The diagnosis of a giant cardiac malignant lymphoma in the right ventricle: a case report
Abstract
Cardiac lymphoma is extremely rare in patients with normal immune function and difficult to identify through routine examinations in patients with atypical clinical manifestations, making early diagnosis very difficult. We reported a 71-year-old male patient who was repeatedly diagnosed of pulmonary infection, suspected lung tumour, and Kimura disease in other hospital due to cough, expectoration, and dyspnoea. Later on, the patient visited our hospital due to heart failure and epistaxis. Transthoracic echocardiogram confirmed the presence of a space-occupying lesion in the right heart, cardiac magnetic resonance imaging preliminarily determined the nature of this lesion, finally, 18F-fluorodeoxyglucose positron-emission tomography/computed tomography, and nasal mass biopsy confirmed the diagnosis of cardiac malignant lymphoma, and the pathological type was diffuse large B-cell lymphoma.
Introduction
Primary cardiac lymphoma (PCL) is a rare malignancy originating from the heart. It is an extra-nodal lymphoma or giant cardiac lymphoma that only involves the heart and/or pericardium or causes cardiac symptoms.1, 2 It is extremely rare in patients with normal immune function, accounting for about 2% of primary cardiac tumours and 0.3% of lymphomas.3 In the broad pathological spectrum of PCL, diffuse large B-cell lymphoma (DLBCL) is the most common subtype, accounting for about 63–85% of cardiac lymphomas.4, 5 The clinical and radiological manifestations of DLBCL may resemble non-haematopoietic neoplasms, and the initial biopsies can lead to a clinical or radiological impression favouring metastatic or other primary tumours.6 So the possibility of cardiac malignant lymphoma should be considered when a giant space-occupying lesion is found in heart chambers.
Case report
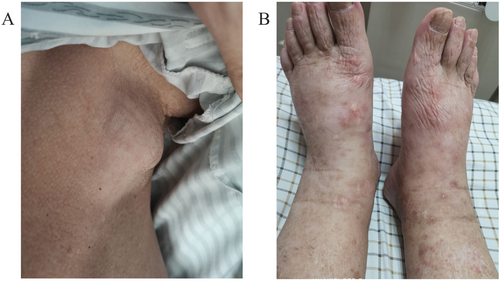
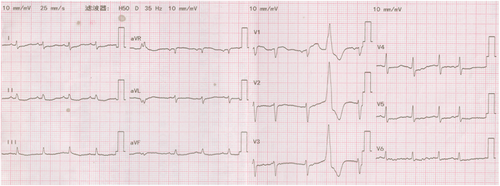
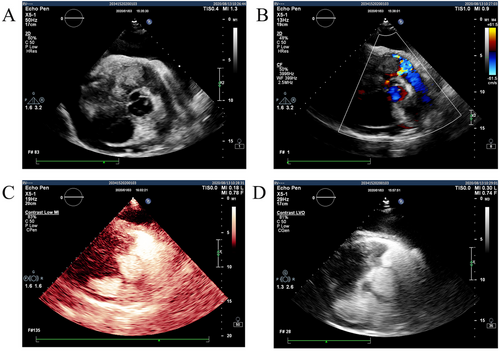
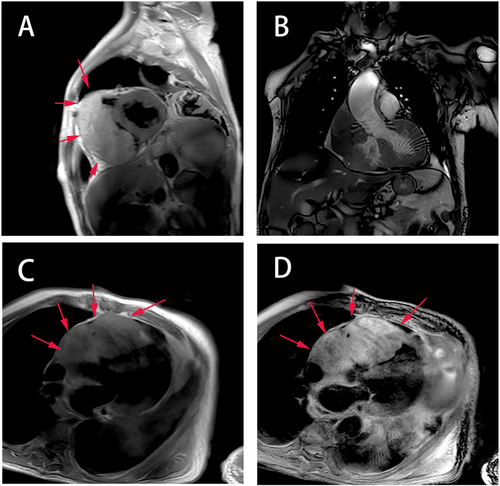
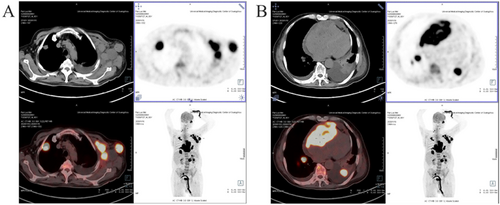
A 71-year-old male patient with normal immune function visited our cardiology department in January 2020 due to progressive dyspnoea, cough and expectoration for a year, and aggravated oedema of both lower limbs and occasional epistaxis. There was no history of fever and weight loss, and there was no significant finding in family history. The patient had a history of chronic obstructive pulmonary disease. In December 2018, the patient repeatedly visited local hospitals due to cough, expectoration, and dyspnoea. Chest computed tomography (CT) scan showed pulmonary infection, which was slightly ameliorated after anti-infective treatment. In March 2019, this patient visited the hospital again due to aggravation of the above symptoms; chest CT scan indicated pulmonary infection with left axillary lymph node enlargement. Pulmonary tumour or metastasis was considered at that time, but no clear lesion, pericardial, or pleural effusion was observed on chest CT. In June 2019, the patient was re-admitted due to limb skin pruritus in addition to aggravation of the above symptoms, blood routine showed slightly elevated eosinophil counts (0.7 × 109/L) (0.02 ~ 0.52 × 109/L), increased hypersensitive C-reactive protein (9.67 mg/L) (0 ~ 3 mg/L) and increased erythrocyte sedimentation rate (44 mm/h) (0 ~ 28 mm/h). Bone marrow aspiration indicated active hyperplasia, increased granulocytic eosinophils, decreased megakaryocyte distribution, and platelet distribution in aggregates, but no abnormality was found in bone marrow chromosome karyotyping and genetic testing. Biopsy of left axillary lymph nodes showed active lymphoid hyperplasia; in the background of hyperplastic lymphoid tissues, the inflammatory cell components were mixed, including eosinophils, neutrophils and a small number of plasma cells and histiocytes, formation of focal lymphoid follicles and germinal centre, and stroma rich in mural small vessels. Combining with eosinophil counts, serum immunoglobulin E (IgE) (the details were unknown) level and immunohistochemical markers, angiolymphoid tissue hyperplasia with eosinophilia (Kimura disease) was considered. Accordingly, Kimura disease was diagnosed, and the following hormone pulse therapy (unknown usage and dosage) led to shrinkage of the lymph nodes and alleviation of dyspnoea symptom. The patient was diagnosed and treated in the respiratory department outside the hospital and was not evaluated by a cardiologist, nor did he have an electrocardiogram or an echocardiogram examination. In January 2020, He visited our cardiology department. Physical examination showed blood pressure 124/68 mmHg, regular cardiac rhythm, heart rate 105 beats per minute, both lungs were clear, left axillary lymph node enlargement, severe oedema of the lower limbs (Figure 1). Laboratory examinations: white blood cell counts 19.25 × 109/L (3.5 ~ 9.5 × 109/L), neutrophil counts 0.02 × 109/L, alanine transaminase148 IU/L (4 ~ 40 IU/L), aspartate transaminase123 IU/L (4 ~ 40 IU/L), creatinine 165 μmol/L (70 ~ 115 μmol/L), hypersensitive C-reactive protein 25.28 mg/L, Interleukin-6 (IL-6) 225.0 ng/L (<7.0 ng/L), erythrocyte sedimentation rate 88 mm/L. N-terminal pro-brain natriuretic peptide 12423 ng/L (<300 ng/L). Tumour index, vasculitis index, autoantinuclear antibody spectrum, blood coagulation function, thromboelastogram, and HIV were all negative. Imaging and pathological examinations: electrocardiogram showed sinus tachycardia, atrial extrasystole, ventricular extrasystole, limb lead depression, and ST-T changes (Figure 2). Transthoracic echocardiogram (TTE) and contrast echocardiography revealed a giant mass in the right ventricle (RV) (Figure 3). This mass occupied 80–90% of the RV and invaded the right atrium, affecting cardiac reflux and causing massive pericardial effusion. The mass was localized to the tricuspid and penetrated into the right atrium, meanwhile small masses were also observed on the atrial side of tricuspid. Its perfusion was abundant but slightly lower than normal myocardial perfusion. A large amount of pericardial effusion could cause pericardial tamponade. Left ventricular ejection fraction was 56%, and no obvious valvular disease was found. Pericardiocentesis was performed to relieve the symptoms such as progressive dyspnoea and distention of bilateral jugular veins. Chest X-ray showed cardiomegaly and a small amount of pleural effusion on the left side. Cardiac magnetic resonance imaging (CMRI) revealed a 6.2 × 12 × 9.8 cm mass in the anterior wall of the RV protruding into the chamber. Because it showed a uniform signal slightly higher than normal myocardial signal on T1-weighted and T2-weighted images, RV tumour was considered. However, gadolinium-enhanced scan was not performed (Figure 4). Pathological examination of paraffin-embedded pericardial effusion samples found adenoid or small clusters of epithelioid cells and redundant small round atypical cells with less cytoplasm and visible nuclei. Immunohistochemical examination showed epithelioid cells CK (+), CK7 (+), CR (+), MC partially (+), CD68 (+), TTF-1 (−), mesothelial cells, and diffusely distributed atypical cells CK (−), CD20 (+), CD3 (−), CD138 (−), Ki-67 ~ 10% (+) (Figure 5). Combined with the results of immunohistochemistry, the possibility of lymphoproliferative lesions cannot be ruled out. Because the specific type of malignancy could not be determined, we conducted further investigation. Because axillary lymph node biopsy performed in other hospitals could not confirm the diagnosis, axillary lymph node resection for biopsy was planned. Consultation with multiple departments of this hospital suggested that right heart failure could occur during the anaesthesia process due to the patient's poor general state, which is clearly a contraindication for anaesthesia. Surgical resection of the heart mass for pathological biopsy, however, required the support of extracorporeal membrane oxygenation (ECMO), in addition to costly manpower and material resources, there was also a possibility of regeneration, so the surgery was not recommended for the time being. Later on, the patient experienced epistaxis again. As soon as a mass in the left nasal cavity was found, biopsy of the nasal mass was performed using transnasal endoscopy. Meanwhile,18F-Fluorodeoxyglucose positron-emission tomography/computed tomography18F-FDG PET/CT reported multiple enlarged lymph nodes in the left axilla, mediastinum, and celiac mesentery. Given the increased glucose metabolism inside the above lesions in the right heart and left nasal cavity, malignant lymphoma was considered (Figure 6). Pathological examination of nasal mass showed redundant fat fusiform histiocytes, small lymphocytes, and scattered or aggregated atypical cells, which were medium-sized with large and round deep-stained nuclei; in some cells, nucleoli and mitosis were visible without necrosis. Immunohistochemical examination showed CD3 (+ mainly small lymphocytes), large CD5 (+ mainly small lymphocytes); large atypical cells CD20, CD79a (+), MUM-1 (+), BCLE6 (+), a few CD10 (+), CD30 (−), ALK (−) Cyclin D1 low level (+), BCL-2 (+), CK (−), VIM (−), CK5/6 (−), p63 (−), TDT (−), CD34 blood vessels (+), CD68 redundant histocytes (+), Ki-67 ~ 50% (+); in situ hybridization showed EBER (−). Pathological diagnosis: non-Hodgkin's lymphoma, consistent with T-cell/histiocytic diffuse large B-cell lymphoma, non-germinal centre (Figure 7). Kimura disease usually manifests progressive painless masses in the head and neck, and elevated eosinophil counts and IgE level. The pathological findings include proliferation of lymphoid follicles, formation of germinal centre, and small blood vessels. Combined with current laboratory, imaging, and pathological findings, Kimura disease was ruled out. We recommended chemotherapy, but the patient declined.
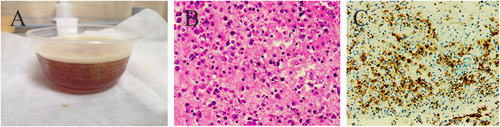

Discussion
An overview of primary cardiac lymphoma
Primary cardiac lymphoma was first described in the 1930s; despite its unknown pathogenesis, it often occurs in immunosuppressive population such as patients with HIV/AIDS and patients receiving immunosuppressants or heart transplant.7, 8 DLBCL, plasmablastic lymphoma, immunoblastic lymphoma, and some low-grade lymphomas are more common.
Predilection site and clinical manifestation of primary cardiac lymphoma
Structural and functional changes of the involved heart are the most prominent characteristics of PCL. The most common sites of PCL are the right atrium, followed by the RV, left ventricle, left atrium, and finally the atrial septum and interventricular septum.5 In addition to atria and ventricles, primary cardiac DLBCL can also invade any parts of the heart including pericardium, heart valves, atrioventricular node, macrovessels, and atrioventricular septum. Pericardium is the common site of involvement around the heart, characterized by pericardial effusion and/or pericardial mass, and pericardial tamponade in severe cases. In this case, the patient manifested pericardial effusion. Primary cardiac DLBCL often has a single mass, which can grow into the heart and plug the chambers or infiltrate into the myocardium. The clinical symptoms are mainly related to the differential sites of tumour invasion. The tumour can often cause chest tightness, shortness of breath, oedema of the face and/or lower extremities, palpitation, chest pain, and other non-specific symptoms. It may also cause various arrhythmias such as sinus tachycardia, atrioventricular block, atrial fibrillation, and ventricular fibrillation. Cases with syncope as the initial manifestation were also reported.9, 10 Congestive heart failure was observed in more than half of PCL patients according to the report.
Differential diagnosis of cardiac space-occupying lesions
Common differential diagnosis of cardiac space-occupying lesions includes (i) thrombus: often in the left atrium, commonly observed in patients with rheumatic heart disease, mitral stenosis or insufficiency, and atrial fibrillation. It was also reported in acute myocardial infarction;11 (ii) Vegetation: typically irregular, mobile, and attached to the valve, commonly observed in patients with valvular disease, congenital vascular malformation, and artificial valve replacement; (iii) tumour: myxoma is often a movable benign tumour attached to the endocardium in the left atrium. Primary cardiac sarcoma is a more challenging type; it is more common than PCL, but usually occurring in the left chambers without concomitant pericarditis or extracardiac lesions. Unlike other sarcomas, angiosarcoma typically originated from the right atrium and was often accompanied by right heart failure, haemorrhagic pericardial effusions, or metastases. Due to the necrosis of blood vessels, CMRI often found uneven signals. This often requires pathological examination to confirm the diagnosis. Cardiac lymphoma is extremely rare and often detected during autopsy; pathological results are the ‘gold standard’ for diagnosis.12 With the advancement in medical technologies, especially imaging technology, the antemortem diagnosis rate has increased significantly. Echocardiography, CT, and CMRI are non-invasive examinations of cardiac masses. Echocardiography can reveal the anatomic location, extent, and consequence of intracardiac mass through dynamic assessment during each cycle.13 CMRI can better identify the nature of lesions.14 The 18F-FDG PET/CT, however, can simultaneously detect anatomical and metabolic indexes, giving rise to a higher accuracy in the diagnosis of non-Hodgkin's lymphoma in the neck, supraclavicular, and extranodal areas.15
Conclusion
In summary, primary cardiac DLBCL is an extremely rare tumour and difficult to diagnose. Correct identification of the nature of cardiac mass through non-invasive imaging is critically important for the diagnosis, management, and prognosis. When a cardiac mass is detected, effective evidence on the localization and structure of the mass can be provided by transthoracic and transesophageal echocardiography, CT, and MRI, then properties of the mass are evaluated by18F-FDG PET/CT, eventually, the pathological results will confirm the nature of masses.
Acknowledgements
Fei Miao and Yingfeng Liu designed the report; Jiafeng Yu, Xin Zhao, and Qingwei Yan collected patient's clinical data; Jiafeng Yu analysed the data and wrote this report.
Conflict of interest
None declared.
Funding
This work was supported by the Guangdong Basic and Applied Basic Research Foundation (Item No. 2020A1515010288).
Informed consent statement
A written informed consent was obtained from the patient.




