Iron deficiency impacts prognosis but less exercise capacity in heart failure with preserved ejection fraction
Abstract
Aims
Whether and how iron deficiency (ID) impacts patients with heart failure (HF) with preserved ejection fraction (HFpEF) remain unclear. The aim of our study was to investigate the impact of ID on functional status, exercise capacity, and prognosis in HFpEF.
Methods and results
The study population consisted of 300 HFpEF patients. ID was defined as serum ferritin <100 μg/L or 100–300 μg/L and transferrin-saturation <20%. Baseline functional status, quality of life (HADS score and EQ 5D index), 6 min walking test, echocardiography, and outcome (all-cause mortality and combined all cause-mortality and HF hospitalization) were evaluated. ID was found in 159 (53%) patients. Patients with ID had a worse prognosis with a higher combined endpoint of all-cause mortality and HF hospitalization after 4 years of follow-up (log rank = 0.008). Pulmonary hypertension, depression, and thyroid disease were more prevalent in the ID group. Multivariable analysis showed that ID was independently associated with body mass index (P = 0.003), pulmonary hypertension (P = 0.008), and thyroid disease (P = 0.01). Although patients with ID had a lower exercise capacity compared with patients without ID (393 m [294–455] vs. 344 m [260–441], P = 0.008), there was no significant correlation after multivariable correction for age, BMI, NT-proBNP, DM, and depression.
Conclusions
Heart failure with preserved ejection fraction patients with ID have a worse prognosis and impaired exercise capacity compared with those without ID. However, although a trend was observed, after multivariable correction ID was no longer significantly associated with a reduced exercise capacity. This reflects that impaired exercise capacity in HFpEF is complex and seems multifactorial. Interestingly, pulmonary hypertension was an independent predictor of both ID and exercise capacity.
Introduction
Iron deficiency (ID) is highly prevalent in heart failure (HF) patients. The effect of ID has been extensively studied in HF with reduced ejection fraction (HFrEF) where ID has shown to be associated with reduced exercise capacity1 and poor outcome.2 Intravenous iron supplementation is recommended in those patients as it reverses impaired exercise capacity, improves quality of life, and reduces HF hospitalizations.3-5 The role of ID in HF with preserved ejection fraction (HFpEF) remains poorly understood.6, 7 Because no therapy has had a clear benefit in HFpEF patients so far, ID could be a promising therapeutic target. Recently, a meta-analysis showed that the prevalence of ID may be even higher in HFpEF compared with HFrEF.7 To date, only four studies have investigated the relationship between ID and exercise capacity in HFpEF, obtaining conflicting results.8-11 Although the majority of the studies suggest that ID is related to decreased exercise capacity (lower peak VO2 max and lower 6 min walking distance) in HFpEF, the small size of these studies (the largest study included 190 HFpEF patients) and the inconsistent criteria used to define HFpEF do not allow to generalize these results to the overall HFpEF population. Furthermore, data on prognosis are lacking.
Reduced intake, systemic inflammation, and blood loss probably play an important link between ID and HF.12 Also, whether ID affects exercise capacity directly or whether it is just a bystander or marker of advanced disease remains to be clarified.
Therefore, the aim of this large prospective cohort study is to evaluate the impact of ID on functional status, exercise capacity, and prognosis in HFpEF. Moreover, our deeply phenotyped HFpEF cohort allows us to identify which factors associate with ID and influence the impact of ID on exercise capacity.
Methods
Patient population
Between May 2015 and June 2019, we prospectively included 300 consecutive patients diagnosed with HFpEF. All patients underwent a standard set of diagnostic tests, including electrocardiogram, echocardiography, pulmonary function test, exercise testing, 6 min walking test (6MWT), 24-h holter monitoring, sleep apnoea screening and blood tests. Patients were evaluated every 6 months for follow-up; intercurrent heart failure hospitalizations and deaths (all-cause mortality) were scored. Date of last outpatient contact or death was defined as date of last follow-up. Heart failure hospitalization and all-cause mortality were recorded during a follow-up period up to 4 years.
Heart failure with preserved ejection fraction was diagnosed according to the 2016 European Society of cardiology (ESC) guidelines,13 using the following criteria: (i) the presence of symptoms of HF; (ii) a preserved ejection fraction, defined as a left ventricular ejection fraction (LVEF) ≥ 50%; (iii) elevated levels of natriuretic peptides [NT-proBNP >15 pmol/L (>125 pg/mL)]; (iv) objective evidence of cardiac structural [i.e. increased left atrial volume index (LAVI >34 mL/m2] or increased left ventricular mass index (LVMI ≥115 g/m2 for men or ≥95 g/m2 for women) and/or functional alterations (E/e′ ≥ 13 and/or mean e′ septal and lateral wall <9 cm/s). A right heart catheterization was performed in cases of clinical uncertainty; a pulmonary capillary wedge pressure ≥15 mmHg was considered diagnostic for HFpEF. From the total of 300 HFpEF patients, 33 underwent an RHC. In general, clinical, echocardiographic, and laboratory parameters, including iron status, were similar in both groups (Supporting Information, Table S4).
Patients with a documented previous LVEF ≤ 40% (i.e. recovered EF), diagnosis of a cardiomyopathy (e.g. dilated or hypertrophic cardiomyopathy), a greater than moderate valvular disease, haemodynamically significant congenital disease, restrictive cardiomyopathies, constrictive pericarditis, or a history of heart transplantation were excluded.
Pulmonary hypertension (PH) was defined as an increased estimated right ventricular pressure >45 mmHg on echocardiography or a mean pulmonary arterial pressure (PAP) ≥ 25 mmHg at rest during right heart catheterization when available. Depression was defined by the presence of at least an episode of depression diagnosed by the general practitioner, clinical psychologist, or psychiatrist. Furthermore, a score >7 points in the depression domain of the Hospital Anxiety and Depression Scale (HADS) score was also diagnostic for depression. The study protocol was approved by the local ethics committee, and all subjects gave written informed consent. The study was conducted in accordance with the Declaration of Helsinki.
6 min walking test
The 6MWT was performed according to the 2002 guidelines of the American Thoracic Society (ATS).14 A well-trained technician supervised each test and used a stopwatch to monitor the time and a mechanical lap counter to measure the walker distance. Blood pressure and pulse oxygen saturation (SpO2) measurement was performed at baseline and directly after completion of the test. The 6MWD % predicted was calculated by using the reference equations provided by Enright and Sherrill for men and women.15
Quality of life
All patients were asked to self-complete the following questionnaires: HADS score and EuroQoL 5-Dimensional Descriptive System index (EQ 5D-index) (Supporting Information).
Iron status and anaemia
Iron deficiency was defined in line with the Kidney Disease Outcomes Quality Initiative16 and with previous studies evaluating ID in HF11, 17, 18 as serum ferritin <100 μg/L (absolute ID) or serum ferritin of 100–299 μg/L in combination with a transferrin saturation <20% (functional ID). Anaemia was defined as haemoglobin <7.5 mmol/L (<12.0 g/dL) for women and <8.0 mmol/L (<13.0 g/dL) for men.19, 20
Statistical analysis
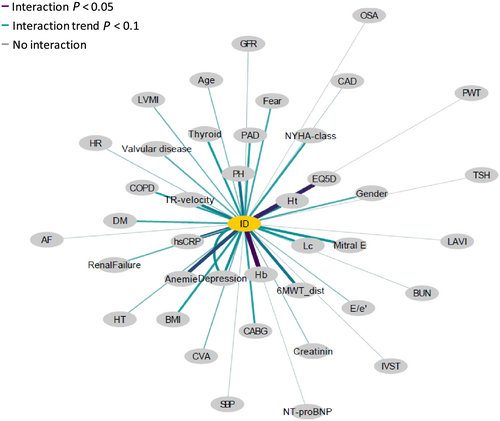
Continuous variables are expressed as mean ± standard deviation or as median (interquartile range) as appropriate. Categorical variables are expressed as numbers with percentages. Intergroup differences were tested using χ2 method, Student's t-test, or Mann–Whitney U-test as appropriate. Normality was visually analysed using QQ-plots and not normally distributed data [6MWT distance, 6MWT predicted distance (%), NT-proNP, and hs-CRP] were underwent logarithmic transformation before all statistical analyses were performed. Mortality and hospitalization rates were compared between patients with and without ID using Cox regression analysis and visualized using Kaplan–Meier curves. Univariable associations were analysed using the Pearson's or Spearman's correlation coefficient, depending if the variables were normally distributed or not. Besides age and sex, statistically significant parameters (P < 0.05) of the univariable analysis [body mass index (BMI), 6MWT distance, HADS-depression, EQ 5D-index, haematocrit, leucocytes, high sensitivity CRP (hs-CRP), PH, depression, anaemia, thyroid disease, e′ average, and TR-speed] were analysed in a multivariable logistic regression analysis using the backward method, to evaluate which variables were independent predictors of ID. Network analysis was performed to visualize the relationship between the different variables and ID. The graphical representation of the network analysis shows how close (distance of the variable to ID) and how strong (thickness of the line connecting the variables and ID) the relationship of the different variables and ID is.
Multicollinearity testing was used to exclude highly correlated variables and selected a single representative and clinical relevant variable for each domain. A P-value of <0.05 was considered to be significant and for interaction tests a P of 0.10 were considered statistically significant. All calculations were performed using the SPSS statistical package version 24 SPSS Inc. Chicago, IL USA.
Results
Iron deficiency was diagnosed in 159 (53%) HFpEF patients, absolute ID in 120 (40%), and functional ID in 39 (13%). Baseline characteristics are summarized in Table 1. Patients with ID had a higher BMI, more often PH, depression, anaemia, and thyroid disease. Laboratory tests showed that haematocrit levels were lower, while leucocyte and hs-CRP levels were higher in ID compared with non-ID patients. Network analyses that evaluated the interaction between different variables and ID displayed that haemoglobin, haematocrit, EQ-5D index, depression, anaemia, TR-velocity, PH, and hs-CRP were the strongest variables correlated to ID (Figure 1). Multivariable logistic regression analysis showed that BMI, PH, and thyroid disease were independent predictors for ID (Table 2).
| Demographic and clinical variables | N | No ID (N = 141, 47%) | N | ID (N = 159, 53%) | P-value |
|---|---|---|---|---|---|
| Age, years | 141 | 76.2 (7.1) | 159 | 75.3 (8.0) | 0.32 |
| BMI, kg/m2 | 141 | 29.7 (5.4) | 159 | 31.1 (6.2) | 0.04 |
| Female, % | 141 | 88 (62.4) | 159 | 111 (70.3) | 0.15 |
| Systolic BP, mmHg | 141 | 146.5 (12.8) | 159 | 147.6 (21.7) | 0.68 |
| Diastolic BP, mmHg | 141 | 77.5 (12.8) | 159 | 77.0 (13.2) | 0.74 |
| Heart rate, b.p.m. | 141 | 71.3 (12.2) | 159 | 72.5 (12.7) | 0.37 |
| Functional class | 0.18 | ||||
| NYHA I-II, % | 141 | 70 (49.6) | 159 | 64 (40.3) | |
| NYHA III, % | 141 | 68 (48.2) | 159 | 88 (55.3) | |
| NYHA IV, % | 141 | 3 (2.1) | 159 | 7 (4.4) | |
| Test results | |||||
| 6MWT distance (m) | 130 | 378.9 (112.4) | 142 | 341.5 (125.7) | 0.01 |
| 392.5 [294–455] | 344.0 [260–441] | 0.008 | |||
| 6MWT % predicted | 130 | 66.6 [52–78] | 142 | 59.2 [44–73] | 0.01 |
| Baseline O2 sat 6MWT | 128 | 94.9 (2.4) | 142 | 94.5 (2.7) | 0.24 |
| Minimum O2 sat 6MWT | 128 | 91.4 (5.2) | 142 | 91.6 (4.3) | 0.73 |
| Quality of life | |||||
| HADS | |||||
| Distress | 141 | 4.0 [0–7.0] | 159 | 5.0 [1.0–8.0] | 0.11 |
| Depression | 141 | 3.2 [0–6.0] | 159 | 4.0 [1.0–8.0] | 0.01 |
| EQ 5D-index (0–100) | 89 | 64.8 (13.6) | 111 | 58.4 (15.0) | 0.01 |
| Laboratory | |||||
| Hb, mmol/L | 137 | 8.4 (0.9) | 157 | 7.9 (0.9) | 0.85 |
| Ht, L/L | 135 | 0.41 (0.04) | 156 | 0.40 (0.04) | 0.02 |
| Leukocytes, 10E9/L | 132 | 7.0 () | 151 | 8.0 () | 0.02 |
| Urea, mmol/L | 136 | 8.0 [8.0–10.0] | 159 | 7.9 [6.1–10.4] | 0.71 |
| Creatinine, μmol/L | 140 | 106.9 (44.3) | 159 | 108.6 (39.0) | 0.71 |
| eGFR, mL/min/1.73/min | 134 | 52.0 (15.6) | 159 | 51.1 (17.4) | 0.68 |
| NT-proBNP, pmol/L | 139 | 88.0 [36.0–195.0] | 159 | 84.0 [41.0–185.0] | 0.71 |
| Cholesterol, mmol/L | 139 | 4.7 (1.2) | 157 | 4.5 (1.2) | 0.18 |
| HDL, mmol/L | 139 | 1.4 (0.4) | 157 | 1.3 (0.5) | 0.25 |
| LDL/mmol/L | 139 | 2.5 (1.2) | 157 | 2.4 (1.0) | 0.36 |
| Tsat, % | 141 | 25.0 [21.0–31.5] | 159 | 17.0 [13.0–20] | <0.01 |
| Ferritin, μg/L | 141 | 228.0 [153.5–371.5] | 159 | 65.0 [38.0–96.0] | <0.01 |
| TSH, mU/L | 75 | 1.9 (1.1) | 94 | 2.6 (4.2) | 0.27 |
| hs-CRP | 130 | 2.0 [1.1–4.3] | 158 | 3.6 [1.4–6.6] | 0.001 |
| Co-morbidities | |||||
| Myocardial infarction, % | 141 | 10 (7.1) | 159 | 18 (11.3) | 0.20 |
| CAD, % | 141 | 37 (26.2) | 159 | 38 (23.9) | 0.64 |
| PCI, % | 141 | 23 (16.3) | 159 | 22 (13.8) | 0.54 |
| CABG, % | 141 | 5 (3.5) | 159 | 14 (8.8) | 0.06 |
| Valvular disease (mild–moderate), % | 141 | 21 (14.9) | 159 | 33 (20.8) | 0.18 |
| CVA, % | 141 | 22 (15.6) | 159 | 18 (11.3) | 0.27 |
| Peripheral vascular disease, % | 141 | 10 (7.1) | 22 (13.8) | 0.05 | |
| Diabetes mellitus, % | 141 | 40 (28.4) | 159 | 59 (37.1) | 0.10 |
| Hypertension, % | 141 | 124 (87.9) | 159 | 133 (83.6) | 0.28 |
| Hypercholesterolaemia, % | 141 | 83 (58.9) | 159 | 92 (57.9) | 0.86 |
| COPD, % | 141 | 16 (11.3) | 159 | 30 (18.9) | 0.07 |
| Sleep apnoea, % | 141 | 55 (39.0) | 159 | 61 (38.4) | 0.90 |
| Pulmonary hypertension, % | 141 | 21 (14.9) | 159 | 45 (28.3) | 0.005 |
| Depression | 141 | 11 (7.8) | 159 | 27 (17.0) | 0.01 |
| Rheumatoid arthritis | 141 | 6 (4.3) | 159 | 10 (6.3) | 0.43 |
| AF | 141 | 81 (57.4) | 88 (55.3) | 0.71 | |
| Kidney failure (eGFR <60 mL/min/1.73/min, %) | 141 | 49 (34.8) | 159 | 66 (41.5) | 0.23 |
| Anaemia, % | 137 | 32 (22.7) | 158 | 74 (46.5) | 0.001 |
| Thyroid disease, % | 141 | 12 (8.5) | 159 | 26 (16.4) | 0.04 |
| Echocardiography | |||||
| LVEF (Teichholz) | 141 | 60.3 (4.9) | 159 | 60.0 (5.1) | 0.70 |
| LVEDd (mm) | 141 | 46.9 (5.4) | 159 | 47.9 (5.6) | 0.11 |
| IVS (mm) | 140 | 9.0 [8.2–10.0] | 157 | 9.0 [8.0–10.0] | 0.73 |
| PW (mm) | 140 | 9.0 [8.0–10.0] | 157 | 9.0 [8.0–10.0] | 0.97 |
| LAVI (mL/m2) | 135 | 45.7 [36.1–56.3] | 156 | 44.9 [35.6–57.3] | 0.82 |
| LVMI (g/m2) | 120 | 81.4 [67.2–96.7] | 132 | 82.6 [72.0–101.0] | 0.28 |
| EA ratio | 86 | 0.87 [0.72–1.35] | 99 | 0.88 [0.71–1.32] | 0.94 |
| e′ average | 118 | 7.5 [6.1–9.2] | 136 | 8.4 [6.7–9.5] | 0.03 |
| E/e average | 118 | 10.2 [8.0–13.6] | 136 | 10.9 [8.3–13.9] | 0.43 |
| TR-speed (m/s) | 131 | 2.5 [2.3–2.9] | 148 | 2.7 [2.3–3.1] | 0.01 |
- Data are n (%), mean ± SD or median [IQR].
- AF, atrial fibrillation; BMI, body mass index; BP, blood pressure; CABG, coronary artery bypass grafting; CAD, coronary artery disease; COPD, chronic obstructive pulmonary disease; CVA, cerebrovascular accident; EQ 5D, European quality of life 5 Dimensional Questionnaire; eGFR, estimated glomerular filtration rate; HADS, Hospital Anxiety and Depression Scale; Hb, haemoglobin; HDL, high density lipoprotein; Ht, haematocrit; hs-CRP, high sensitivity C-reactive protein; ID, iron deficiency; IVS, intraventricular septum thickness; LAVI, left atrium volume index; LVEDd, left ventricular end-diastolic diameter; LVEF, left ventricular ejection fraction; LVESd, left ventricular end-systolic diameter; LVMI, left ventricle mass index; LDL, low density lipoprotein; 6MWT, 6 min walking test; NT-proBNP, N-terminal pro-B-type natriuretic peptide; NYHA, New York Heart Association; PCI, percutaneous coronary intervention; PW, posterior wall thickness; Tsat, transferrin saturation; TR, tricuspid regurgitation; TSH, thyroid stimulating hormone.
- All statistically significant values (p < 0.05) are provided in bold.
| Univariable | Multivariable | ||
|---|---|---|---|
| P-value | P-value | OR (95% CI) | |
| Age | 0.32 | ns | |
| Sex | 0.15 | ns | |
| BMI | 0.04 | 0.003 | 1.0 (1.0, 1.1) |
| 6MWT distance (m) | 0.01 | ns | |
| HADS-depression | 0.01 | ns | |
| EQ 5D-index | 0.01 | ns | |
| Ht | 0.02 | ns | |
| Leukocytes | 0.02 | ns | |
| hs-CRP | 0.001 | ns | |
| Pulmonary hypertension | 0.005 | 0.008 | 3.0 (1.2,7.6) |
| Depression | 0.01 | ns | |
| Anaemia | 0.001 | ns | |
| Thyroid disease | 0.04 | 0.01 | 4.2 (1.3,13.6) |
| e′ average | 0.03 | ns | |
| TR-speed | 0.01 | ns | |
- BMI, body mass index; CI, confidence interval; EQ 5D, European quality of life 5 Dimensional Questionnaire; hs-CRP, high sensitivity C-reactive protein; Ht, haematocrit; HADS, Hospital Anxiety and Depression Scale; 6MWD, 6 min walking distance; OR, odds ratio; TR, tricuspid regurgitation.
- All statistically significant values (p < 0.05) are provided in bold.
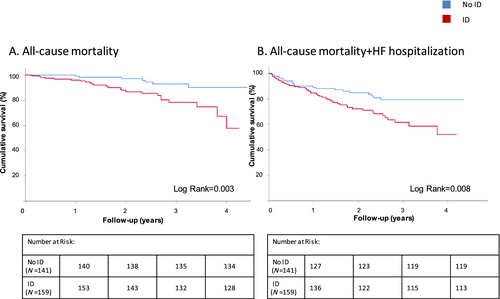
Iron deficiency and prognosis
The median follow-up was 47 months (95% CI: 46–49). A total of 31 deaths and 48 HF hospitalizations were observed within this period of time. The combined endpoint (all-cause mortality and HF hospitalization) was significantly higher in ID versus non-ID HFpEF patients (HR = 1.99, 95% CI: 1.19, 3.32, P = 0.008, Figure 2). Cox regression and Kaplan–Meier survival analysis showed that ID was significantly associated with an increased all-cause mortality (HR = 3.55, 95% CI: 1.52, 8.25, P = 0.003) but not with HF hospitalizations (HR = 1.6, 95% CI: 0.90, 2.95, P = 0.10, Supporting Information, Table S1).
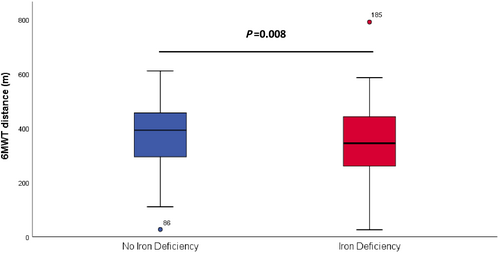
Iron deficiency and exercise capacity
Patients with ID had a lower exercise capacity compared with those without ID [6MWT distance 393 m (294–455) vs. 344 m (260–441), P = 0.008, Figure 3]. Linear regression analyses did not show a correlation between 6MWT and ferritin (R = 0.07, P = 0.23) but did show a very weak correlation between 6MWT and transferrin saturation (R = 0.16, P = 0.07).
Univariable regression analysis showed that multiple clinical, laboratory, and echocardiographic parameters predicted exercise capacity [6MWT distance (m) and predicted distance (%); Supporting Information, Table S2]. In the multivariable analysis, only the most clinically relevant variables were included (age, sex, BMI, ID, haemoglobin, creatinine, log NT-proBNP, log hs-CRP, DM, depression, average E/e′, and TR-speed). This analysis showed that a trend; however, ID was not a significant independent predictor of exercise capacity measured by 6MWT distance (m) (β: −26.4, 95% CI: −55.14 to 2.28, P = 0.070), while other factors such as age, BMI, NT-proBNP, DM, and depression remained as independent predictors of exercise capacity in the model (Supporting Information, Table S3). Similar results were found using 6MWT predicted distance (%) (data not shown).
Iron deficiency and quality of life
Patients with ID scored higher on the HADS questionnaire for depression [4.0 (1.0–8.0) vs. 3.4 (0–6.0), P = 0.01] and lower on the EQ 5D-index [58.4 (15.0) vs. 64.8 (13.6), P ≤ 0.01, Table 1]. In contrast, multivariable analysis demonstrated that the HADS score for depression and EQ 5D-index were not independent predictors for ID (Table 2).
Discussion
This study demonstrates that ID in HFpEF is associated with higher all-cause mortality and combined endpoint (all-cause mortality and HF hospitalization) rates. Our results show that although ID influences exercise capacity, ID is not independently associated with a reduced exercise capacity at baseline and impaired exercise capacity in HFpEF seems multifactorial. Additionally, this study shows body mass index, pulmonary hypertension, and thyroid disease are independent predictors of ID in HFpEF.
As far as we know, our study is the first showing the impaired prognosis of ID-HFpEF patients. The recent meta-analysis of Beale et al. summarizes all abstracts presented until date, in which ID is related with a poorer prognosis in HFpEF.7 Unfortunately, these abstracts are only presented at conferences. The complete data of these abstracts have not been published yet. The findings of our study have important clinical and therapeutic implications and support the need for further prospective studies analysing the impact of iron supplementation in patients with HFpEF.
The first study reporting the association between ID and exercise capacity in HFpEF dates back to 2013. In that study, the authors did not found any difference in exercise capacity and peak VO2 max between 15 ID and 11 non-ID HFpEF patients.11 In contrast, the following three studies obtained opposite results. One study that included 637 ID HF patients showed that ID was associated with a decreased VO2max. Unfortunately, only 43 of the total of 637 patients were diagnosed with HFpEF, making it impossible to extrapolate these results to the HFpEF population.10 A small study by Nuñez et al. found a positive correlation between VO2max, ferritin (r = 0.30, P = 0.008), and transferrin saturation (r = 0.46, P = 0.003), irrespective of haemoglobin levels, in 40 advanced HFpEF patients.8 Bekfani et al. recently demonstrated in 190 HFpEF patients (58.6% of them with ID) that patients with ID performed worse during the 6MWT (420 ± 137 vs. 344 ± 124 m, P = 0.008) and had a shorter exercise time during cardiopulmonary exercise testing (645 s ± 168 s vs. 538 s ± 178 s, P = 0.03).21 It is important to underline that from all the studies mentioned earlier, only the study of Bekfani et al. performed a multivariable analysis to determine independent predictors of ID.9 However, this study had several methodological limitations. First, only 88 (46%) of the ID patients underwent 6MWT, which could possibly include a selection bias that was not discussed by the authors. Also, a multivariable logistic regression (with an arbitrary 6MWT cut off value) was used to determine which variables were independent predictors of impaired exercise capacity in HFpEF, instead of linear regression model with 6MWT as a continuous variable; the approach we used in our study. Moreover, the number of variables in the final logistic regression model was limited [ID (yes/no), haemoglobin, NT-proBNP, hsCRP, and E/e′], and important factors such as age of sex were not included. With the aim of comparing the results of both studies, we performed additional analyses using the same statistical approach with our data, obtaining comparable results, where ID, NT-proBNP, and hs-CRP were independent predictors of impaired exercise capacity in HFpEF (data not shown). All in all, the results of our study show that although ID plays a role, ID it is not independently associated with impaired exercise capacity in HFpEF.
This study shows that the impaired exercise capacity in HFpEF is multifactorial. It is related to multiple clinical factors including age, BMI, NT-proBNP, and important cardiovascular co-morbid conditions as DM and depression. Interestingly, PH, a well-known predictor of poor prognosis and impaired exercise capacity in HFpEF, was highly prevalent and independently associated with ID. The correlation between ID and PH in HFpEF has not been described before. Animal and human studies have shown that hypoxia inducible factor pathway plays a central role in the exaggerated response to hypoxia observed in ID, which could contribute to pulmonary hypertension.22-24 A couple of small human studies have corroborated this, showing that patients with ID have a greater increase in systolic pulmonary artery pressure (SPAP) after a period of hypoxia compared with controls, reversible after intravenous iron supplementation.23, 25
Inflammatory cytokines could play an essential in the exaggerated response to hypoxia produced by ID in HFpEF. The activation of inflammatory cytokines, especially IL-6, are responsible for the activation of the JAK–STAT signalling pathway, responsible of the increase of hepatic hepcidin production. Hepcidin levels are responsible for the internalization and degradation of ferroportin, a necessary transporter for intracellular iron excretion into the blood.26, 27 A co-morbidity driven generalized low grade inflammation has been postulated as the main pathophysiological mechanism responsible for HFpEF, ratified by the increased level of inflammatory biomarkers found in these patients.28-30 The combination of HFpEF and ID may enhance the inflammatory state present in both entities and could suppress even more compensatory mechanisms activated in iron-deficient states, as the increase in circulating hepcidin levels, worsening the effects of ID. Furthermore, hypoxia present in ID could result in a profound pulmonary vascular remodelling in ID-HFpEF patients. This process has already been demonstrated in iron-deficient diet rats. After 4 weeks, the authors observed that ID rats developed PH and important pulmonary vascular abnormalities, that is, smooth muscle hypertrophy, lumen reduction, and inflammatory cell infiltration.31
The relationship between ID and PH could also be the other way around. It is well known that PH is one of the most significant determinants and modifiers of HF severity and prognosis.32, 33 A more severe PH-HFpEF phenotype could suffer more frequently from ID, and this would be a secondary and reinforcing co-morbid condition. This ‘chicken and egg’ calls for further research, preferably in mechanistic studies and longitudinal cohorts.
Furthermore, our study shows that ID-HFpEF patients suffer more frequently from thyroid disease. Different studies have shown how ID may affect thyroid hormone metabolism.34-36 The pathophysiological mechanism responsible for this alteration is still poorly understood. Several theories could explain this alteration, including ID driven impaired erythropoiesis, hepatic typhoid hormone inactivation, and even reduced thyroperoxidase activity with consequently thyroid hormone production.34-36 The mechanism and the therapeutic consequence of thyroid disease in ID-HFpEF patients has not been studied yet. Our finding provides a new opportunity of enhancing future research.
Study limitations
There are study limitations to acknowledge. This is a single-centre study of patients that were referred to the Maastricht University Medical Centre, which serves both as a local and as a tertiary medical centre. Thus, our result may not be generalizable to the patients in the general community, other countries, and ethnicities. However, the baseline characteristics of our HFpEF cohort are comparable with the ones presented by others.37, 38 Furthermore, we currently do not have data on the use of iron supplementation during the follow up period. Although this is the largest study published until date analysing the effect of ID in HFpEF, the limited amount of patients still can affect the study results. In particular, the results obtained by the multiple regression analysis may be affected by the limited amount of patients that underwent the 6MWT 130 from the total of non-ID and 142 from the 159 patients in the ID group. This study shows for the first time that impaired exercise capacity in HFpEF is a multifactorial issue and its related to multiple clinical factors such as age, BMI, NT-proBNP levels and importnat cardiovascular co-morbid conditions such as DM and depression. Furthermore, this conclusion was not affected by the limited amount of patients that underwent the 6MWT. Anyway, the trend between ID and impaired exercise capacity observed in this study as well as the rest of the results should be validated in an external validation cohort. Finally, one of the limitations of this study is the lack of power to correct the survival analysis for possible confounders such as age or PH.
In conclusion, ID-HFpEF patients have a poorer prognosis, and ID is an independent predictor for all-cause mortality. Reduced exercise capacity in HFpEF is multifactorial and not independently related to an ID state. Moreover, some specific clinical factors such as BMI, pulmonary hypertension, and thyroid disease are independently associated with ID. Although iron supplement treatments have showed to improve exercise capacity, quality of life, and prognosis in HFrEF, it is uncertain whether iron supplement will have the same positive effects in HFpEF.3, 4, 39 This study is the first to highlight reduced exercise capacity in HFpEF as multifactorial and not independent associated with ID. Thus, the results suggest that isolated treatment of ID could be insufficient to affect exercise capacity in HFpEF and stress out the importance of the systematic and deep phenotyping in HFpEF. The selection of the right clinical phenotype of ID-HFpEF patients (younger, less severe forms of HF, and less CV co-morbid conditions especially DM and depression) for future prospective trials designed for testing the effect intravenous iron supplement is key.
Acknowledgements
None.
Conflict of interest
None declared.
Funding
This work was funded by Health Foundation Limburg and the Dutch Heart Foundation and with an unrestricted grant of Vifor Pharma.




