Left ventricular dimensions and cardiovascular outcomes in systolic heart failure: the WARCEF trial
Abstract
Aims
There is limited information on the association between left ventricular (LV) dimensions and cardiovascular (CV) outcomes in patients with heart failure (HF) with reduced LV ejection fraction (HFrEF) receiving recommended HF treatment. We investigated the association between LV dimensions and CV outcomes in HFrEF patients receiving recommended HF treatment.
Methods and results
We investigated the association between LV echocardiographic dimensions and CV outcomes using conventional Cox models in 1138 HFrEF patients in sinus rhythm randomized to warfarin or aspirin treatment in the Warfarin vs. Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) trial. LV enlargement, whether by diameter [LV end-diastolic diameter index (LVEDDI) and LV end-systolic diameter index (LVESDI)] or volume [LV end-diastolic volume index (LVEDVI) and LV end-systolic volume index (LVESVI)], was independently associated with all-cause death [LVEDDI: hazard ratio (HR) per cm/m2 1.53, LVESDI: HR per cm/m2 1.65, LVEDVI: HR per 10 mL/m2 1.07, and LVESVI: HR per 10 mL/m2 1.10; all P values < 0.001], CV death (HR 1.68, 1.79, 1.09, and 1.12, respectively; all P values < 0.001), and HF hospitalization (HR 1.59, 1.79, 1.06, and 1.08, respectively; all P values < 0.001). No association was observed with myocardial infarction or stroke. The associations were independent of LV ejection fraction values, and incremental to them. LV volumes conferred additional predictive value over LV diameters.
Conclusions
Left ventricular enlargement is an independent predictor of CV events in patients with HFrEF and recommended HF treatment. LV dimensions should be considered in the risk assessment.
Introduction
Left ventricular (LV) enlargement is a powerful predictor of adverse outcomes such as all-cause death, cardiovascular (CV) death, heart failure (HF) hospitalization, myocardial infarction (MI) in patients with HF with reduced LV ejection fraction (HFrEF).1-8 In most studies on the topic, however, the frequencies of recommended HF medications, such as beta-blocker and angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB), were low.1-10 As a result, no recent large studies have investigated the association between LV dimensions and CV outcomes in patients with HFrEF and recommended HF treatment; also, the possible interaction of LV enlargement and systemic anticoagulation on outcome has not been investigated.
The primary aim of this study was to investigate the association between LV dimensions and CV outcomes (all-cause death, CV death, MI, stroke, and HF hospitalization) in patients with HFrEF receiving recommended HF treatment. Additional aims were to investigate (i) the interaction between LV dimensions and left ventricular ejection fraction (LVEF) on CV outcomes, (ii) whether LV volumes were superior to LV diameters for risk prediction, and (iii) whether antithrombotic treatment (warfarin or aspirin) modified the association between LV dimensions and CV outcomes.
Methods
Study patients
Details of the Warfarin vs. Aspirin in Reduced Cardiac Ejection Fraction (WARCEF) trial have been published previously.11 In this randomized, double-blind trial, 2305 patients with LVEF ≤ 35% in sinus rhythm were randomly assigned to warfarin (target international normalized ratio 2.75, with acceptable target range of 2.0 to 3.5) or aspirin (325 mg/day). Patients were enrolled at 168 centres in 11 countries from October 2002 to January 2010. The mean follow-up time was 3.5 ± 1.8 years. Patients who had a clear indication for warfarin or aspirin were not eligible. Additional eligibility criteria were a modified Rankin score of 4 or less (on a scale of 0 to 6, with higher scores indicating more severe disability), and planned treatment with a beta-blocker, an ACE inhibitor (or, if the side-effect profile with an ACE inhibitor was unacceptable, with an ARB), or hydralazine and nitrates. Patients were ineligible if they had a condition that conferred a high risk of cardiac embolism, such as presence of atrial fibrillation, a mechanical cardiac valve, endocarditis, or an intracardiac mobile or pedunculated thrombus. The study conforms with the principles outlined in the Declaration of Helsinki. Patients provided informed consent, and the study was approved by the international review boards and ethics boards of participating centres.
Echocardiography
Left ventricular ejection fraction assessment was performed by echocardiography using the method of discs at the individual sites. Mean time from echocardiogram performance to enrolment was 6.5 days. All echocardiographic studies were reinterpreted, blinded to treatment assignment, at a core echocardiography laboratory to confirm LVEF assessment and measure other pertinent echocardiographic variables. LV diameters were measured from the parasternal long-axis view and divided by body surface area (LV end-diastolic diameter index and LV end-systolic diameter index). LV volumes were measured from an apical view using the method of discs and divided by body surface area [LV end-diastolic volume index (LVEDVI) and LV end-systolic volume index (LVESVI)].12 Overall, 1138 patients in whom all echocardiographic and clinical parameters were available were included in the present analysis.
Follow-up and assessment of cardiovascular outcomes
Follow-up was performed monthly by telephone or in person. A follow-up assessment in person was also conducted quarterly for a clinical evaluation and annually for a detailed examination. In WARCEF, an independent endpoint adjudication committee, whose members were unaware of the treatment assignments, adjudicated the primary and other outcomes. The primary outcome was the time to the first event in a composite endpoint of ischaemic stroke, intracerebral haemorrhage, or all-cause death. The secondary outcome was the first event in a composite of the primary outcome, MI, or HF hospitalization. The present study focused on individual CV outcomes. Sudden death was defined as (i) death witnessed or occurring within 15 min of observed collapse or new cardiac symptoms, without preceding other modes of death, or (ii) death unwitnessed but known to have occurred in the prior 72 h in the absence of other modes of death or (iii) patient resuscitated from cardiac arrest and dying within 24 h or prior to discharge, in case neurologic function was not restored. CV death included sudden death, documented ventricular tachycardia or fibrillation, documented bradyarrhythmia, MI, and circulatory failure. The diagnosis of MI was based on two of the following: (i) typical cardiac pain or its equivalent, (ii) electrocardiogram evidence of acute MI, or (iii) positive cardiac biomarkers. Stroke was defined as a clinically relevant new lesion detected on computed tomography or magnetic resonance imaging or, in its absence, clinical findings consistent with clinical stroke and lasting over 24 h. HF hospitalization during the follow-up were defined as admissions with typical symptoms; intravenous diuretics, vasodilator, or inotropic therapy; and at least 24 h of hospital stay. We also investigated major haemorrhage as a clinical event. Major haemorrhage was defined as intracerebral, epidural, subdural, subarachnoid, spinal intramedullary, or retinal haemorrhage; any other bleeding causing a decrease in the haemoglobin level of >2 g/dL in 48 h; or bleeding requiring transfusion of two units of whole blood, hospitalization, or surgical intervention.
Statistical analysis
The analysis is restricted to patients who have all four LV dimension parameters (n = 1138). Mean values ± standard deviation for continuous variables and frequencies for categorical variables are presented. Univariable Cox models were used to evaluate the association between clinical outcomes and different LV dimension parameters. The models were then adjusted for baseline covariates that are associated with each outcome in univariable Cox models. A threshold of P value ≤ 0.10 in the univariable model was used instead of 0.05 to allow the inclusion of more variables that might be clinically relevant to the outcomes. The proportional hazard assumption was tested using a Kolmogorov-type supremum test.13 The likelihood ratio test and the concordance statistic (C-index) were used to evaluate the additional benefit of LV volume parameter in addition to the corresponding LV diameter parameter in predicting the risk of each clinical outcome.
To investigate whether the risk associated with LV enlargement was independent of LVEF, we assessed the relationship between LV dimensions and outcomes using Cox models stratified by LVEF categories with a cut-off at 25%. Similarly, Cox models were used to assess whether there is any interaction between antithrombic treatment (aspirin or warfarin) and LV dimension parameters.
Missing values of baseline covariates with less than 10% missingness were imputed using mean for continuous variables and mode for categorical variables. Baseline variables with more than 10% missingness were excluded from the analysis. All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC).
Results
Among 2305 WARCEF patients, 1138 had measures of all four LV dimension parameters and were included in the analysis. Baseline characteristics of the study cohort are shown in Table 1. Mean age was 61 years; 980 patients (79.8%) were men. ACE inhibitor or ARB use and beta-blocker use were present in 1123 (98.9%) and 1021 (89.9), respectively. Overall, 269 patients (23.6%) experienced all-cause death, 187 patients (16.4%) CV death, 33 patients (2.9%) MI, 41 patients (3.6%) stroke, and 229 patients (20.1%) HF hospitalization. Forty-nine patients (6.6%) experienced major haemorrhage. There was no significant difference regarding CV outcomes or major haemorrhage between patients included and excluded in the analysis (shown in Supporting Information, Table S1).
| Baseline characteristics | n = 1138 | |
|---|---|---|
| Treatment group, no. (%) | ||
| Warfarin | 555 (48.8) | |
| Aspirin | 583 (51.2) | |
| Age (year) | 61 ± 11.5 | |
| Location, no. (%) | ||
| North America | 455 (40.0) | |
| Europe | 657 (57.7) | |
| Argentina | 26 (2.3) | |
| Sex, no. (%) | ||
| Male | 908 (79.8) | |
| Female | 230 (20.2) | |
| Race or ethnic group, no. (%) | ||
| Non-Hispanic white | 900 (79.1) | |
| Non-Hispanic black | 148 (13.0) | |
| Hispanic | 54 (4.8) | |
| Other | 36 (3.2) | |
| Hypertension, no./total no. (%) | 661/1106 (59.8) | |
| Diabetes mellitus, no./total no. (%) | 341/1136 (30.0) | |
| History of atrial fibrillation, no./total no. (%) | 38/1136 (3.4) | |
| Prior myocardial infarction, no./total no. (%) | 555/1136 (48.9) | |
| Ischaemic cardiomyopathy, no./total no. (%) | 501/1136 (44.1) | |
| Peripheral vascular disease, no. (%) | 126 (11.1) | |
| Prior stroke or TIA, no./total no. (%) | 146/1136 (12.9) | |
| Smoking status, no./total no. (%) | ||
| Current smoker | 196/1136 (17.3) | |
| Former smoker | 604/1136 (53.2) | |
| Never smoked | 336/1136 (29.6) | |
| Alcohol consumption, no. (%) | ||
| Current consumption, >2 oz/day | 270 (23.7) | |
| Previous consumption, >2 oz/day | 220 (19.3) | |
| Never consumed alcohol | 648 (56.9) | |
| NYHA classification, no. (%) | ||
| I | 150 (13.2) | |
| II | 617 (54.2) | |
| III | 352 (30.9) | |
| V | 19 (1.7) | |
| Distance covered on 6-min walk (m) | 353 ± 145.6 | (n = 1037) |
| History of aspirin or other antiplatelet agent, no./total no. (%) | 639/850 (75.2) | |
| History of warfarin or other oral anticoagulant, no. (%) | 96 (8.4) | |
| ACE inhibitor or ARB, no./total no. (%) | 1123/1135 (98.9) | |
| Beta-blocker, no./total no. (%) | 1021/1136 (89.9) | |
| Aldosterone blocker, no./total no. (%) | 409/672 (60.9) | |
| Nitrate, no./total no. (%) | 289/1136 (25.4) | |
| Calcium-channel blocker, no./total no. (%) | 90/1135 (7.9) | |
| Diuretic, no./total no. (%) | 931/1136 (82.0) | |
| Statin, no./total no. (%) | 669/812 (82.4) | |
| Pacemaker or defibrillator, no./total no. (%) | 263/1136 (23.2) | |
| BUN (mg/dL) | 24 ± 12.7 | (n = 1093) |
| Creatinine (mg/dL) | 1 ± 0.3 | (n = 1130) |
| eGFR (mL/min/1.73 m2) | 69 ± 20.4 | (n = 1130) |
| Haemoglobin (g/dL) | 14 ± 1.5 | (n = 1057) |
| Haematocrit (%) | 42 ± 4.4 | (n = 1068) |
| Sodium (mEq/L) | 140 ± 3.3 | (n = 1131) |
| White blood cell count (× 109/L) | 7 ± 2.0 | (n = 1131) |
| LVEF (%) | 24.5 ± 7.4 | |
| LVEDDI (cm/m2) | 3.3 ± 0.6 | |
| LVESDI (cm/m2) | 2.8 ± 0.6 | |
| LVEDVI (mL/m2) | 103.0 ± 36.7 | |
| LVESVI (mL/m2) | 78.1 ± 31.5 | |
- ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; BUN, blood urea nitrogen; EDDI, end-diastolic diameter index; EDVI, end-diastolic volume index; EF, ejection fraction; eGFR, estimated glomerular filtration rate; ESDI, end-systolic diameter index; ESVI, end-systolic volume index; LV, left ventricular; NYHA, New York Heart Association; TIA, transient ischemic attack.
- Mean ± SD were calculated for continuous variables, and number/total number (%) for categorical variables.
Left ventricular dimensions and cardiovascular outcomes
The associations between each LV dimension and CV outcomes using unadjusted and adjusted Cox proportional hazards regression analysis are shown in Table 2. After adjustment for pertinent covariates, LV dimensions were independently associated with risk of all-cause death, CV death, and HF hospitalization, but not MI or stroke. Both systolic and diastolic dimensions predicted CV outcomes to a similar extent.
| Outcomes | LVEDDI (per 1 cm/m2 increase) | LVESDI (per 1 cm/m2 increase) | LVEDVI (per 10 mL/m2 increase) | LVESVI (per 10 mL/m2 increase) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | ||||||
| All-cause death (n = 269) | Unadjusted | 1.59 | 1.31 | 1.93 | <0.001 | 1.69 | 1.40 | 2.05 | <0.001 | 1.08 | 1.05 | 1.12 | <0.001 | 1.11 | 1.07 | 1.15 | <0.001 |
| Adjusteda | 1.53 | 1.22 | 1.92 | <0.001 | 1.65 | 1.31 | 2.07 | <0.001 | 1.07 | 1.04 | 1.11 | <0.001 | 1.10 | 1.06 | 1.15 | <0.001 | |
| CV death (n = 187) | Unadjusted | 1.85 | 1.47 | 2.32 | <0.001 | 1.96 | 1.56 | 2.45 | <0.001 | 1.11 | 1.07 | 1.15 | <0.001 | 1.14 | 1.10 | 1.18 | <0.001 |
| Adjusteda | 1.68 | 1.29 | 2.19 | <0.001 | 1.79 | 1.38 | 2.34 | <0.001 | 1.09 | 1.05 | 1.14 | <0.001 | 1.12 | 1.07 | 1.18 | <0.001 | |
| MI (n = 33) | Unadjusted | 0.95 | 0.52 | 1.71 | 0.857 | 0.81 | 0.45 | 1.47 | 0.495 | 1.03 | 0.94 | 1.13 | 0.511 | 1.03 | 0.92 | 1.14 | 0.646 |
| Adjusteda | 1.01 | 0.55 | 1.85 | 0.979 | 0.88 | 0.48 | 1.62 | 0.688 | 1.03 | 0.94 | 1.13 | 0.488 | 1.03 | 0.92 | 1.15 | 0.591 | |
| Stroke (n = 41) | Unadjusted | 1.49 | 0.90 | 2.48 | 0.120 | 1.62 | 0.99 | 2.67 | 0.056 | 1.02 | 0.94 | 1.11 | 0.633 | 1.03 | 0.93 | 1.13 | 0.610 |
| Adjusteda | 1.46 | 0.86 | 2.46 | 0.159 | 1.60 | 0.96 | 2.68 | 0.073 | 1.02 | 0.94 | 1.10 | 0.655 | 1.03 | 0.93 | 1.13 | 0.613 | |
| HF hospitalization (n = 229) | Unadjusted | 1.74 | 1.41 | 2.15 | <0.001 | 1.94 | 1.57 | 2.38 | <0.001 | 1.08 | 1.05 | 1.12 | <0.001 | 1.11 | 1.07 | 1.15 | <0.001 |
| Adjusteda | 1.59 | 1.25 | 2.02 | <0.001 | 1.79 | 1.41 | 2.27 | <0.001 | 1.06 | 1.02 | 1.10 | 0.001 | 1.08 | 1.03 | 1.12 | <0.001 | |
- CI, confidence interval; CV, cardiovascular; EDDI, end-diastolic diameter index; EDVI, end-diastolic volume index; EF, ejection fraction; ESDI, end-systolic diameter index; ESVI, end-systolic volume index; HF, heart failure; HR, hazard ratio; LV, left ventricular; MI, myocardial infarction; other abbreviations as in Table 1.
- a Adjusted for age, location, sex, diabetes mellitus, history of atrial fibrillation, ischemic cardiomyopathy, peripheral vascular disease, smoking (current or former), NYHA classification (III, IV vs. I, II), distance covered 6-min walk, beta-blocker, diuretic, BUN, creatinine, eGFR, haemoglobin, haematocrit, and LVEF for all-cause death; adjusted for age, location, sex, ischemic cardiomyopathy, peripheral vascular disease, NYHA classification (III, IV vs. I, II), distance covered 6-min walk, beta-blocker, diuretic, BUN, creatinine, eGFR, haemoglobin, and LVEF for CV death; adjusted for prior MI, ischemic cardiomyopathy, and nitrates for MI; adjusted for location, prior stroke or TIA, nitrates, and BUN for stroke; adjusted for diabetes mellitus, ischemic cardiomyopathy, peripheral vascular disease, NYHA classification (III, IV vs. I, II), distance covered 6-min walk, history of aspirin or other antiplatelet agent, history of warfarin or other anticoagulant, diuretic, pace maker or defibrillator, BUN, creatinine, eGFR, haemoglobin, haematocrit, sodium, and LVEF for HF hospitalization.
Interaction between left ventricular dimensions and left ventricular ejection fraction on cardiovascular outcomes
To investigate whether the risk associated with LV enlargement was independent of LVEF, we assessed the relationship between LV dimensions and outcomes stratified by LVEF value. LV dimensions, whether by diameter or volume, were independently associated with risk of all-cause death both in patients with LVEF ≥ 25% and in those with LVEF < 25% after adjustment for pertinent covariates (Table 3). In both groups, each LV dimension parameter was also independently associated with risk of CV death and HF hospitalization. No association with MI or stroke was observed, regardless of LVEF level. There was no significant interaction between LV dimensions and LVEF category on the risk of any of the CV outcomes (also Table 3).
| Outcomes | LVEDDI (per 1 cm/m2 increase) | LVESDI (per 1 cm/m2 increase) | LVEDVI (per 10 mL/m2 increase) | LVESVI (per 10 mL/m2 increase) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | ||||||
| All-cause death (n = 269) | |||||||||||||||||
| Unadjusted | LVEF ≥ 25% (n = 556) | 1.71 | 1.24 | 2.36 | 0.001 | 1.78 | 1.28 | 2.46 | <0.001 | 1.10 | 1.05 | 1.16 | <0.001 | 1.14 | 1.07 | 1.22 | <0.001 |
| LVEF < 25% (n = 582) | 1.40 | 1.09 | 1.81 | 0.009 | 1.53 | 1.19 | 1.97 | 0.001 | 1.06 | 1.02 | 1.10 | 0.002 | 1.08 | 1.04 | 1.13 | <0.001 | |
| Interaction | 0.344 | 0.475 | 0.252 | 0.184 | |||||||||||||
| Adjusteda | LVEF ≥ 25% (n = 556) | 1.66 | 1.16 | 2.36 | 0.005 | 1.72 | 1.20 | 2.47 | 0.003 | 1.09 | 1.04 | 1.15 | <0.001 | 1.14 | 1.06 | 1.22 | <0.001 |
| LVEF < 25% (n = 582) | 1.45 | 1.10 | 1.93 | 0.009 | 1.61 | 1.22 | 2.13 | <0.001 | 1.06 | 1.02 | 1.11 | 0.006 | 1.09 | 1.03 | 1.15 | 0.001 | |
| Interaction | 0.556 | 0.768 | 0.382 | 0.315 | |||||||||||||
| CV death (n = 187) | |||||||||||||||||
| Unadjusted | LVEF ≥ 25% (n = 556) | 2.18 | 1.49 | 3.20 | <0.001 | 2.16 | 1.46 | 3.21 | <0.001 | 1.13 | 1.07 | 1.20 | <0.001 | 1.18 | 1.10 | 1.28 | <0.001 |
| LVEF < 25% (n = 582) | 1.52 | 1.13 | 2.04 | 0.006 | 1.67 | 1.25 | 2.24 | <0.001 | 1.08 | 1.04 | 1.13 | <0.001 | 1.11 | 1.05 | 1.16 | <0.000 | |
| Interaction | 0.141 | 0.304 | 0.258 | 0.153 | |||||||||||||
| Adjusteda | LVEF ≥ 25% (n = 556) | 2.14 | 1.39 | 3.29 | <0.001 | 2.13 | 1.36 | 3.32 | <0.001 | 1.13 | 1.06 | 1.20 | <0.001 | 1.19 | 1.10 | 1.30 | <0.001 |
| LVEF < 25% (n = 582) | 1.49 | 1.09 | 2.05 | 0.014 | 1.65 | 1.21 | 2.27 | 0.002 | 1.07 | 1.02 | 1.13 | 0.004 | 1.10 | 1.04 | 1.16 | 0.001 | |
| Interaction | 0.177 | 0.355 | 0.184 | 0.104 | |||||||||||||
| MI (n = 33) | |||||||||||||||||
| Unadjusted | LVEF ≥ 25% (n = 556) | 0.88 | 0.34 | 2.25 | 0.788 | 0.61 | 0.23 | 1.61 | 0.318 | 1.07 | 0.94 | 1.22 | 0.338 | 1.09 | 0.91 | 1.30 | 0.335 |
| LVEF < 25% (n = 582) | 0.98 | 0.44 | 2.19 | 0.962 | 0.95 | 0.43 | 2.10 | 0.900 | 1.00 | 0.88 | 1.14 | 0.977 | 0.99 | 0.85 | 1.15 | 0.874 | |
| Interaction | 0.862 | 0.488 | 0.505 | 0.404 | |||||||||||||
| Adjusteda | LVEF ≥ 25% (n = 556) | 0.89 | 0.34 | 2.31 | 0.805 | 0.61 | 0.23 | 1.65 | 0.330 | 1.05 | 0.92 | 1.20 | 0.435 | 1.08 | 0.90 | 1.29 | 0.422 |
| LVEF < 25% (n = 582) | 1.09 | 0.48 | 2.46 | 0.834 | 1.10 | 0.49 | 2.47 | 0.818 | 1.01 | 0.89 | 1.16 | 0.842 | 1.00 | 0.86 | 1.17 | 0.985 | |
| Interaction | 0.746 | 0.366 | 0.684 | 0.557 | |||||||||||||
| Stroke (n = 41) | |||||||||||||||||
| Unadjusted | LVEF ≥ 25% (n = 556) | 1.26 | 0.54 | 2.94 | 0.602 | 1.37 | 0.58 | 3.23 | 0.466 | 1.01 | 0.88 | 1.16 | 0.907 | 0.99 | 0.82 | 1.20 | 0.911 |
| LVEF < 25% (n = 582) | 1.61 | 0.83 | 3.13 | 0.160 | 1.77 | 0.913 | 3.43 | 0.091 | 1.02 | 0.91 | 1.13 | 0.757 | 1.03 | 0.91 | 1.16 | 0.692 | |
| Interaction | 0.651 | 0.646 | 0.923 | 0.757 | |||||||||||||
| Adjusteda | LVEF ≥ 25% (n = 556) | 1.17 | 0.49 | 2.80 | 0.729 | 1.32 | 0.55 | 3.12 | 0.534 | 1.01 | 0.88 | 1.16 | 0.884 | 0.99 | 0.82 | 1.21 | 0.953 |
| LVEF < 25% (n = 582) | 1.57 | 0.79 | 3.12 | 0.194 | 1.74 | 0.87 | 3.50 | 0.120 | 1.01 | 0.90 | 1.12 | 0.882 | 1.01 | 0.89 | 1.15 | 0.829 | |
| Interaction | 0.592 | 0.616 | 0.982 | 0.868 | |||||||||||||
| HF hospitalization (n = 229) | |||||||||||||||||
| Unadjusted | LVEF ≥ 25% (n = 556) | 1.67 | 1.16 | 2.42 | 0.006 | 1.72 | 1.18 | 2.49 | 0.005 | 1.09 | 1.03 | 1.15 | 0.003 | 1.13 | 1.05 | 1.22 | 0.001 |
| LVEF < 25% (n = 582) | 1.58 | 1.21 | 2.06 | <0.001 | 1.83 | 1.40 | 2.39 | <0.001 | 1.06 | 1.02 | 1.10 | 0.003 | 1.07 | 1.03 | 1.12 | 0.003 | |
| Interaction | 0.812 | 0.787 | 0.474 | 0.250 | |||||||||||||
| Adjusteda | LVEF ≥ 25% (n = 556) | 1.61 | 1.09 | 2.39 | 0.017 | 1.64 | 1.10 | 2.45 | 0.015 | 1.08 | 1.02 | 1.15 | 0.008 | 1.12 | 1.03 | 1.21 | 0.006 |
| LVEF < 25% (n = 582) | 1.58 | 1.19 | 2.10 | 0.002 | 1.87 | 1.40 | 2.49 | <0.001 | 1.05 | 1.01 | 1.10 | 0.024 | 1.06 | 1.01 | 1.12 | 0.019 | |
| Interaction | 0.924 | 0.600 | 0.407 | 0.282 | |||||||||||||
- CI, confidence interval; CV, cardiovascular; EDDI, end-diastolic diameter index; EDVI, end-diastolic volume index; EF, ejection fraction; ESDI, end-systolic diameter index; ESVI, end-systolic volume index; HF, heart failure; HR, hazard ratio; LV, left ventricular; MI, myocardial infarction.
- a Adjusted for covariates as in Table 2.
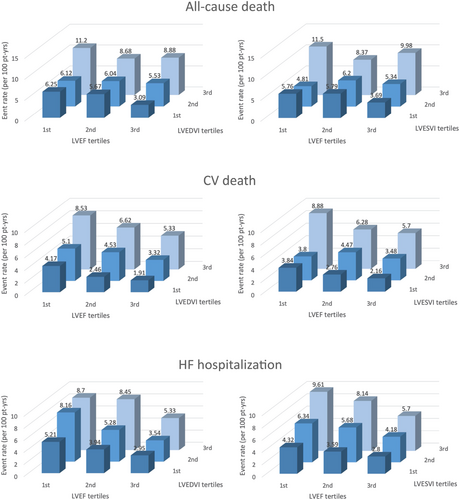
For the CV outcomes that were significantly associated with LV enlargement, Figure 1 shows the CV event rate stratified by LVEF and LV volume tertiles. Larger LV volume tended to be associated with CV events in each LVEF category, and a progressive rate increase was observed for both parameters; the effect of decreasing LVEF and increasing LV volumes was incremental to that of each condition alone, with the combination of lowest LVEF tertile and highest LV volume tertile being associated with the highest rates of all events.
Left ventricular diameters vs. left ventricular volumes for the prediction of cardiovascular outcomes
The results of the likelihood ratio test for nested models comparing LV diameters only vs. LV diameters plus corresponding LV volumes for the prediction of CV outcomes are shown in Table 4. The addition of LV volume measurement significantly improved the risk prediction compared with LV diameter alone in both diastole and systole in adjusted models for all-cause death (P = 0.005 and P = 0.005, respectively) and CV death (P = 0.003 and P = 0.004, respectively). For HF hospitalization, the addition of LV volumes significantly improved risk prediction compared with LV diameters alone at both diastole and systole in unadjusted analysis (P = 0.009 and P = 0.024, respectively), but not after adjustment for covariates. Similar results were obtained by concordance statistic; in adjusted analyses, the addition of LV volumes to LV diameters improved the prediction at both diastole and systole for all-cause death (C-index 0.7107 vs. 0.7143 and 0.7131 vs. 0.7169, respectively), CV death (C-index 0.7317 vs. 0.7390 and 0.7328 vs. 0.7388, respectively), and HF hospitalization (C-index 0.7242 vs. 0.7260 and 0.7282 vs. 0.7292, respectively) (shown in Table S3).
| Outcomes | Model type | Diastolic LV parameters | Systolic LV parameters | ||||||
|---|---|---|---|---|---|---|---|---|---|
| –2logL of diameter | –2logL of diameter & volume | LRT statistic | P value | –2logL of diameter | –2logL of diameter & volume | LRT statistic | P value | ||
| All-cause death (n = 269) | Unadjusted | 3502.922 | 3491.177 | 11.745 | <0.001 | 3496.028 | 3484.626 | 11.402 | <0.001 |
| Adjusteda | 3374.042 | 3366.108 | 7.934 | 0.005 | 3368.935 | 3360.988 | 7.947 | 0.005 | |
| CV death (n = 187) | Unadjusted | 2450.259 | 2436.814 | 13.445 | <0.001 | 2444.274 | 2431.051 | 13.223 | <0.001 |
| Adjusteda | 2357.655 | 2348.818 | 8.837 | 0.003 | 2353.991 | 2345.549 | 8.442 | 0.004 | |
| MI (n = 33) | Unadjusted | 441.129 | 440.426 | 0.703 | 0.402 | 440.691 | 439.694 | 0.997 | 0.318 |
| Adjusteda | 432.234 | 431.663 | 0.571 | 0.450 | 432.073 | 431.312 | 0.761 | 0.383 | |
| Stroke (n = 41) | Unadjusted | 551.540 | 551.449 | 0.091 | 0.763 | 550.340 | 549.939 | 0.401 | 0.527 |
| Adjusteda | 529.048 | 528.955 | 0.093 | 0.760 | 527.852 | 527.477 | 0.375 | 0.540 | |
| HF hospitalization (n = 229) | Unadjusted | 2998.886 | 2991.994 | 6.892 | 0.009 | 2987.349 | 2982.231 | 5.118 | 0.024 |
| Adjusteda | 2889.416 | 2886.666 | 2.750 | 0.097 | 2881.325 | 2880.126 | 1.199 | 0.274 | |
- CV, cardiovascular; HF, heart failure; LRT, likelihood ratio test; LV, left ventricular; MI, myocardial infarction.
- a Adjusted for covariates as in Table 2.
Effect of antithrombotic treatment
Table 5 shows the association between LV dimensions and outcomes stratified by warfarin or aspirin treatment. All LV dimensions were associated with all-cause death, CV death, and HF hospitalization, but not MI or stroke, in both treatment arms, as already observed in the overall study cohort. The deleterious effect of LV enlargement tended to be stronger in warfarin-treated than in aspirin-treated patients for all-cause death, CV death, stroke, and HF hospitalization. A significant interaction between LV volumes, but not LV diameters, and treatment type was observed (on all-cause death and CV death for LVEDVI; on all-cause death, CV death, and stroke for LVESVI; also Table 5). LV enlargement, especially when defined by LV volumes, was significantly associated with outcomes in warfarin-treated patients in both patients with adequate (>60%) time in therapeutic range (TTR) and those with inadequate TTR in adjusted models for all-cause death (LVEDVI: P = 0.003 and P = 0.007; LVESVI: P = 0.007 and P = 0.002, respectively) and CV death (LVEDVI: P < 0.001 and P = 0.006; LVESVI: P = 0.001 and P = 0.002, respectively). For HF hospitalization, LV volumes were significantly associated with outcomes in both warfarin-treated patients with adequate TTR and those with inadequate TTR in unadjusted models (LVEDVI: P = 0.018 and P = 0.003; LVESVI: P = 0.011 and P < 0.001, respectively). No significant interaction of LV dimensions and TTR on the risk of outcomes was observed.
| Outcomes | LVEDDI (per 1 cm/m2 increase) | LVESDI (per 1 cm/m2 increase) | LVEDVI (per 10 mL/m2 increase) | LVESVI (per 10 mL/m2 increase) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | HR | 95% CI | P value | ||||||
| All-cause death (n = 269) | |||||||||||||||||
| Unadjusted | Warfarin (n = 555) | 1.58 | 1.19 | 2.11 | 0.002 | 1.65 | 1.25 | 2.18 | <0.001 | 1.10 | 1.06 | 1.15 | <0.001 | 1.13 | 1.08 | 1.19 | <0.001 |
| Aspirin (n = 583) | 1.59 | 1.22 | 2.08 | <0.001 | 1.74 | 1.33 | 2.27 | <0.001 | 1.07 | 1.02 | 1.11 | 0.002 | 1.09 | 1.04 | 1.14 | <0.001 | |
| Interaction | 0.968 | 0.782 | 0.234 | 0.217 | |||||||||||||
| Adjusteda | Warfarin (n = 555) | 1.53 | 1.11 | 2.10 | 0.009 | 1.61 | 1.18 | 2.20 | 0.003 | 1.11 | 1.06 | 1.17 | <0.001 | 1.15 | 1.09 | 1.22 | <0.001 |
| Aspirin (n = 583) | 1.53 | 1.13 | 2.07 | 0.006 | 1.70 | 1.25 | 2.31 | <0.001 | 1.04 | 1.00 | 1.09 | 0.074 | 1.06 | 1.01 | 1.12 | 0.028 | |
| Interaction | 1.000 | 0.804 | 0.040 | 0.034 | |||||||||||||
| CV death (n = 187) | |||||||||||||||||
| Unadjusted | Warfarin (n = 555) | 1.86 | 1.34 | 2.57 | <0.001 | 1.89 | 1.38 | 2.61 | <0.001 | 1.14 | 1.09 | 1.19 | <0.001 | 1.17 | 1.11 | 1.23 | <0.001 |
| Aspirin (n = 583) | 1.84 | 1.33 | 2.53 | <0.001 | 2.03 | 1.47 | 2.80 | <0.001 | 1.08 | 1.03 | 1.13 | 0.002 | 1.10 | 1.04 | 1.17 | <0.001 | |
| Interaction | 0.970 | 0.771 | 0.128 | 0.127 | |||||||||||||
| Adjusteda | Warfarin (n = 555) | 1.69 | 1.18 | 2.43 | 0.004 | 1.75 | 1.23 | 2.48 | 0.002 | 1.14 | 1.09 | 1.20 | <0.001 | 1.18 | 1.11 | 1.26 | <0.001 |
| Aspirin (n = 583) | 1.68 | 1.17 | 2.40 | 0.005 | 1.86 | 1.29 | 2.68 | <0.001 | 1.05 | 0.99 | 1.10 | 0.095 | 1.07 | 1.00 | 1.14 | 0.047 | |
| Interaction | 0.973 | 0.800 | 0.018 | 0.014 | |||||||||||||
| MI (n = 33) | |||||||||||||||||
| Unadjusted | Warfarin (n = 555) | 1.03 | 0.42 | 2.53 | 0.947 | 0.72 | 0.29 | 1.78 | 0.477 | 0.90 | 0.76 | 1.08 | 0.270 | 0.88 | 0.71 | 1.09 | 0.255 |
| Aspirin (n = 583) | 0.88 | 0.40 | 1.95 | 0.758 | 0.89 | 0.41 | 1.95 | 0.776 | 1.10 | 0.99 | 1.22 | 0.090 | 1.10 | 0.97 | 1.25 | 0.143 | |
| Interaction | 0.799 | 0.723 | 0.069 | 0.084 | |||||||||||||
| Adjusteda | Warfarin (n = 555) | 1.15 | 0.47 | 2.85 | 0.761 | 0.81 | 0.32 | 2.04 | 0.649 | 0.91 | 0.76 | 1.10 | 0.327 | 0.89 | 0.71 | 1.11 | 0.300 |
| Aspirin (n = 583) | 0.89 | 0.40 | 2.02 | 0.787 | 0.94 | 0.42 | 2.08 | 0.872 | 1.09 | 0.98 | 1.22 | 0.121 | 1.10 | 0.96 | 1.25 | 0.165 | |
| Interaction | 0.683 | 0.809 | 0.101 | 0.110 | |||||||||||||
| Stroke (n = 41) | |||||||||||||||||
| Unadjusted | Warfarin (n = 555) | 1.98 | 0.89 | 4.38 | 0.092 | 1.76 | 0.80 | 3.89 | 0.159 | 1.09 | 0.97 | 1.23 | 0.139 | 1.13 | 0.99 | 1.29 | 0.061 |
| Aspirin (n = 583) | 1.24 | 0.65 | 2.39 | 0.516 | 1.55 | 0.81 | 2.94 | 0.183 | 0.970 | 0.87 | 1.08 | 0.569 | 0.95 | 0.83 | 1.08 | 0.406 | |
| Interaction | 0.376 | 0.800 | 0.140 | 0.056 | |||||||||||||
| Adjusteda | Warfarin (n = 555) | 2.04 | 0.92 | 4.52 | 0.079 | 1.91 | 0.86 | 4.23 | 0.113 | 1.10 | 0.99 | 1.24 | 0.086 | 1.14 | 1.01 | 1.29 | 0.035 |
| Aspirin (n = 583) | 1.13 | 0.58 | 2.24 | 0.718 | 1.37 | 0.70 | 2.67 | 0.357 | 0.95 | 0.86 | 1.06 | 0.384 | 0.93 | 0.82 | 1.06 | 0.271 | |
| Interaction | 0.267 | 0.529 | 0.065 | 0.025 | |||||||||||||
| HF hospitalization (n = 229) | |||||||||||||||||
| Unadjusted | Warfarin (n = 555) | 1.66 | 1.25 | 2.20 | <0.001 | 1.87 | 1.42 | 2.46 | <0.001 | 1.08 | 1.04 | 1.13 | <0.001 | 1.11 | 1.06 | 1.16 | <0.001 |
| Aspirin (n = 583) | 1.86 | 1.36 | 2.53 | <0.001 | 2.02 | 1.47 | 2.76 | <0.001 | 1.08 | 1.04 | 1.14 | <0.001 | 1.11 | 1.05 | 1.17 | <0.001 | |
| Interaction | 0.595 | 0.720 | 0.999 | 0.988 | |||||||||||||
| Adjusteda | Warfarin (n = 555) | 1.62 | 1.18 | 2.22 | 0.003 | 1.90 | 1.40 | 2.58 | <0.001 | 1.08 | 1.03 | 1.13 | 0.0017 | 1.10 | 1.04 | 1.16 | 0.001 |
| Aspirin (n = 583) | 1.56 | 1.13 | 2.12 | 0.008 | 1.66 | 1.18 | 2.33 | 0.003 | 1.04 | 0.99 | 1.10 | 0.103 | 1.05 | 0.99 | 1.12 | 0.092 | |
| Interaction | 0.884 | 0.549 | 0.359 | 0.313 | |||||||||||||
- CI, confidence interval; CV, cardiovascular; EDDI, end-diastolic diameter index; EDVI, end-diastolic volume index; ESDI, end-systolic diameter index; ESVI, end-systolic volume index; HF, heart failure; HR, hazard ratio; LV, left ventricular; MI, myocardial infarction.
- a Adjusted for covariates as in Table 2.
Discussion
Left ventricular dimensions and outcomes
In the present study, we describe how LV enlargement was significantly associated with all-cause death, CV death and HF hospitalization in a cohort of patients with HFrEF and sinus rhythm who were treated with recommended HF medications and randomized to different antithrombotic treatments. No significant association was observed between LV enlargement and stroke or MI.
The observation of a relationship between LV dimensions and CV outcomes is consistent with previous studies. Yeboah et al. reported that LV diastolic dysfunction by cardiac magnetic resonance imaging was a predictor of incident HF in 4974 patients with subclinical CV disease and without known CV disease in a MESA study subanalysis.5 LV diastolic dysfunction was a significant predictor even in the subgroup with low LVEF (n = 85). McManus et al. also reported on LVESVI as a predictor of incident HF in patients with stable coronary artery disease.4 In patients with low LVEF, Solomon et al. also reported that LVEDV and LVESV were independent predictors for the combined end points of death or HF or the combined end point of death, HF, MI, cardiac arrest, or stroke in 603 patients after MI enrolled in the VALIANT Echo Study.6 The present study provides similar results in a more recently enrolled large cohort, but notable differences do exist. The present study includes a far larger number of patients with low LVEF (1138 vs. 603) and patients on beta-blockers even compared with VALIANT Echo Study (89.9% of patients vs. 73.4%). Several other studies also reported on the association between LV dimensions and CV outcomes in patients with HFrEF.1, 2 However, their use of recommended HF medications, including beta-blockers and ACE inhibitors or ARB, was infrequent.
The most widely accepted explanation of LV enlargement as a predictor of CV outcomes is that LV enlargement is a compensatory mechanism for LV systolic dysfunction. The myocardium has been shown to remodel after an injury14; after MI, outward remodelling of the LV myocardium and consequent LV enlargement is often observed.15 Enlarged LV may not be able to compensate for increasing afterload as preload reserve may already be exceeded even at baseline. This can result in afterload mismatch.16 LV enlargement was, hence, an independent predictor of CV outcomes not only in patients with MI, but also in those with dilated cardiomyopathy, and valvular disease.3, 5, 7 LV enlargement precedes clinical symptoms of HF and has been the target for disease-modifying therapies such as beta-blockers, ACE inhibitors, ARB, and aldosterone antagonists.17-25 Our study demonstrates that the independent effect of LV enlargement on death and HF hospitalization persists even in the presence of these treatments, which were highly prevalent in our cohort, except for aldosterone antagonists.
No significant association was observed between LV enlargement and the risk of MI or stroke. This may have been driven in part by the low number of such events, which confirms that these adverse outcomes are infrequent in systolic HF patients in sinus rhythm treated with optimal HF therapy. In addition, the presence of warfarin or aspirin treatment in all patients may have contributed to the lower number of MI and stroke outcomes, which may more often recognize an embolic aetiology and be less affected by LV dimension than other CV outcomes.
Interaction between left ventricular dimensions and left ventricular ejection fraction on cardiovascular outcomes
Left ventricular ejection fraction was inversely associated with all-cause death, CV death, and HF hospitalization [hazard ratio (HR) 0.97; 95% confidence interval (CI) 0.96 to 0.99; P < 0.001, HR 0.96; 95% CI 0.94 to 0.98; P < 0.001 and HR 0.96; 95% CI 0.94 to 0.98; P < 0.001, respectively], but not with MI and stroke, confirming a previous analysis on the same cohort.26 LVEF is the most widely accepted indicator of LV systolic function and is associated with CV outcomes.6, 8, 26-29 Because LV enlargement and lower LVEF tend to be associated, the risk of outcome associated with LV enlargement may reflect the coexistence of severely reduced LVEF. In the present study, LV enlargement was a significant predictor of all-cause death, CV death and HF hospitalization in both patients with less (≥ 25%) or more (< 25%) severe LVEF reduction (Table 3). Moreover, there was no significant interaction between any LV dimension parameter and LVEF on all CV outcomes, although the highest frequencies of outcome events were observed in patients in the lowest tertile of LVEF and highest tertile of LV volumes (Figure 1). These results suggest that the association between LV dimensions and CV outcomes is not merely a reflection of concomitant differences in LVEF and that LV enlargement should be regarded as an additional risk factor over lower LVEF, possibly signalling the need for more intensive HF treatment for any given LVEF value when LV dilation is also present.
Prognostic value of left ventricular diameters vs. left ventricular volumes
Although the first studies on the prognostic role of LV dimension in HF were based on LV diameters,3, 7 the use of LV volumes has become the gold standard for the assessment of LV dimensions.30, 31 The measurement of LV volumes is however more time consuming and may not be as feasible as a linear measurement in some patients. In the present study, LV enlargement, both by diameters or volumes, was an independent predictor of CV outcomes; however, the addition of LV volumes to LV diameters increased the predictive value for CV outcomes. LV volumes more accurately represent actual LV dimensions than LV diameters parameters because they correct for LV shape distortions that may not be accounted for when using a linear dimension, especially in patients with regional LV dysfunction and/or LV remodelling.30, 31 This circumstance may have driven the observed difference in prediction ability between LV diameter parameters and LV volume parameters. The modern three-dimensional echocardiographic assessment of LV volumes may further refine the predictive power for outcomes. LV volume addition also unmasked possible differences in the effect of LV enlargement in patients treated with warfarin or aspirin (refer to the next section). Therefore, our results suggest that, while the measurements of LV diameters may be sufficient for a screening for CV risk, the addition of LV volumes may result in a refinement of the prediction that is desirable whenever their measurement is technically feasible.
Effect of antithrombotic treatment
Because some CV events in HFrEF may recognize an embolic aetiology, systemic anticoagulation might be expected to decrease the risk; in the WARCEF trial, warfarin treatment appeared to decrease the stroke risk, although this effect was counteracted by an increase in risk of major haemorrhagic events.11 There was no significant difference in major haemorrhagic events between patients included or excluded from the analysis (shown in Table S1), and there was no significant association between LV dimension parameters and major haemorrhage (shown in Table S4). Because LV chamber enlargement may predispose to blood stasis and thrombus formation, warfarin treatment might be expected to reduce the risk of CV events related to LV enlargement to a greater extent than aspirin. Our results showed that LV enlargement was associated with death and HF hospitalization in both treatment arms, but its effect seemed to be stronger in the warfarin than in the aspirin arm; a significant interaction between LV volumes and antithrombotic treatment was observed all-cause death, CV death, stroke, and HF hospitalization. This finding might be secondary to the fact that an adequate TTR was not uniformly achieved in warfarin-treated patients, thus conceivably reducing the treatment effect on embolic events; an adequate TTR (>60%) was achieved in only 38.8% of patients, which may have diluted the effect of warfarin treatment on the results. This observation raises the question of whether achieving a better TTR might have affected the observed treatment differences; also, it suggests the need to assess the effect on the association between LV dimensions and CV outcomes of direct oral anticoagulants (DOAC). The efficacy of DOAC in reducing the risk of thromboembolic events in patients with atrial fibrillation is well documented; DOAC might achieve a more consistent anticoagulation level than warfarin, and their use might provide new insights on preventing CV outcomes in HFrEF patients with sinus rhythm and LV enlargement.
Limitations
Our study has some limitations. First, approximately half of the original WARCEF cohort had adequate information on LV dimensions both by diameters and volumes, as the present investigation is ad hoc analysis. This smaller sample size may have decreased the ability to detect significant associations between LV enlargement and low-frequency events such as MI and stroke. Additionally, information on other possible contributors to outcome, such as degree of functional mitral regurgitation and LV diastolic dysfunction, was not uniformly available in the study. On the other hand, the central interpretation of the echocardiographic studies assured a standardized assessment of LV dimensions.
Second, as per WARCEF protocol, only patients with HFrEF (LVEF ≤ 35%) were included in the study; therefore, the relationship between LV dimensions and CV outcomes in patient with HF with preserved LVEF could not be investigated.
The patients in this study may be at a more advanced stage of HF than normally encountered in clinical practice, as mean LV volumes seem to be larger than those commonly observed in HF patients. Also, the LVEF inclusion criterion of the study (≤35%) is lower than that included in HF guidelines (LVEF ≤ 40%). The mean values of LV volumes, however, are similar to those of previous studies in HFrEF patients.32-34 Although guidelines-recommended treatment of HFrEF was present in the vast majority of patients, more modern drugs, such as angiotensin receptor–neprilysin inhibitor or sodium-glucose cotransporter-2 inhibitors, were not available at the time the trial took place. Finally, this is a retrospective analysis from a prospectively designed clinical trial that was not originally designed to evaluate the association between LV dimensions and CV outcomes. Nevertheless, the present study is one of the largest investigations on this topic in patients with HFrEF and recommended HF therapy.
Conclusions
In conclusion, (i) LV enlargement remains associated with all-cause death, CV death, and HF hospitalization, but not MI or stroke, in patients with HFrEF and sinus rhythm treated with recommended HF medications and antithrombotic medications; (ii) the association between LV dimensions and CV outcomes is not merely a reflection of differences in LVEF, but is additional to it; (iii) LV volumes determination confers incremental predictive value over LV diameters alone; (iv) the effect of LV volumes on CV outcomes persists despite the presence of antithrombotic treatment, but may be affected by its type.
In summary, LV enlargement was independently associated with CV outcomes in patients with HFrEF even when treated with recommended HF therapy. LV dimensions may represent an additional indication over LVEF for more intensive HF treatment.
Conflict of interest
S.D.A. reports receiving fees from Abbott Vascular, Bayer, Boehringer Ingelheim, Cardiac Dimension, Impulse Dynamics, Novartis, Servier and Vifor Pharma, and grant support from Abbott Vascular and Vifor Pharma. The other authors report no conflicts.
Funding
This work was supported by the National Institute of Neurological Disorders and Stroke (U01-NS-043975 to S.H. and U01-NS-039143 to J.L.P.T.).




