 0.012 vs. 0.63–0.98, P = 0.006, respectively) (Figure 2).
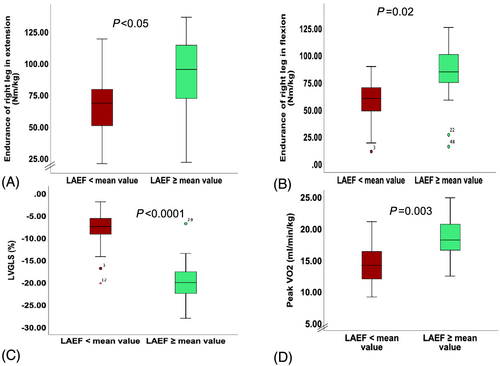
To show the profile of patients with reduced LAEF (< mean value of the cohort = 34.5%), we divided the cohort of patients into two groups according to the mean value of LAEF. Accordingly, patients with reduced LAEF had reduced exercise capacity (peak VO2: 14.3 + 3.5 vs. 18.5 ± 3.5 mL/min/kg, P = 0.003, VE/VCO2: 39.3 ± 8.7 vs. 31.8 ± 4.9, P = 0.007) and reduced muscle function measured as peak torque and endurance (all P < 0.05) (Table 3). The atrioventricular coupling was reflected in reduced LVGLS in patients with reduced LAEF (−8.5 ± 5 vs. −19.6 ± 5.3%, P < 0.0001) (Figure 3). Considering the recommended cut-off value of 48% for LAEF, we divided the cohort into two groups (21 patients with reduced LAEF vs. 9 patients with preserved LAEF) and found that two-thirds of patients with RME of left leg in extension had reduced LAEF vs. one-third without reduced LAEF [14/21 (66.7%) vs. 7/21 (33.3%), P = 0.03]. We were able to show similar results by dividing the cohort according to the mean value of LAS (Table 4).
| LAEF < 36.5% (mean value) | LAEF ≥ 36.5% (mean value) | P-value | |
|---|---|---|---|
| N = 15 | N = 15 | ||
| Age (years) | 73 ± 7 | 69 ± 7 | 0.1 |
| Sex (m/f) f % | (10/5) 33.3 | (9/6) 40.0 | 1.00 |
| LVGLS % | −8.5 ± 5.0 | −19.6 ± 5.3 | <0.0001 |
| Peak VO2 (mL/min/kg) | 14.3 + 3.5 | 18.5 ± 3.5 | 0.003 |
| VE/VCO2 | 39.3 ± 8.7 | 31.8 ± 4.9 | 0.007 |
| Blood pressure at rest (mmHg) | 106 ± 17 | 123 ± 12 | 0.002 |
| Blood pressure at peak VO2 (mmHg) | 133 ± 34 | 184 ± 37 | 0.007 |
| BNP (pg/mL) | 435 ± 313 | 124 ± 64.2 | 0.001 |
| GFR (mL/min) | 61.1 ± 20.4 | 77.1 ± 11.0 | 0.012 |
| Peak torque of left leg in flexion (Nm/kg) | 6.0 ± 2.1 | 7.6 ± 2.2 | 0.038 |
| Peak torque of right leg in extension (Nm/kg) | 9.2 ± 2.3 | 11.0 ± 2.7 | 0.055 |
| Muscle endurance of the right leg in extension (Nm/kg) | 67.2 ± 24.9 | 90.4 ± 34.8 | 0.045 |
| Muscle endurance of the right leg in flexion (Nm/kg) | 57.1 ± 22.1 | 81.8 ± 29.3 | 0.015 |
| Muscle endurance of the left leg in extension (Nm/kg) | 64.3 ± 23.9 | 88.5 ± 32.3 | 0.028 |
| Muscle endurance of the left leg in flexion (Nm/kg) | 56.0 ± 23.1 | 76.1 ± 28.3 | 0.045 |
- BNP, brain natriuretic peptide; GFR, glomerular filtration rate; LAEF, left atrial emptying fraction; LVGLS, left ventricular global longitudinal strain; PVO2, maximal oxygen uptake; VE/VCO2, ventilatory efficiency slope.
| LAS < 19.8% (mean value) | LAS ≥ 19.8% (mean value) | P-value | |
|---|---|---|---|
| N = 17 | N = 13 | ||
| Age (years) | 72 ± 7 | 69 ± 7 | 0.4 |
| Sex (m/f) f % | (11/6) 35.3 | (8/5) 38.5 | 1.00 |
| LVGLS % | −9.0 ± 5.3 | −19.9 ± 5.3 | <0.0001 |
| Peak VO2 (mL/min/kg) | 14.7 ± 3.6 | 18.6 ± 3.6 | 0.006 |
| Blood pressure at rest (mmHg) | 108 ± 19 | 123 ± 8 | 0.012 |
| Blood pressure at peak VO2 (mmHg) | 143 ± 46 | 179 ± 31 | 0.022 |
| BNP (pg/mL) | 386 ± 309 | 133 ± 105 | 0.009 |
| GFR (mL/min) | 65.4 ± 21.9 | 74.01 ± 10.1 | 0.20 |
| Reduced muscle endurance of the right leg in extension (Nm/kg) (yes/no) % | 12/17 (70.6) | 5/13 (38.5) | 0.028 |
- BNP, brain natriuretic peptide; GFR, glomerular filtration rate; LAS, left atrial strain; LVGLS, left ventricular global longitudinal strain; PVO2, maximal oxygen uptake.
In a multiple linear regression, LAEF and working load measured during CPET (watt) were independent factors for RME after adjusting for age, LVGLS, and 6MWT [LAEF (B: 0.09, 95% CI: 1.01; 1.18, P = 0.024), exercise capacity (watt) (B: 0.05, 95% CI: 1.01; 1.08, P = 0.006)] (Table 5).
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| B | 95% CI | P-value | B | 95% CI | P-value | |
| Age | 0.07 | 0.87–1.01 | 0.08 | 0.09 | 0.95–1.29 | 0.21 |
| Sex (male/female) | 0.34 | 0.45–4.13 | 0.54 | |||
| LVGLS | −0.10 | 0.82–0.99 | 0.041 | 0.05 | 0.88–1.26 | 0.62 |
| LAEF | 0.08 | 1.03–1.14 | 0.004 | 0.09 | 1.01–1.18 | 0.024 |
| Working load (W) | 0.04 | 1.01–1.06 | 0.002 | 0.05 | 1.01–1.08 | 0.006 |
| 6MWT | 0.01 | 1.00–1.02 | 0.008 | 0.01 | 0.99–1.02 | 0.21 |
| LVEF | 0.03 | 0.99–1.07 | 0.08 | |||
| LAVI | −0.02 | 0.94–1.01 | 0.20 | |||
| LAS | 0.09 | 1.03–1.17 | 0.004 | |||
- 6MWT, 6 min walk test; CI, confidence interval; LAEF, left atrial emptying fraction; LAS, left atrial strain; LAVI, left atrial volume index; LVEF, left ventricular ejection fraction; LVGLS, left ventricular global longitudinal strain.
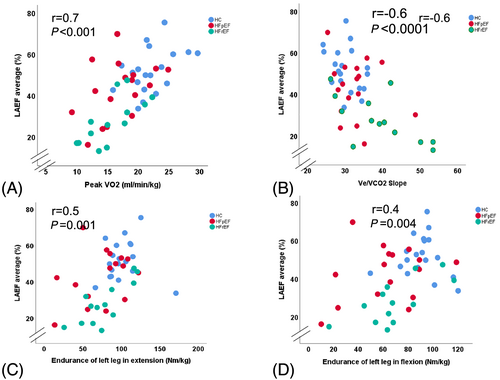
In a simple regression analysis in the whole cohort, LAEF and LAS were associated with LVGLS (r = 0.7, P < 0.0001; r = 0.6, P < 0.0001), as well as with peak VO2 (r = 0.7, P < 0.0001; r = 0.5, P < 0.0001), and with VE/VCO2 (r = −0.6, P < 0.001; r = 0.4, P = 0.004), respectively. LAEF was similarly associated with muscle endurance (Figure 4). LVGLS and left atrial volume index (LAVI) were not associated with muscle function (P = 0.13, P = 0.20, respectively). Peak torque of the left leg in extension was associated with E/LAS in patients with HFpEF (r = −0.6, P = 0.020). LAEF and LAS were associated with endurance of left leg in extension and with peak VO2 in HFrEF patients (Tables 6A–6C).
| BNP | Muscle endurance | Peak VO2 | ||||
|---|---|---|---|---|---|---|
| P-value | r-value | P-value | r-value | P-value | r-value | |
| LAVI | 0.007 | 0.4 | 0.20 | 0.16 | <0.0001 | 0.52 |
| LAEF | <0.0001 | 0.6 | 0.001 | 0.46 | <0.0001 | 0.65 |
| LAS | 0.002 | 0.4 | 0.002 | 0.43 | <0.0001 | 0.53 |
| LVGLS | <0.0001 | 0.5 | 0.13 | 0.22 | <0.0001 | 0.49 |
- P-values < 0.05 and their associated r-values were marked in bold.
| BNP | Muscle endurance | Peak VO2 | ||||
|---|---|---|---|---|---|---|
| P-value | r-value | P-value | r-value | P-value | r-value | |
| LAVI | 0.46 | 0.19 | 0.05 | 0.49 | 0.51 | 0.17 |
| LAEF | 0.05 | 0.48 | 0.18 | 0.35 | 0.09 | 0.42 |
| LAS | 0.19 | 0.33 | 0.09 | 0.44 | 0.045 | 0.49 |
| LVGLS | 0.68 | 0.11 | 0.64 | 0.13 | 0.06 | 0.46 |
- P-values < 0.05 and their associated r-values were marked in bold.
| BNP | Muscle endurance | Peak VO2 | ||||
|---|---|---|---|---|---|---|
| P-value | r-value | P-value | r-value | P-value | r-value | |
| LAVI | 0.99 | 0.01 | 0.99 | 0.01 | 0.46 | 0.19 |
| LAEF | 0.18 | 0.44 | 0.001 | 0.79 | 0.01 | 0.67 |
| LAS | 0.35 | 0.32 | 0.02 | 0.64 | 0.02 | 0.62 |
| LVGLS | 0.81 | 0.01 | 0.39 | 0.27 | 0.64 | 0.15 |
- BNP, brain natriuretic peptide; LAEF, left atrial emptying fraction; LAS, left atrial strain; LAVI, left atrial volume index; LVGLS, left ventricular global longitudinal strain.
- P-values < 0.05 and their associated r-values were marked in bold.
Measurements of LAS (LAVI: P = 0.01, r = 0.36; E/e′: P = 0.03, r = 0.34) and LAEF (LAVI: P < 0.0001, r = 0.49; E/e′: P = 0.003, r = 0.50) correlated significantly to traditional echocardiographic measurements such as LAVI and E/e′.
In a sub-analysis, by excluding patients with atrial fibrillation (a. fib), the results of our study remained unchanged. However, by focusing on patients with a. fib (excluding sinus rhythm), we found occasionally significant correlations between LAEF and peak VO2 (r = 0.57, P = 0.03) and between LAEF and LVGLS (r = 0.79, P = 0.001). All other results turned insignificant.
Discussion
To our knowledge, this is the first study that shows a link between central (LAEF and LAS) and peripheral factors (skeletal muscle function) involved in the pathophysiology of reduced exercise capacity in patients with HF. We found that patients with HFpEF and HFrEF have reduced LAEF and LAS compared with HC. Furthermore, we showed a high AUC of both LAEF and LAS in association with RME. In a multiple linear regression, LAEF and working load measured during CPET (watt) were independent factors for predicting RME after adjusting for age, LVGLS, and 6MWT.
To describe the profile of patients with reduced LAEF, we divided the cohort into two groups according to the mean value of LAEF and found that patients with reduced LAEF have reduced exercise capacity measured as peak VO2 and elevated VE/VCO2, as well as reduced muscle function measured as peak torque and muscle endurance of legs both in flexion and in extension. Similar results were shown by dividing the cohort according to the mean value of LAS. In other words, we showed for the first time a relationship between central and peripheral limitations of exercise capacity in patients with HF both with HFpEF and with HFrEF. Neither LVGLS nor LAVI was as sensitive and did not show any relation to muscle endurance.
Peak torque of the left leg in patients with HFpEF was inversely associated with E/LAS. The elevated novel LA filling index (E/LAS ratio) was recently shown to be an effective and useful parameter to determine the elevated LV filling pressure in patients with HFpEF.26 Accordingly, our findings show that HFpEF patients with elevated LV filling pressure (elevated E/LAS ratio) correlated with reduced muscle strength of legs.
Patients with HF suffer mainly from dyspnoea and reduced exercise capacity measured in the CPET as reduced peak VO2.1, 27, 28 The pathophysiology beyond dyspnoea and exercise intolerance in patients with HF is multifactorial and includes both central (cardiac) and peripheral (skeletal muscle) factors.11, 29, 30 A link between cardiac and muscular function contributing to the reduced peak VO2 in HF, as a result to the systemic involvement of HF, is expected. In other words, we hypothesized that central novel parameters (LAEF and LAS) are capable to detect the peripheral limiting factors (reduced skeletal muscle function).
The role of peripheral factors such as skeletal muscle mass and function in explaining the reduced exercise capacity in patients with HF has been shown in several studies.11, 12, 14
Centrally, elevated filling pressure of the left ventricle was suggested as an important mechanism in explaining dyspnoea and reduced exercise capacity.1, 29 LAS is a surrogate of elevated LV filling pressure and an indicator of cardiovascular performance through regulating pulmonary venous return and LV filling.5, 31 Recent studies and guidelines have defined the normal values of LAEF (>48%) and LAS (>26%).3, 21
Additionally, recent studies showed a link between LA function measured by 2D-STE and reduced exercise capacity with CPET.6, 8, 32 The latest relationship could be explained by the anatomical location and function of the LA. The LA functions as a reservoir during systole, conduit during early diastole, and a blood pump in the late diastole.33 The harmony of all of these three phases is very important to keep the atrioventricular coupling intact during exercise and therefore maintaining the best possible cardiac output and exercise capacity. One of the adaptive mechanisms of the LA to maintain the atrioventricular coupling is to increase LA volume through LA dilation and keeping as a result the LV filing pressure optimally as low as possible,34 which leads finally to increase LA volume and reduce LA function. HF guidelines recommend the evaluation of LAVI.16 However, the relationship between LA function using 2D-STE and exercise capacity (peak VO2) is stronger than LAVI.8 Furthermore, LA dysfunction was documented in patients with hypertension or diabetes even with normal LA size.35 Recently, LA function has also been shown to be an independent predictor for HF hospitalization.36 Frydas et al. found recently in an analysis in 300 patients with HF that LAS is more sensitive than LAVI in detecting LA impairment in HF and that LAS is superior to LAEF, LAVI, or E/e′ in predicting the presence elevated LV filling pressures. Furthermore, the diagnostic value of LAS was independent from LVEF.37
In spite of the strong correlation shown in our results between LVGLS and LAS/LAEF, LVGLS failed to predict the reduced exercise capacity measured evaluated both as peak VO2 and as RME. Lundberg et al. found in a simultaneous echocardiography and invasive haemodynamic measurements that LAS correlates with pulmonary capillary wedge pressure (PCWP) but not LVGLS.38 This all emphasizes the importance of using LAS/LAEF and not LAVI or LVGLS in evaluating LA function and LV filling pressure. This was explained by the fact that LAS quantifies mechanical events at the LA level associated with PCWP, as opposed to LVGLS, which might better reflect LV end-diastolic pressure. Furthermore, previous experimental studies have shown distinct cellular responses with more pronounced pro-fibrotic changes detected in the LA as compared with the LV wall,39 which supports the diagnostic importance of LAS/LAEF independent of the LVGLS.
Limitations
We investigated a small group of patients with HFpEF and HFrEF. Future studies should focus on cohorts of patients either with HFpEF or with HFrEF alone, because these two groups of patients present different cohorts with different pathophysiology. Larger and perspective studies are required to confirm and validate our results and prove the causality between LAS/LAEF and skeletal muscle function.
Although we mainly performed the majority of the echocardiography tests using GE technology, just few of these tests were performed with the Philips technology. Ideally, all investigations should be performed with a machine from one manufacture. Furthermore, LAS is not widely used yet and requires special training and experience. Image quality is very essential requirement for performing 2D-STE. Furthermore, the assessment of LA mechanical function was performed at rest and subsequently associated with measures performed during physical activity (muscle function and peak VO2). Data on LA function during exercise would be more relevant and should be measured in future research parallel to CPET or during the measurement of muscle endurance. Additionally, strain studies on patients with a. fib need to be further validated and different cut-off values might be required. Further, missing nutritional data present a limitation, as these might influence muscle mass and muscle function. An additional challenge was recruiting elderly HC without any diseases such arterial hypertension and diabetes.
Conclusions
Deteriorations on different levels of exercise capacity and skeletal muscle function in patients with HF are detectable efficiently centrally by measuring changes taking place in the LAEF and LAS. Using a sensitive cardiac measurement (LAEF/LAS), we proved a link between central and peripheral limitations in exercise capacity. Our findings might have diagnostic and therapeutic impact on the management of HF and its comorbidities. Developing scores that integrate central and peripheral factors in evaluating and staging the exercise intolerance in patients with HF could be very helpful. Accordingly, integrating the evaluation of LAEF and LAS in the assessment of functional capacity in patients with HF could be of special importance for staging the reduced exercise capacity and might be a potential therapeutic target or a marker to control the success of HF therapies.
Acknowledgement
Open Access funding enabled and organized by Projekt DEAL.




