Heart Failure Association of the European Society of Cardiology position paper on the management of left ventricular assist device-supported patients for the non-left ventricular assist device specialist healthcare provider: Part 2: at the emergency department
The authors warrant that the article is original and does not infringe upon any copyright or other proprietary right of any third party. The article is not under consideration by another publication and has not been previously published.
[Correction added on 05 March 2022, after first online publication: The affiliation for Prof. Giuseppe Rosano was previously wrong and has been corrected in this version.]
Abstract
The improvement in left ventricular assist device (LVAD) technology and scarcity of donor hearts have increased dramatically the population of the LVAD-supported patients and the probability of those patients to present to the emergency department with expected and non-expected device-related and patient–device interaction complications. The ageing of the LVAD-supported patients, mainly those supported with the ‘destination therapy’ indication, increases the risk for those patients to suffer from other co-morbidities common in the older population. In this second part of the trilogy on the management of LVAD-supported patients for the non-LVAD specialist healthcare provider, definitions and structured approach to the LVAD-supported patient presenting to the emergency department with bleeding, neurological event, pump thrombosis, chest pain, syncope, and other events are presented. The very challenging issue of declaring death in an LVAD-supported patient, as the circulation is artificially preserved by the device despite no other signs of life, is also discussed in detail.
Bleeding: gastrointestinal and non-gastrointestinal
Several quality of life-limiting complications are frequently seen during left ventricular assist device (LVAD) support, one of the most prominent being bleeding complications.
Incidence
Significant bleeding complications are quite common in LVAD carriers, incidence varying depending on the source, but ranging between 22% and 32% during LVAD support or 0.35–0.65 events per patient-year.1, 2 These bleeding episodes were rarely fatal: major bleeding was the cause of death in 2% of the total number of deaths in LVAD patients.3 Recently, a special focus has been placed on the interaction of blood components and the device, that is, haemocompatibility, with increasing awareness of the importance of these interactions on adverse events.4
Comparisons of the haemocompatibility parameters of different LVADs have been examined, and while pump thrombosis has been present less often in the newest centrifugal-flow device, the incidence of bleeding was quite similar between the investigated devices (HMII™, HM3™, and HW™).5, 6 On the other hand, the recent analysis by Kormos et al. noted a significantly lesser incidence of gastrointestinal bleeding (GIB) in patients implanted with a centrifugal-flow pump compared with those who received an axial flow pump.3, 4 Similar findings were observed in the MOMENTUM trial, where a significantly lower incidence of any bleeding, and GIB in particular, was seen with the centrifugal pump (HM3 vs. HMII).4
Significance and long-term repercussions of bleeding
Although bleeding complications are usually not fatal, they are yet clinically significant. Aside from the obvious impairment of quality of life, bleeding usually leads to adjustments in anticoagulant and anti-platelet therapy, which could potentially increase the probability of pump thrombosis.
Additionally, some of the bleeding episodes will be significant enough to require blood transfusions at an average of 2–4 units of packed red blood cells per admission.7 This is particularly relevant for the bridge-to-transplant candidates, in whom leuco-irradiated products should be used to reduce the risk of allo-sensitization caused by the excessive exposure to blood products that could result in low availability of appropriate heart donors or higher risk for humoral rejection after heart transplantation (HTx).8, 9
Pathophysiology of bleeding
An obvious contributing factor to the bleeding diathesis is the mandatory anticoagulant and anti-platelet therapy, required for the normal function of the devices.
Other possible contributing factors have been investigated. A lot of attention has been devoted to the interplay of the pump with the blood, unified under the name haemocompatibility.6
One of the examined pathohistological mechanisms for the induction of bleeding in ventricular assist device-supported patients is the acquired von Willebrand syndrome (AvWS). von Willebrand factor (vWF) is large multimeric glycoprotein, crucial for the process of haemostasis at the site of vascular injury, where it promotes platelet aggregation and the formation of platelet plug, also serving as a carrier protein for FVIII and protecting it from proteolysis.10, 11 Patients with impaired function of vWF, that is, patients with von Willebrand disease, are prone to bleeding complications. This has previously been described also in patients with aortic stenosis (Heyde's syndrome) and severe heart failure (HF) patients: vWF may already be degraded in some patients with severe HF at the time of LVAD implant, and these patients tend to bleed more.12 A similar pathological process is seen in LVAD carriers.13
According to the current literature, most all of the LVAD patients have loss of vWF activity,14 which seems to resolve after LVAD explant or HTx.15 Different mechanisms have been hypothesized to contribute to the AvWS in LVAD patients. It is thought that increased shear stress in the LVAD circulation may cause the vWF to disentangle, thus exposing domains promoting proteolysis and degradation of vWF,11 which then leads to reduced platelet aggregation. But despite some extent of AvWS being present in most LVAD patients,13 not all experience bleeding events.
von Willebrand factor is also considered an inhibitor of angiogenesis,16 meaning loss of function of vWF could be implicated in promotion of angiodysplasia and arteriovenous malformations.17 Bansal et al. conducted a prospective multicentre clinical trial, which demonstrated lower amount of vWF monomer degradation in the HM3 group.12 Despite all that, it is yet unclear if assessment for inherited or acquired coagulation disorders is warranted.
Platelet damage and dysfunction observed in LVAD patients,18-20 thought to be related to shear forces in the LVAD, could potentially add to the increased risk of bleeding in this population.
Arteriovenous malformation is anatomically the most frequently reported cause of GIB. Right HF is another potential risk factor for GIB through the enhanced arteriovenous malformation formation due to elevated portal vein pressure and coagulopathy associated with congestive hepatic failure. Non-pulsatile flow was considered a potential predisposing factor for the development of GIB through the formation of small intestinal angiodysplasia21; however, studies with partial ventricular support with fully preserved arterial pulse still reported a high incidence of GIB.22
Improved haemodynamics might have a favourable effect on suppression of GIB, achieved through the use of optimal medical therapy or optimizing LVAD speed through ramp testing23 and medications to control the blood pressure such us angiotensin-converting enzyme inhibitor and angiotensin receptor blocker24 and digoxin that was found to be associated with a decreased incidence of angiodysplasia-related GIB in patients with continuous-flow (CF)-LVADs.25
The favourable effects of omega-3 unsaturated fatty acids on angiogenesis and inflammation have been previously described,26 and in a small study, authors demonstrated that LVAD patients regularly receiving omega-3 acids had a greater freedom from GIB compared with controls.27
Risk factors for bleeding in the left ventricular assist device patient
Some patient populations are at increased risk of bleeding. In the case of GIB, risk factors include older age,28 lower body mass index, history of GIB prior to LVAD implant, smoking, elevated international normalized ratio (INR), and a low platelet count.29 Patients with pre-implant right ventricular failure have been prone to development of bleeding complications, possibly due to hepatic congestion and subsequent coagulopathy. Female sex has also been associated with an elevated incidence of all bleeding complications after LVAD implant.28
Diagnosis and treatment options
The extent and the selection of diagnostic and therapeutic modalities will vary according to the bleeding location, as well as its severity and the clinical presentation of the patient.
General conditions
- Depending on the bleeding severity, a temporary interruption of anticoagulation might be necessary, and, if deemed clinically justified, reversal of anticoagulation might be considered.30 These decisions are to be made in close collaboration with experienced HF cardiologists, due to the possible adverse outcomes (i.e. device thrombosis).
- Depending on the bleeding site, different specialists need to be consulted.
- Previously suggested reduction of pump speed in order to reduce the sheer stress on blood components30 has been proven to be ineffective,31 as well as in real life where we have seen similar bleeding incidence in the newest generation of devices despite their functioning at lower speeds.11
Gastrointestinal bleeding
Gastrointestinal bleeding is most frequently seen in LVAD patients,10 with the incidence ranging between 25% and 35% of patients with events on LVAD support or 0.31–0.56 events per patient-year,4, 5, 32 and is one of the major causes of readmission of LVAD patients.10 GIB can occur in any part of the gastrointestinal (GI) tract, but most often in the upper portion, proximal to the Treitz ligament.30
Invasive procedures
Diagnostic and treatment options include upper and lower tract endoscopies, video capsule endoscopy, or deep enteroscopy.21, 30 If these are not able to locate the bleeding site, angiography is advised (if the patient is unstable, consult with interventional radiology), or scintigraphy with tagged red blood cells.
Despite best efforts, sometimes the locus of the GIB remains obscure, even after best endoscopic efforts. Patel et al. found that hypervascularity of the nasal mucosa was associated with GI bleeding in LVAD-supported patients suggesting nasal endoscopy as a potential surrogate for diagnosing AV malformations in the GI tract as in other mucosal sites in LVAD carriers.33
Medical treatment
- This includes octreotide, a synthetic somatostatin analogue, which acts through decreased splanchnic blood flow, increased vascular resistance, improved platelet aggregation, and inhibition of angiogenesis.34 Studies with octreotide in LVAD carriers showed varying results.29, 35 A protocol of intramuscular application of octreotide in monthly visits has been evaluated, and authors demonstrated significant reduction in the frequency of GIB without associated complications and without the need to alter anticoagulant and anti-platelet therapy.36
Preventive therapies
- Initiation of intravenous proton pump inhibitor is indicated,10 especially when involvement of the gastric mucosa is suspected.
- Consider cessation of aspirin and adjustment or cessation of warfarin depending on the severity of the bleeding episode and ongoing risk for re-bleeding. This should be performed in close consultation with the LVAD unit. The presumed assumption that combined use of aspirin and anticoagulants will lower the risk of thrombus formation in LVAD-supported patients has been recently questioned with the low incidence of thrombus formation in the HM3-supported patients. The ARIES study37 will examine whether anti-platelet therapy can be safely avoided in HM3-supported patients, thus reducing the risk of bleeding.
- The beneficial effect of thalidomide in GIB is associated to its antiangiogenic properties through the suppression of vascular endothelial growth factor.38 This therapy for GIB in LVAD-supported patients is not supported by trials. Therefore, the potential beneficial effects must outweigh the potential adverse events associated with thalidomide therapy (pancytopenia, neuropathy, and thrombo-embolic events).
- Danazole, a derivative of testosterone, reduces the risk of bleeding through enhanced concentration of red blood cells, but has adverse androgenic effects and when administered with statins may increase the risk of rhabdomyolysis.39
- Factor VIII concentrates containing FVIII and vWF have been utilized in some studies, but they have been associated with device thrombosis.40
- Because several studies have shown resolution of bleeding complications after HTx, this could also be seen as a management strategy for LVAD patients with incessant bleeding.7, 32
Non-gastrointestinal bleeding
Intracranial haemorrhage
Several large-scale studies found intracranial haemorrhage (ICH) in 3–11% of LVAD patients represented also as 0.05–0.07 events per patient-year.41, 42 The important issue of ICH is discussed in detail in the section on ‘Neurological events’ in the subsection on ‘Haemorrhagic stroke’.
Gynaecological bleeding
Given the relatively small proportion of female LVAD patients: around 20%,3, 43 the data on management of gynaecological bleeding are understandably scarce.
Women seem to have a greater incidence of non-gynaecological types of bleeding complications (including gastrointestinal, naso-oropharyngeal, and ICH) compared with men, but this did not translate into worse survival.2, 28, 44 In an analysis by Yavar et al., 10% of patients experienced gynaecological bleeding requiring transfusions or surgical interventions.28 Depending on the severity, such episodes might require interruption of anticoagulant and anti-platelet treatment, hormonal therapy, or, in extreme cases, surgical treatment.
The aetiology of increased bleeding diathesis in women is not yet elucidated.
Other sources of bleeding
Other forms of bleeding that might be encountered in LVAD carriers include mostly bleeding from the nasal or oral mucosa, seen usually as epistaxis, after teeth extraction, skin bruises, or haematuria.28 Although these bleeding episodes are usually treated by other specialists (otolaryngology, oral surgeons, urologists, etc.), it is advised to include a cardiologist specialized in managing LVAD patients in the team responsible for deciding on interruption of anticoagulant or anti-platelet therapy.
Prevention
Several preventive measures can be undertaken in order to reduce the number and intensity of bleeding episodes.
Optimal management of anticoagulation is of utmost importance: keeping the INR values within the therapeutic range could help decrease the risk of bleeding complications. For this end, patient education and nutritional counselling are very helpful if the oscillations of INR levels are suspected to be associated with food interactions (e.g. green and leafy vegetables). Detailed guidelines on the management of oral anticoagulation in this population are available.45, 46
Coumarin oral anticoagulants such as warfarin are metabolized via the CYP2C9,47 which is also a common pathway for several other drugs commonly used in this patient population (antidiabetic medication, angiotensin receptor blockers, anticonvulsive medication, etc.).
Given the common occurrence of polypharmacy in these patients, caution must be exercised regarding the drug–drug interactions. In patients with special difficulties maintaining the INR range, genetic counselling with the identification of the patient's metabolic profile is possible.47 Devices for home monitoring of INR are available and could potentially be beneficial in achieving optimal INR control.
The excellent results of the MOMENTUM trials,4 demonstrating significantly reduced burden of device thrombosis in HM3 carriers, raised the potential need for decreased level of anticoagulation. This was examined in the MAGENTUM 1 trial, which evaluated lower target INR levels (target INR 1.5–1.9) in 15 HM3 carriers after the sixth post-operative week. Primary endpoint (event-free survival) was achieved in 93 ± 6%, but due to the small sample size. This study needs further validation.48
- GIB is the most frequent source of bleeding in LVAD-supported patients.
- Considering reversal of the anticoagulation should be weighed against the risk of pump thrombosis.
- When the blood loss results in low haemoglobin level, try to avoid blood transfusion in the bridge to transplantation group, but if needed, use leuco-irradiated blood products. Try giving iron transfusion and consult the LVAD centre.
- To lower the incidence of ICH, keep the mean arterial pressure (MAP) below 80 and watch the anticoagulation level closely keeping it within the advised limits and not earlier.
Neurological events
Stroke is a leading cause of significant morbidity in patients with LVAD and can be an important contraindication for cardiac transplantation.52, 53 At this point, little is known about stroke in LVAD patients beyond descriptive epidemiology, including associated neurological complications and available treatment strategies. Patients with LVAD are likely to be at high risk for recurrent stroke and may have a poor recovery prognosis than undifferentiated stroke patients due to baseline severity of illness.
Despite generational advances in engineering of such devices, from large pulsatile mechanisms to smaller CF devices, the complications related to neurological adverse effects have not improved substantially. Haemorrhagic and ischaemic strokes continue to occur, with a frequency greater than that observed in patients with advanced HF. This complication has limited the expansion of such devices to patients with less severe stages of illness, and the quest to understand these devastating events and to prevent them is ongoing.
The older devices were larger and had a higher incidence of thrombosis, and therefore, anticoagulation targets were different compared with today's devices. Yet the general incidence of neurological complications, principally stroke, has not decreased in the contemporary era. This suggests that the underlying reasons for stroke may be changing as a function of device era, with causative factors that may have more to do with vascular biology than simply anticoagulation strategies. The rate of adverse events associated with CF-LVAD use—specifically, strokes—is as high as 10% of individuals who are affected by stroke in the first year of support alone.52 Furthermore, according to the INTERMACS registry, stroke remains the primary cause of death in the 6–24 months of the LVAD implantation.53 The stroke rate associated with pulsatile LVADs was 4 times higher than the rate of patients managed medically over 2 years.54
In the original trials evaluating the first CF-LVAD (HMII), the rate of disabling stroke was similar among individuals managed with the HII CF-LVAD vs. pulsatile devices (17% vs. 14%, respectively, P = 0.56) over 2 years.41 The HW LVAD, a newer CF-LVAD currently in use, was associated with a higher stroke rate compared with the HII (29.7% vs. 12.1%, P < 0.001) over 2 years.5 Finally, in the MOMENTUM trial, the newest CF-LVAD, the HM3, was found to have a much lower stroke rate than the HII device (10.1% vs. 19.2%, P = 0.02) over a 2 year period.55 Thus, refinement of device technology has led to a reduction in the incidence of stroke among these patients compared with older pumps.
The causes of strokes with the newer LVADs are unclear and could relate to (i) clots that pass through the device (as with atrial fibrillation in patients with a device or with clots that form within the device or in the proximate ventricle), (ii) vascular changes as a result of non-pulsatile flow (we know that reduced pulse pressure with the newer devices increases vascular fragility, so even lower levels of BP can cause vascular loss of integrity) especially with relation to cerebral amyloid angiopathy,56 and (iii) rheological causes (new devices create an acquired von Willebrand syndrome and can predispose to bleeding).14, 57
Three types of neurological events are clinically relevant in LVAD patients: ischaemic strokes, haemorrhagic conversion, and intra-cerebral haemorrhage.
Ischaemic stroke
Ischaemic strokes can occur because of thrombi that pass from the heart through the device and into the brain or could develop in unique locations, such as the aortic surface of the valves within the sinus of Valsalva, the carotid bulb, and in some cases septic emboli as a result of infections.58, 59
The most consistent risk factor for ischaemic stroke in CF-LVADs is systemic infection, which has increased the risk of stroke by nearly two-fold (39.5% vs. 19.3%, P = 0.003).60-62 In the INTERMACS registry, independent preoperative predictors of ischaemic stroke were female sex and previous cardiac operation.52 However, when considering post-operative risk factors, infection and GIB significantly increased the risk of stroke.52
One of the most feared complications of any ischaemic stroke is bleeding into the area of the infarcted brain (haemorrhagic conversion/transformation). Consequently, the risk assessment for resuming anti-thrombotic agents is often guided by known risk factors in the undifferentiated ischaemic stroke patient, including infarct size, cardioembolic aetiology, vessel recanalization, and heparin treatment.63-65
Haemorrhagic stroke
Intracranial haemorrhage is a complication, which usually results in significant long-term consequences for the patient. An INTERMACS analysis looking into the incidence of stroke in LVAD carriers showed that 10% had at least one stroke, of which nearly 50% were haemorrhagic, leading to a significantly worse survival compared with those who suffered an ischaemic stroke.52 In this analysis, 45% of patients survived 1 month after the ICH, while only 30% survived 1 year. In a multivariable analysis, heparin-induced thrombocytopenia, use of intra-aortic balloon pump, female sex, and primary cardiac diagnosis were significant predictors of ICH. Female sex has been a predisposing factor in other analysis.8, 28, 66 Whereas GIB might facilitate a sooner transplantation in LVAD carriers, ICH more often renders the patient ineligible for transplant, depending on the residual morbidity.52
There are several possible causes of haemorrhagic stroke in patients with LVAD, such as anticoagulation, haemorrhagic conversion after infarction, the acquired von Willebrand syndrome as a result of mechanical destruction, and proteolysis of high-molecular-weight multimers of von Willebrand factor 8 and rupture of histologically fragile vessels caused by the non-physiological continuous pulseless flow.8, 67 It is often unclear why a specific CF-LVAD patient develops a specific ICH, as coagulopathy alone appears neither necessary nor specific. Intra-cerebral haemorrhages occur in the absence of endocarditis with subtherapeutic INRs, whereas supratherapeutic INR levels do not necessarily lead to haemorrhagic stroke.67
The aetiology of ICH is not completely elucidated. The presented rates of haemorrhagic strokes are significantly higher compared with other populations treated with warfarin, and other studies demonstrated that spontaneous ICH has been noted in all INR ranges.68-70 Interestingly, all the patients who developed ICH had concomitant AvWS.71
The incidence of haemorrhagic stroke has also been shown to reduce with the newest generation of centrifugal-flow devices.4 This leads to the conclusion that there could be several predisposing factors for ICH.
While it is long known from normal pulsatile physiology, elevated BP during LVAD support has recently been associated with a higher incidence of intracranial bleeding, and subsequent mortality, which could indicate BP control as a beneficial measure in preventing ICH.72, 73 Given the established difficulties in obtaining BP measurements in LVAD carriers, an alternative to the traditional electronic BP measuring devices has been investigated, and Doppler-derived BP measurements have shown to correlate well to invasive systolic BP measuring.74 According to the International Society for Heart and Lung Transplantation guidelines for mechanical circulatory support, level of evidence C (expert opinion), the Doppler-derived BP is to be maintained at ≤80 mmHg.75
The correlation between hypertension and neurological events was not so obvious in the INTERMACS analysis. Such a correlation might be device specific as presented in the ENDURANCE supplemental study showing that BP management is associated with reduced stroke rates in HW subjects.61 Maintaining BP in the reference range was associated with better overall outcomes.51 The authors stress the importance of avoiding too aggressive BP management with hypotension as well as hypertension.
Strict management of BP maintaining MAP at 70–90 mmHg49 and anticoagulation targeting INR levels of 2–2.549 are expected to be beneficial preventive strategies for several complications of LVAD treatment, but this does not necessarily guarantee absence of bleeding complications49 (see Part 3 for more on anticoagulation in LVAD-supported patients).
A study by Trachtenberg et al. indicated blood stream infections as a predisposing factor for neurological events, both ischaemic and haemorrhagic.76
Survival after the haemorrhagic stroke is significantly reduced and was 45.3%, 34.8%, and 30.3% at 1 month, 6 months, and 1 year, respectively.52
Cerebral microbleeds shown by brain magnetic resonance imaging are predictive of symptomatic cerebral haemorrhage in patients with various causes.77
In general population studies, it has been reported that several factors (e.g. male sex, older age, and elevated BP) show positive correlations with the number of cerebral microbleeds,67, 78 while correlations with aspirin and warfarin administration, which are mandatory for patients with LVAD, remain controversial.79
One small-scale study assessed a treatment protocol for ICH in LVAD patients, which included observation alone, observation with suspension of anticoagulation, observation with reversal of anticoagulation, or reversal of anticoagulation plus surgical intervention.
Reversal of warfarin was performed with fresh frozen plasma (FFP), prothrombin complex concentrate, vitamin K, or a combination. Prothrombin complex concentrate is preferred in neurosurgical emergencies, given its faster effect.71
Depending on the severity of presentation, some patients will require surgical treatment, such as a craniotomy with drainage.53 Ramey et al. suggest terminating anti-platelet treatment in patients with established AvWS, but this is not supported by data.71
Routine administration of platelets is debatable in ICH, because adverse outcomes have been noted, but it might be requested in patients undergoing neurosurgical intervention.71
The significance of radiological screening of potential risk factors for ICH is not established. A multidisciplinary approach to treatment and decision-making might be optimal in this setting.
Treatment
The assessment of all stroke patients is performed using standardized examination tools, such as the National Institutes of Health Stroke Scale, and a measure of consciousness, such as the Glasgow Coma Scale, for the sake of monitoring the patient with reproducible, objective measurement tools. Intravenous recombinant tissue plasminogen activator is highly effective for improving functional outcomes in general acute ischaemic stroke patients, but there are no trials in LVAD-related stroke patients. Thrombolysis may be ineffective because thrombi from within the CF-LVADs may have a composition (fibrin and denatured protein) that is not been amenable to thrombolysis.
Patients with CF-LVADs are likely to be at significantly higher risk of symptomatic haemorrhagic transformation after systemic thrombolysis. Firstly, patients with CF-LVADs are often taking warfarin and typically at least one anti-platelet agent, and tend to have larger territorial infarcts, which are all associated with a higher risk of haemorrhagic conversion in several prediction scores. Secondly, the association of systemic infection with ischaemic stroke increases the likelihood a septic embolism, and thrombolysis in patients with bacterial endocarditis leads to high rate of haemorrhagic complications (close to 20%).80 Thirdly, patients with CF-LVADs have an acquired vWF deficiency that would increase the risk of haemorrhagic transformation.8
Because of the high risk for haemorrhagic transformation, endovascular stroke therapy may provide specific clinical and diagnostic benefits in patients with a CF-LVAD, avoiding systemic fibrinolytic therapy in patients treated with anticoagulation and anti-platelets. However, the risk of haemorrhagic transformation with endovascular stroke therapy appears like that of systemic thrombolysis.81
Treatment decisions for acute haemorrhagic stroke patients are based on whether the patient has had a primary haemorrhagic stroke (intra-cerebral haemorrhage and subarachnoid haemorrhage) or haemorrhagic transformation. Other factors include the anti-thrombotic agents used, coagulation profile, and platelet count. Regardless of aetiology, the first focus of treatment is to establish the need for mechanical ventilation due to impaired alertness, in parallel with preventing expansion of the haematoma. ICH is associated with a very high in-hospital mortality of about 20%.67
Currently, the mainstay of therapy in haemorrhagic stroke is reduction of BP and reversing coagulopathy, in line with safety concerns regarding BP targets with specific devices. The acute treatment of BP in the CF-LVAD patient with an ICH is not well established in the guidelines for stroke, but it would be reasonable to lower mean arterial pressure to <90 mmHg, as suggested for primary stroke prevention in CF-LVAD.82
The decision to reverse a coagulopathy is more complex. The most recent ICH guidelines from the American Stroke Association suggest reversing warfarin with prothrombin complex concentrates or FFP without a specific INR target.83
The decision about when to resume anticoagulation in the ischaemic stroke patient without endocarditis is driven by the presence, and likelihood, of developing haemorrhagic conversion in the general ischaemic stroke patient. Generally, resume warfarin at the same time as aspirin (without a heparin bridge) within 24 h if haemorrhagic conversion is absent and the total infarct size is <50% of the middle cerebral artery territory (given the association of large infarct size with haemorrhagic conversion).67
If the patient is at high risk for haemorrhagic conversion, such as with large infarcts with petechial bleeding, a head computed tomography (CT) is repeated at 5 days and warfarin is resumed at that point if there is no evidence of haemorrhagic conversion.67
After a primary ICH in the general stroke patient, long-term anticoagulation can be resumed for clear indications if there is no lobar ICH due to cerebral amyloid angiopathy.
Early decompressive haemicraniectomy for large hemispheric ischaemic strokes with somnolence and intact brainstem reflexes (except loss of one pupillary response) provides substantial mortality benefit (absolute risk reduction 78–29%) in patients <60 years of age.84 However, confounding the care of patients with CF-LVADs, haemicraniectomy requires withholding anticoagulation to prevent surgical site bleeding until 4–8 weeks afterward when the cranioplasty is placed. Post-operative management with anti-thrombotic agents and long-term functional prognosis in large strokes increases the complexity of the decision on whether to perform haemicraniectomy.
There is a strong need to continue research into the risk factors, natural history, complication rates, and acute stroke treatment for patients with CF-LVADs.
The efficacy of treatment after stroke is also not well known, with small series documenting results after reversing anti coagulation in ICH, but not outcomes beyond death such as functional statistics.85 This is notable because stroke can directly lead to death but more frequently leads to significant disability. The latter can be particularly important to patients with LVAD in whom complications from limited mobility can be substantial, including loss of eligibility for transplantation and infection.
- When a patient with LVAD presents with ischaemic stroke, rule out background infection or even sepsis as a contributing factor.
- Because of anticoagulation issues of this group of patients, beware of haemorrhagic conversion.
- Treatment of haemorrhagic stroke first includes lowering MAP to below 90, and conversion of the anticoagulation with FFP, or prothrombin complex concentrates.
Suspected pump thrombosis
Pump thrombosis is still a dreaded complication of both short-term and long-term use of LVADs as it occurs in 2–13% of the adult patients.86 Pump thrombosis is almost non-existent with the use HM3 device unless ingestion of material occurs within the device. In the first HM3-implanted patients, the clip used to secure the swivel point was not used increasing the risk of a rare but catastrophic complication of outflow graft twist with thrombus formation in the graft in those patients.87
Pump thrombosis is the development of a clot within the flow path of any component of the pump: the titanium inflow cannula/the rotor or the outflow graft. The thrombus can be created within the LVAD, or it can travel from either the left atrium or the left ventricle or lodge in any of the pump components.
Pump thrombosis is a life-threatening event, and thus, prompt diagnosis and successful management are crucial, as it can lead to one of the following: neurological event, peripheral thrombo-embolism, LVAD malfunction with reduced flows and life-threatening haemodynamic impairment, cardiogenic shock, and death.
When an LVAD patient arrives to the emergency department (ED) with suspected pump thrombosis, first thrive for patient stabilization; next, the source and location of the thrombus must be identified, and the proper therapy must be initiated.
Several therapeutic options are available according to the location of the thrombus. While one can address both the inflow cannula and the outflow graft thrombus either medically or surgically, for the outflow graft, the stenting approach is also available.
In any scenario, the LVAD implanting centre must be approached and transferring the patient to the LVAD centre is preferred.
Diagnosis
Inflow cannula and intra-device thrombus
Left ventricular assist device thrombus that is located within the pump has a very distinct appearance (Figure 1); once seen, one can better understand the catastrophic consequences of this event.88
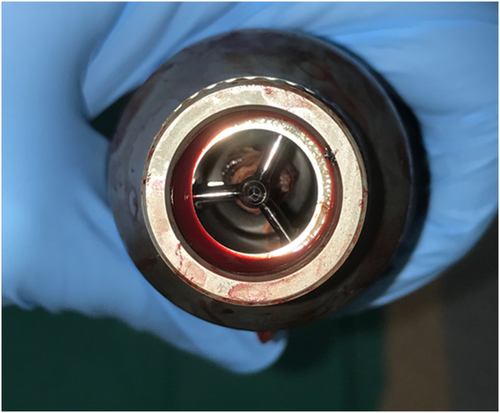
Inflow cannula and intra-pump thrombus are suspected in the setting of any of the following: signs of worsening HF (progressive dyspnoea on exertion), acute changes in the LVAD parameters (‘low-flow’ alarms and LVAD power spikes), haemolysis (lactic dehydrogenase increase, haemoglobin drop plasma-free haemoglobin rise, and darker urine), and signs of distal embolization.
-
Transthoracic echocardiogram—ramp studies:
- Left ventricle enlargement
- Opening of the aortic valve in each cycle
- Worsening mitral regurgitation
- Inflow cannula turbulence
- Concern for a possible seen thrombus
- CT with angiography that will demonstrate no outflow graft obstruction
Outflow graft obstruction/thrombosis
Although the outflow graft is the less prevalent site of a pump thrombosis, the diagnostic process is very similar to the one of an inflow cannula thrombosis with some modification.
First, one should understand that while inflow cannula obstruction can occur only from the ‘inside’, outflow graft obstruction can occur also from an external pressure on the graft.
The patient presentation will be very similar with progressive dyspnoea on exertion, ‘low-flow’ alarms, and LVAD power spikes. However, in this case, the decrease of power and flow happens in a relatively ‘longer’ period of time.
While the blood and urine haemolysis pattern may be like the one seen in an inflow pump thrombosis, it might be ‘softer’; for example, the values will not be as high as seen in an inflow pump thrombosis. Moreover, one should remember that outflow graft obstruction causing HF symptoms can be also caused from external pressure on the graft; hence, in some cases, no haemolysis will take place.
-
Transthoracic echocardiogram—ramp studies:
- Left ventricle enlargement
- Opening of the aortic valve in every cycle
- Worsening mitral regurgitation
- Outflow graft turbulence
- No thrombus is seen inside the left ventricle
- CT with angiography—outflow graft obstruction/stenosis present
- Intravascular ultrasound—narrowing of the internal diameter due to external pressure
Treatment
- The INR levels adjusted to 2.5–3.5.
- If no change has occurred during the next 24 h, start continuous heparin infusion to an activated partial thromboplastin time goal of 80–100 s. Activated partial thromboplastin times tend to overestimate anticoagulation, and anti-XA monitoring might be used as well to assess adequacy of therapy.
- If non-responsive or unstable, try thrombolysis with tissue plasminogen activator started as 30 mg bolus followed by infusion of the next 20 mg at the rate of 1 mg/min for the next 20 min, followed with eptifibatide and intravenous heparin 2 h after the tissue plasminogen activator infusion.
- If yet non-responsive or unstable, pump exchange might be needed. If the patient is unstable, skip the medical treatment and go straight for a pump replacement or put on venoarterial extracorporeal membrane oxygenation for metabolic and end-organ rescue prior to pump exchange.
If an outflow graft obstruction was diagnosed and a percutaneous approach is applied, when balloon inflating does not suffice, the use of Wallstent is preferred. The physiological effect of reliving the outflow graft occlusion can be seen immediately after deploying the stent by a reduction of the power consumption and an increase in the pump flow.
A word of caution regarding the use of thrombolysis must be cleared. Its efficacy in treating LVAD thrombosis has been questioned along the years and was debated because of increased risk of bleeding; however, data were scarce. Lately, Seese et al.84 have demonstrated that out of 26 patients that received initial thrombolytic therapy, in only three patients a successful treatment was achieved. Conversely, three patients suffered from major bleeding and two patients had a new stroke. Furthermore, most patients undergoing thrombolytic therapy underwent subsequent device exchange.84 Hence, thrombolysis for an HMII pump thrombosis should only be applied as a last resort due to the limited efficacy of thrombolysis and the related increased risk of major bleeding or stroke and surgical pump exchange is preferred. Regarding the management of an HW pump thrombosis, as of 3 June 2021, the distribution of the HW has been discontinued; therefore, in case of the need for pump exchange, the HW would have to be replaced with the HM3. A surgical procedure for such an LVAD exchange is not established yet, and most centres do not have the needed experience in performing such an exchange. Thus, the non-surgical approach to HW pump thrombosis must be exploited as far as possible.
- Pump thrombosis is a life-threatening event that must be addressed immediately.
- Once the patient is stabilized and diagnosed, promptly initiate medical treatment.
- Use increased anticoagulant therapy and stenting in outflow graft thrombus.
- If medical treatment fails, contact the implanting centre for an advanced therapy.
Chest pain
Chest pain in patients with LVADs is encountered commonly not only immediately after surgery but also long after the device implantation. Chest pain was in some centres the second most common ED presentation but lead to hospitalization in only in 50% of cases. Chest pain can be caused by both cardiac and non-cardiac causes.90 In another large single-centre observational study, chest pain accounted for 4% of all the unplanned readmissions.91
- Although less frequent, chest pain in LVAD-supported patients can be also from cardiac origin like myocardial infarction, pericarditis, aortic dissection, pulmonary embolism, or implantable cardioverter defibrillator discharge. The seventh INTERMACS report indicates myocardial infarction rate of 0.06 (events per 100 patient-months) during first 12 months after implantation.92 Myocardial infarction may be caused not only by a rupture of atherosclerotic plaque but also by a thrombo-embolic complication. Embolus may originate from the left ventricle or atria but may be due to pump thrombosis or thrombosis in the aortic root. An evolving thrombus in the aortic root or in the aortic valve itself can cause acute obstruction of the coronary arteries. An echocardiography or even CT angiography should be used to rule out this potentially catastrophic event. Optimal treatment of myocardial infarction in LVAD patients is not clear. Primary coronary intervention approach favouring rapid symptomatic relief, reducing risk of malignant arrhythmia, and protection of right ventricular function have been reported.88 Although the levels of the two biomarkers: N-terminal pro-brain natriuretic peptide and troponin are expected to decline or increase respectively because of left ventricular unloading or the occurrence of myocardial infarction, the clinical significance of these two biomarkers in LVAD-supported patients has not been thoroughly explored yet.
- Chest pain in most cases is of non-cardiac origin and difficult to diagnose.
- Chest pain in LVAD-supported patients' needs to be thoroughly assessed.
- Chest pain from cardiac origin in LVAD patients is not common but can be life-threatening despite the LVAD support.
- Have acute myocardial infarction treatment as in non-LVAD patients with primary coronary intervention after consulting an LVAD specialist.
Syncope in left ventricular assist device-supported patients
Syncope is defined as a total loss of consciousness, due to cerebral hypoperfusion, characterized by a rapid onset, short duration, and spontaneous complete recovery.93 Occurrence of syncope has been described in LVAD patients.75
In daily clinical practice, syncope and orthostatic symptoms like dizziness, blurry vision, confusion, and nausea can be challenging in LVAD patients.
Prevalence of dizziness and syncope has been described to occur in 13% of LVAD patients. Up to 30% patients suffer from orthostatic hypotension.
Aetiology for syncope in these patients includes orthostatic hypotension, cardiac factors like arrhythmias, and vasodepressive neurogenic and device-specific mechanical factors.
Left ventricular assist device patients show an increased susceptibility for orthostatic hypotension, resulting from pathophysiological properties in CF and in HF in general. This includes an abnormal sympathetic tone with a marked decrease in vasoconstrictive function, an offloading of carotid and aortic baroreceptors causing a loss of compensatory mechanisms with positional changes.94
Further contributors to orthostatic hypotension include low intravascular volume state, medications with vasodilator properties, poor right ventricular function, and co-morbidities like diabetes mellitus leading to secondary autonomic failure. Device-specific factors like malposition of the inflow cannula receive special attention. Pathophysiology of syncope in LVAD patients can be complex resulting from different predisposing factors at the same time. Positional changes from supine to upright in even mildly hypovolemic and diabetic LVAD-supported patient can cause dizziness and syncope.
It is crucial to understand the mechanisms behind these events by adequately performing diagnostic procedures to tailor individualized therapeutic regimens.
Diagnosis includes careful history taking, thorough physical examination, supine and standing BP measurements, immediate electrocardiogram (ECG) monitoring when there is suspicion of arrhythmia, and blood tests (haemoglobin, kidney function tests, uric acid, etc.). Advanced evaluation with echocardiography is essential to assess septum position, inflow cannula position, right ventricular function, and volume status. A head-up tilt test can be performed when there is suspicion of syncope due to orthostatic hypotension. Interrogation of LVAD parameters like power, flow, revolutions per minute, pulsatility index, and the occurrence of suction events completes diagnosis. LVAD alarms must be scrutinized. In special situations, interdisciplinary discussion with the implanting cardiac surgeon and technicians can be very helpful.
- The LVAD patients are prone to syncope due to orthostatic hypotension.
- Always evaluate the patients for dehydration, anaemia and arrhythmia with ECG, echocardiography, blood tests, and pacemaker interrogation.
- When suction event is suspected, perform fluid resuscitation and consult the LVAD specialist.
Death declaration in the emergency department
The number of LVADs implanted is expanding all around the globe. Thus, complications that were once seen sporadically are now seen in increasing numbers. Furthermore, LVADs are constantly becoming better and more reliable, and the frequency of LVAD-related side effects is declining. Thus, the life expectancy of the LVAD-supported patients is extended, exposing the patients to new complications and the physicians to new dilemmas. The following chapter will review the process of a patient arriving to the ED addressing the option that the cardiopulmonary resuscitation (CPR) process was not successful.
Left ventricular assist device patients can be brought to the ED by their family members/first responders while being resuscitated. Management of the unresponsive LVAD-supported patient hospitalized in the non-intensive care setting has been described91 but not the management of such a patient brought to the ED been on CPR.
- Continue CPR.
- Ventilate if not yet performed by the emergency medical service.
- Assess LVAD function. If not functioning, reconnect the LVAD to electrical supply if possible.
- Perform an ECG.
- Perform basic echocardiography to assess heart function.
- If not asystole and there is some cardiac function, assess organ perfusion by performing carotid and femoral Doppler.
- If LVAD is functioning and the perfusion is found to be appropriate, call for a stroke code.
- If no perfusion, consider advance therapies as venoarterial extracorporeal membrane oxygenation depending on the patient's status, the duration of the CPR, and other co-morbidities.
- Consider death declaration according to the local law and contact the ventricular assist device unit for disconnecting the device.
The World Health Organization has set Clinical Criteria for the Determination of Death,95 but because the declaration of death relies on the law of each country, the presented protocol is only a general point of view, emphasizing the obligation to declare death according to the local law.
-
Neurological arrest:
-
No other reason was identified:
- Good ventilation, oxygenation, and perfusion
- Not hypothermic
- No metabolic or endocrinological issues
- No acid–base or electrolytes issues
- No toxic substances were used
- No neuromuscular blockers or neurodepressant drugs were given
- Coma, no brain stem reflexes, and apnoea
- Confirmed by electroencephalogram, brain perfusion, and observation
- Declaration of death
-
-
Cardiocirculatory arrest:
- No response, gasping, and no circulation
- CPR failed: no pulse, no heart sounds, no breathing, and no pupil light response
- Echocardiogram, arterial line, or ECG confirmed all the above
- Wait for 5 min to verify no spontaneous recovery and then declare death
- neurological function,
- check pupils for position and response to light,
- check response to tactile stimuli, and
- check for spontaneous respiration.
Some of the WHO criteria needed modification for the LVAD-supported patients. The difference lies within the 2b criteria: ‘CPR failed: no pulse, no heart sounds, no breathing, and no pupil light response’. Checking for pulse and heart sounds is challenging in LVAD patient, and therefore, declaration of death must be verified using advance tools like ECG, echocardiography, and PETCO2 under 10.
- To declare death in an LVAD patient, one must verify whether the LVAD is functioning or not and troubleshoot the device.
- Use advanced tools and the WHO criteria to declare if there is circulatory or neurological arrest.
- Follow the local law to declare death!
Conclusion
Despite the more frequent use of new-generation LVADs, the growing number of LVAD-supported patients and their improved survival is expected to increase their need for ED support due to LVAD-related and non-LVAD-related medical emergencies. The understanding, prompt, and structured approach to the LVAD-supported patient presenting at the ED with bleeding, neurological event, pump thrombosis, chest pain, or syncope is critical for the successful management and longer survival of these patients. When everything fails, the challenging declaration of death in the LVAD-supported patient needs to be made according to the local regulations.
Conflict of interest
The authors have nothing to disclose. The authors confirm that the final manuscript has been read and each author's contribution has been approved by the appropriate author.
Funding
None.




