Managing older patients with heart failure calls for a holistic approach
Abstract
Aims
This study aims to assess the presence of geriatric domain impairments in an older heart failure (HF) outpatient population and to relate these domain impairments with 1 year mortality risk in comparison with a geriatric outpatient population without HF.
Methods and results
Data were used from two different prospective cohort studies: 241 outpatients with HF (mean age 78 ± 9 years, 48% female) and 686 geriatric outpatients (mean age 80 ± 7 years, 55% female). We similarly assessed the following geriatric domains in both cohorts: physical function, nutritional status, polypharmacy, cognitive function, and activities in daily living. Cox proportional hazards analyses were used to relate individual domains to 1 year mortality risk in both populations and to compare 1 year mortality risk between both populations. Of the patients with HF, 34% had impairments in ≥3 domains, compared with 38% in geriatric patients. One-year mortality rates were 13% and 8%, respectively, in the HF and geriatric populations; age-adjusted and sex-adjusted hazard ratio (95% confidence interval) for patients with HF compared with geriatric patients was 1.7 (1.3–2.6). The individual geriatric domains were similarly associated with 1 year mortality risk in both populations. Compared with zero to two impaired domains, age-adjusted and sex-adjusted mortality risk (hazard ratio, 95% confidence interval) for three, four, or five impaired domains ranged from 1.6 (0.6–4.2) to 6.5 (2.1–20.1) in the HF population and from 1.4 (0.7–2.9) to 7.9 (2.9–21.3) in the geriatric population.
Conclusions
In parallel with geriatric patients, patients with HF often have multiple geriatric domain impairments that adversely affect their prognosis. This similarity together with the findings that patients with HF have a higher 1 year mortality risk than a general geriatric population supports the integration of a multi-domain geriatric assessment in outpatient HF care.
Introduction
Heart failure (HF) is a major public health problem as its prevalence and incidence increase with age.1 In addition, HF is associated with accelerated biological ageing resulting in an increased risk for other health issues.2-6
The co-occurrence of HF with other health issues and their association with an increased risk of adverse health outcomes, such as functional decline, institutionalization, and mortality, calls for a holistic approach. The European Society of Cardiology (ESC) 2016 HF guideline states that a more holistic approach, by monitoring frailty and cognitive impairment, should be a key component in managing older patients with HF.7 A multi-domain geriatric assessment can be helpful to identify impairments in geriatric domains to develop a co-ordinated and integrated plan for improving health outcomes for older adults with HF.4
In the majority of HF clinics, a multi-domain geriatric assessment has not yet been incorporated in daily assessment routine, and existing assessments are mainly focused on co-morbidities rather than impairments in geriatric domains. We recently demonstrated the potential added value of a multi-domain geriatric assessment in the evaluation and treatment of patients with HF, as the number of geriatric domain impairments appeared more strongly associated with short-term (3 months) adverse health outcomes than severity of HF or co-morbidities.8 However, the prevalence of geriatric domain impairments in older patients with HF in comparison with geriatric patients, in which geriatric domain impairments are routinely assessed,9 is not precisely known. This study was set up to assess the presence of geriatric domain impairments and to relate geriatric domain impairments with 1 year mortality risk in an outpatient older HF population as compared with a geriatric outpatient population without HF.
Methods
Design and population
This prospective cohort study included 241 consecutive patients aged ≥60 years previously diagnosed with HF (not specified to any underlying cardiac cause) attending outpatient HF clinics between January 2018 and March 2019 in two Dutch hospitals, namely, the Amsterdam University Medical Center, location VUmc, Amsterdam (tertiary hospital) and Amstelland Hospital, Amstelveen (community hospital).
For comparison with a geriatric population, data of 686 geriatric outpatients of the Amsterdam Ageing Cohort were used.10 The Amsterdam Ageing Cohort is an ongoing longitudinal cohort study of patients visiting the outpatient geriatric clinic at the Amsterdam University Medical Center, location VUmc. Patients are referred to the outpatient clinic for medical care. Patients from the Amsterdam Ageing Cohort were excluded if they did not have a full year of follow-up or if they had been diagnosed with HF according to their electronic medical records.
The local medical ethics committees approved both research protocols, and all patients gave written informed consent before study entry.
Demographics and general assessment
Demographics (age and gender) of both cohorts were assessed at baseline. The general assessment included blood pressure and heart rate measurement (measured with Dinamap® automated blood pressure monitor), and presence of diabetes mellitus was assessed according to the medical history. The standard cardiac assessment in the HF population included N-terminal pro-hormone brain natriuretic peptide, the New York Heart Association classification, and the left ventricular ejection fraction as measured with an echocardiogram. Additionally, patients with HF were categorized as HF with reduced ejection fraction (HFrEF), HF with mid-range ejection fraction (HFmrEF), and HF with preserved ejection fraction (HFpEF) if their ejection fraction was <40%, 40–49%, or >50%, respectively.7
Multi-domain geriatric assessment
A multi-domain geriatric assessment was performed at baseline by a trained nurse (either an HF nurse or geriatric nurse, according to the study population) and covered physical function, nutritional status, cognitive function, co-morbidities/polypharmacy, and dependency in activities of daily living (ADL). We scored the different domains impaired as yes/no according to pre-defined cut-off values and calculated the total sum of impaired domains (zero to five).
Co-morbidities/polypharmacy domain
As proxy of presence of co-morbidities, number of prescribed medication was scored according to the prescribed medication in the electronic health record. Polypharmacy was assessed as a continuous value and dichotomized as 0–9 and ≥10 prescribed drugs. Use of ≥10 drugs was used to define polypharmacy instead of use of ≥5 drugs in this study, because chronic use of more than five drugs is almost universal in the HF population due to treatment guidelines and does therefore not discriminate enough as proxy for co-morbidities.11
Physical domain
Physical function was assessed using hand grip strength (kg) and gait speed (m/s). Hand grip strength (kg) was measured using an isometric hand dynamometer (Jamar® hand dynamometer, Sammons Preston) in upright standing position, while holding the dynamometer in one hand. Patients were actively encouraged to squeeze with maximal strength. Two trials were performed alternately for each hand. The best performance out of four measurements was used. Gait speed (m/s) was assessed using a timed 4 m walking test at normal pace from a standing position using a stopwatch. The fastest time of two trials was used for analyses.
The physical domain was considered impaired when a patient had either low hand grip strength or low gait speed. Low handgrip strength was defined as <20 kg for women and <30 kg for men.12 Low gait speed was defined as a walking speed <0.8 m/s.13
Nutritional domain
The Mini Nutritional Assessment-Short Form (MNA-SF) was used to assess the nutritional status.14 The MNA-SF score ranges from 1 to 14 and assesses six items, including weight loss, body mass index, psychological stress or acute disease, mobility, neuropsychological problems, and food intake. The nutritional domain was considered impaired when the MNA-SF score was below 12.15
Cognitive domain
Cognitive function was assessed with the Mini Mental State Examination (MMSE).16 This widely used screening tool has a maximum of 30 points. The cognitive domain was considered impaired when the MMSE score was below 26.17
Dependency domain
The Katz ADL questionnaire was used to evaluate whether a patient needed assistance during one of the following six daily activities: bathing, getting dressed, toileting, transferring from bed to chair, eating/feeding, and continence.18 The total number of daily activities with need for assistance was assessed. The dependency domain was considered impaired if the patient needed assistance in one or more daily activities.
One-year mortality
For the HF population, mortality data and date of death were derived from the patient's electronic health records 1 year after inclusion as part of the study protocol (i.e. end of follow-up). Furthermore, patients were called at 1 year follow-up, and if they or their first contact could not be reached, their general practitioner was contacted to determine their health status (e.g. death or alive).
For the geriatric population of the Amsterdam Ageing Cohort, mortality data and date of death were obtained from the Dutch municipal population register on the 22nd of January 2020. The cause of death could not be obtained via this register. If patients in the Amsterdam Ageing Cohort died more than 365 days after inclusion, they were scored as alive at 1 year follow-up.
For both cohorts, the difference between inclusion date and date of death was calculated based on the mortality data.
Statistical analysis
Statistical analyses were performed using SPSS Version 26 (IBM, Armonk, NY, USA). A P-value < 0.05 was considered statistically significant.
Baseline characteristics were assessed for the HF population and the geriatric population separately. Continuous variables were presented as mean [standard deviation (SD)] or median (interquartile range), depending on the distribution of the variable. Dichotomous variables were presented as frequencies and percentage. Independent sample t-tests, Mann–Whitney U-tests, and χ2 tests were used to assess the differences between population groups in mean, median, and frequencies. Additional analyses were performed to compare baseline characteristics between patients with HFmrEF or HFrEF and patients with HFpEF.
Cox proportional hazards analysis was used in three different ways to assess the 1 year mortality risk [hazard ratios (HRs) and 95% confidence interval (95% CI)].
First, we compared the 1 year mortality risk in the older HF population with the 1 year mortality risk in the geriatric population, taken the latter as reference. All analyses were adjusted for age and gender.
Second, we assessed the relation between impairments in geriatric domains (and its individual items) and 1 year mortality within both populations. We assessed the 1 year mortality risk of the geriatric domain impairments per population, using pre-defined cut-off values. Furthermore, we investigated the association of one SD increase in the individual items of the geriatric assessment on mortality risk. For this, to allow comparison of the individual geriatric assessment items, the different variables were converted into Z-scores and inverted when needed, so that one SD increase in the analysis correlated with a worse performance on the measurement. All these analyses were adjusted for age and sex.
Third, we assessed the influence of the accumulation (i.e. sum) of domains impaired on mortality risk per population, using the presence of zero to two domains impaired as the reference category. These analyses were also adjusted for age and sex.
Results
Table 1 presents the baseline characteristics of the HF population (n = 241) and the geriatric population (n = 686) separately. Patients with HF were younger and less often female and had a lower systolic and diastolic blood pressure. Within patients with HF, the number of patients with HFpEF was lower than patients with either HFmrEF or HFrEF: 18% vs. 82%. Patients with HFpEF were older and more often female, but no significant differences in number of medications, renal function, or number of geriatric domains deficits were found between both groups (Supporting Information, Table S1).
| N | Outpatient heart failure population (N = 241) | Outpatient geriatric population (N = 686) | P-value | |
|---|---|---|---|---|
| Age (mean ± SD) | 927 | 78 ± 9 | 80 ± 7 | 0.01 |
| Female (n, %) | 927 | 115 (48%) | 377 (55%) | 0.05 |
| General assessment | ||||
| Diabetes mellitus (n, %) | 927 | 57 (24%) | 138 (20%) | 0.25 |
| Systolic BP, mmHG (mean ± SD) | 912 | 124 (19) | 145 (20) | <0.01 |
| Diastolic BP, mmHG (mean ± SD) | 912 | 70 (11) | 80 (11) | <0.01 |
| Heart rate, per minute | 905 | 72 (11) | 71 (14) | 0.14 |
| Cardiac assessment | ||||
| Ejection fraction (mean ± SD) | 207 | 38 (13) | n.a. | |
| Type of heart failure | 207 | |||
| HFrEF (n, %) | 112 (54%) | |||
| HFmrEF (n, %) | 56 (27%) | |||
| HFpEF (n, %) | 39 (19%) | |||
| NT-proBNP (median, IQR) | 231 | 1939 (881–3425) | n.a. | |
| NYHA class (n, %) | 235 | n.a. | ||
| Class I | 57 (24%) | |||
| Class II | 110 (47%) | |||
| Class III/IV | 68 (29%) | |||
| Geriatric assessment | ||||
| Prescribed medication, number (mean ± SD) | 925 | 10 ± 4 | 7 ± 4 | <0.01 |
| Hand grip strength, kg (mean ± SD) | 837 | |||
| Male | 31 ± 10 | 30 ± 10 | 0.23 | |
| Female | 17 ± 7 | 18 ± 7 | 0.14 | |
| Gait speed, m/s (mean ± SD) | 927 | 0.91 ± 0.54 | 0.86 ± 0.35 | 0.08 |
| MNA-SF score (median, IQR) | 857 | 12 (11–14) | 12 (10–13) | <0.01 |
| MMSE score (median, IQR) | 904 | 28 (26–29) | 25 (22–28) | <0.01 |
| Katz ADL score (median, IQR) | 855 | 0 (0–1) | 0 (0–1) | 0.48 |
- ADL, activities of daily living; BP, blood pressure; HFmrEF, heart failure with mid-range ejection fraction; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; IQR, interquartile range; MMSE, Mini Mental State Examination; MNA-SF, Mini Nutritional Assessment-Short Form; n.a., not applicable; NT-proBNP, N-terminal pro-hormone brain natriuretic peptide; NYHA, New York Heart Association; SD, standard deviation.
Presence of geriatric domains
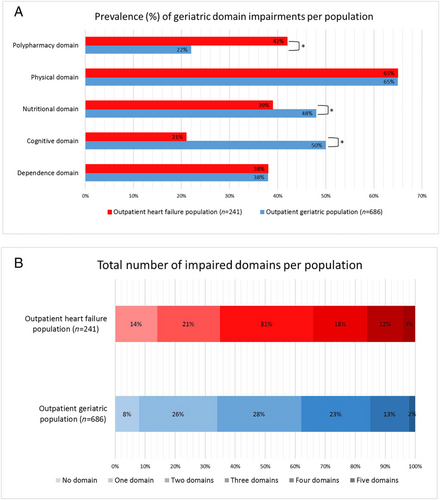
Figure 1 shows the prevalence of impairment in different geriatric domain in the HF and geriatric populations. The prevalence of impairment of the physical and ADL domains was similar for both populations. Polypharmacy was more often present in the HF population, while impairments in the cognitive and nutritional domains were more often present in the geriatric population. A total of 34% in the HF population and 38% in the geriatric population had impairments in three or more domains. The distribution of number of impaired domains did not differ between the HF and geriatric populations (Figure 1).
Mortality risk in heart failure versus geriatric outpatients
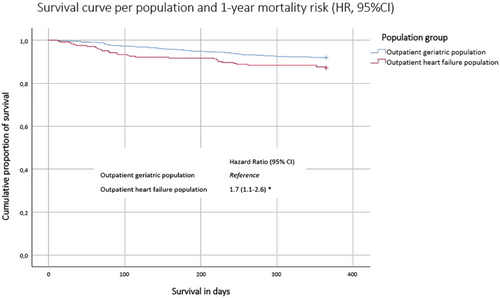
Of the patients with HF, 13% had died within a year, compared with 8% in the geriatric population. Figure 2 shows the Kaplan–Meier survival curve, stratified per population. The survival curve of older patients with HF is significantly steeper than the curve of the geriatric population (log rank test P = 0.027). Cox proportional hazards analyses showed that older patients with HF had a 1.7-fold (95% CI 1.1–2.6) age-adjusted and sex-adjusted 1 year mortality risk compared with the geriatric patients.
Geriatric domain impairments and mortality risk
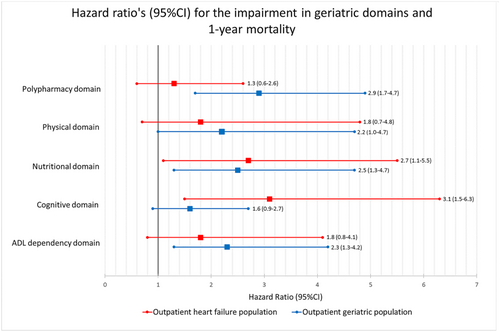
In the HF population, impairments in the nutritional and cognitive domain were significantly associated with a higher 1 year mortality risk (Figure 3). In the geriatric population, impairments in the polypharmacy, physical, nutritional, and ADL dependency domains were significantly associated with 1 year mortality risk (Figure 3). In both populations, similar effect estimates were found for the relation between impairments of the different domains and 1 year mortality risk, except for the polypharmacy domain (HR, 95% CI was 1.3, 0.6–2.6 in the HF population and 2.9, 1.7–4.7 in the geriatric population) and the cognitive domain (HR, 95% CI was 3.1, 1.5–6.3 in the HF population and 1.6, 0.9–2.7 in the geriatric population) (Figure 3).
The age-adjusted and sex-adjusted HRs (95% CI) for one SD increase on the individual geriatric assessment items are shown in the supporting information. In the HF population, one SD increase in handgrip strength, MNA-SF score, MMSE score, number of prescribed drugs, and Katz score showed a 1.3 to 1.8 increased 1 year mortality risk. This was comparable with the results in the geriatric population, where these HRs ranged from 1.5 to 2.2 (Supporting Information, Figure S1).
An increasing number of impaired geriatric domains were associated with an increased 1 year mortality risk in both populations (Table 2); compared with zero to two impaired domains, age-adjusted and sex-adjusted mortality risk (HR, 95% CI) for three, four, or five impaired domains ranged from 1.6 (0.6–4.2) to 6.5 (2.1–20.1) in the HF population and from 1.4 (0.7–2.9) to 7.9 (2.9–21.3) in the geriatric population.
| Outpatient heart failure population | Outpatient geriatric population | |
|---|---|---|
| HR (95% CI)a | HR (95% CI)a | |
| 0–2 domains impaired | Reference | Reference |
| 3 domains impaired | 1.6 (0.6–4.2) | 1.4 (0.7–2.9) |
| 4 domains impaired | 2.8 (1.1–7.3) | 3.4 (1.7–6.8) |
| 5 domains impaired | 6.5 (2.1–20.1) | 7.9 (2.9–21.3) |
- CI, confidence interval; HR, hazard ratio.
- a Model 1: adjusted for age and sex.
Discussion
This study illustrates that, in parallel with geriatric outpatients, outpatients with HF often have multiple geriatric domain impairments that adversely affect their prognosis. This together with the finding that outpatients with HF are even more likely to die within 1 year than geriatric outpatients supports the call for a more holistic approach in HF care, as recommended by 2016 ESC HF guidelines.7 Although a multi-domain geriatric assessment may help to identify impairments across different geriatric domains, the impact of targeted interventions on patient-related outcomes should be subject for future research.
Studies including a multi-domain geriatric assessment in older patients with HF are limited.4, 8 Most studies included hospitalized patients with HF,19, 20 only focused on physical frailty,5, 21, 22 and used age-matched community-dwelling older adults as a comparator group.23-25 When comparing our results with studies in hospitalized settings, prevalence of geriatric domains seems to be less prevalent and less severe in the outpatient HF population.19, 20 This is in line with our expectations that an outpatient population is in better health than those hospitalized. However, performing a multi-domain geriatric assessment at the outpatient clinic has some major advantages. The environment suits better, and identification of vulnerable patients with HF in an earlier phase (before hospitalization) provides more opportunities for setting up individualized care programmes and advance care planning.
In this study, we used widely accessible and easy to use clinical tools to define the different geriatric domains.26 The total multi-domain geriatric assessment used in this study needs minimal training, takes approximately 25 min, could be easily performed by an HF nurse, and is therefore highly feasible in outpatient HF clinics. Similar to the geriatric approach, this assessment should not only function as an inventory for different health problems for which targeted interventions can be applied but also guide physicians to shift focus from a ‘problem-based, disease-orientated’ approach to ‘goal-orientated, integrated’ approach, with attention for personalized treatment goals and advance care planning.27, 28 Earlier research has shown that this holistic approach improves outcomes in older hospitalized patients and patients with hip fractures29-31 and delays progression to frailty in pre-frail outpatients.9 In another outpatient setting, this multidisciplinary approach is currently implemented in regular care for patients with aortic valve stenosis, where a multidisciplinary ‘heart team’ makes treatment decisions in terms of undergoing a transcatheter aortic valve implantation procedure, to the satisfaction of physicians and patients.32 HF care might benefit even more from a holistic approach, as it is more than a ‘go or no go’ for a procedure, being more gradual and having more treatment options. Future research should further investigate how the different domains are most effectively assessed and how to optimize personalized care in older patients with HF. To investigate this, we are evaluating a multidisciplinary care programme in an outpatient HF clinic, which involves a geriatric screening and if indicated a multi-domain geriatric assessment by a geriatric nurse. Patients will be discussed in a multidisciplinary team and given individualized care based on the multi-domain impairments. Care consumption, quality of life, and adverse events will be monitored.
This study has several strengths. This study used a standardized work-up for both the geriatric outpatients and the patients with HF, which resulted in a uniformed data collection, making comparison possible. Furthermore, the populations presented in our study are an accurate representation of all older patients referred to an outpatient clinic for medical care, as hardly any exclusion criteria were applied to both cohorts. Lastly, we obtained, although unintentionally, the mortality data of both cohorts before the COVID-19 pandemic, which has caused excess mortality, especially in this older population.
Limitations of the current study must also be addressed. First, the number of patients with HFpEF in our cohort is lower than expected, and this may explain the underrepresentation of women. Patients with HFrEF are preferentially referred for up-titration of guideline-directed medication, while patients with HFpEF are most often referred back to the general practitioner due to the absence of guideline-directed medical therapy for HFpEF. Second, this study failed to include two other important geriatric domains, for example, the psychological and social domains.28 Although these domains are of added value, especially for quality of life, the domains assessed in this study are more commonly associated with adverse outcomes and thus may be of greater value to assess patients at risk of these adverse outcomes.5, 8 However, assessing these domains to ensure holistic care is recommended in future research and future care.
Third, using polypharmacy as a proxy for multimorbidity might not be sufficient when comparing these specific populations. Due to guideline-directed therapy, the number of prescribed medication is expected to be higher in the HF population. However, using a cut-off value of >10 prescribed medications, we expect to have selected the patients with evident multimorbidity in both cohorts. Another limitation is the use of the MMSE, developed to screen for Alzheimer's disease, to assess impairments in the cognitive domain.33 The aetiology of cognitive impairment in patients with HF is more likely to be vascular, which mostly results in limitations in planning and reasoning, and to lesser extent in memory function, which are not as easily detected with the MMSE.34, 35 This might have influenced the number of patients with cognitive impairment in the HF population and resulted in a mismatch with earlier research.36 Proper detection of cognitive impairment is clinically relevant as it limits a patients' ability to comply with complex medical regimes.
In conclusion, to implement holistic care, as called for in the 2016 ESC HF guideline, our data provide evidence to approach an older patient with HF like a geriatric patient and conduct a similar multi-domain geriatric assessment in patients with HF at the outpatient clinic.
Acknowledgements
We acknowledge the support from the Netherlands CardioVascular Research Initiative: the Dutch Heart Foundation (CVON 2018-28 and 2012-06 Heart Brain Connection), Dutch Federation of University Medical Centres, the Netherlands Organisation for Health Research and Development, and the Royal Netherlands Academy of Sciences. We also thank all the students and nurses working at the HF clinic and geriatric outpatient clinic of Amsterdam UMC, location VUmc, and at the HF clinic of the Amstelland Hospital for their support and contribution to the data collection.
Conflict of interest
H.F.M.R.-M. performs contract research for Combinostics; all funding is paid to her institution.
Funding
Not applicable.




