Myocardial deformation assessed among heart failure entities by cardiovascular magnetic resonance imaging
Abstract
Aims
Although heart failure (HF) is a leading cause for hospitalization and mortality, normalized and comparable non-invasive assessment of haemodynamics and myocardial action remains limited. Moreover, myocardial deformation has not been compared between the guideline-defined HF entities. The distribution of affected and impaired segments within the contracting left ventricular (LV) myocardium have also not been compared. Therefore, we assessed myocardial function impairment by strain in patients with HF and control subjects by magnetic resonance imaging after clinically phenotyping these patients.
Methods and results
This prospective study conducted at two centres in Germany between 2017 and 2018 enrolled stable outpatient subjects with HF [n = 56, including HF with reduced ejection fraction (HFrEF), HF with mid-range ejection fraction (HFmrEF), and HF with preserved ejection fraction (HFpEF)] and a control cohort (n = 12). Parameters assessed included measures for external myocardial function, for example, cardiac index and myocardial deformation measurements by cardiovascular magnetic resonance imaging, left ventricular global longitudinal strain (GLS), the global circumferential strain (GCS) and the regional distribution of segment deformation within the LV myocardium, as well as basic phenotypical characteristics.
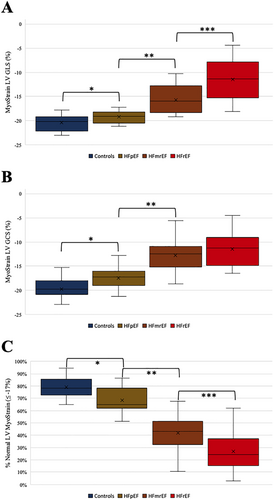
Comparison of the cardiac indices at rest showed no differences neither between the HF groups nor between the control group and HF patients (one-way ANOVA P = 0.70). The analysis of the strain data revealed differences between all groups in both LV GLS (One-way ANOVA: P < 0.01. Controls vs. HFpEF: −20.48 ± 1.62 vs. −19.27 ± 1.25. HFpEF vs. HFmrEF: −19.27 ± 1.25 vs. −15.72 ± 2.76. HFmrEF vs. HFrEF: −15.72 ± 2.76 vs. −11.51 ± 3.97.) and LV GCS (One-way ANOVA: P < 0.01. Controls vs. HFpEF: −19.74 ± 2.18 vs. −17.47 ± 2.10. HFpEF vs. HFmrEF: −17.47 ± 2.10 vs. −12.78 ± 3.47. HFrEF: −11.41 ± 3.27). Comparing the segment deformation distribution patterns highlighted the discriminating effect between the groups was much more prominent between the groups (one-way ANOVA P < 0.01) when compared by a score combining regional effects and a global view on the LV. Further analyses of the patterns among the segments affected showed that while the LVEF is preserved in HFpEF, the segments impaired in their contractility are located in the ventricular septum. The worse the LVEF is, the more segments are affected, but the septum remains an outstanding location with the most severe contractility impairment throughout the HF entities.
Conclusions
While cardiac index at rest did not differ significantly between controls and stable HF patients suffering from HFrEF, HFmrEF, or HFpEF, the groups did differ significantly in LV GLS and LV GCS values. Regional strain analysis revealed that the LV septum is the location affected most, with reduced values already visible in HFpEF and further reductions in HFmrEF and HFrEF.
Introduction
While the causes for heart failure (HF) have been broadly explored and HF is becoming increasingly important due to demographic changes in the western world, mechanisms and haemodynamic have remained insufficiently understood.1, 2 Patients with HF include those with reduced left ventricular (LV) ejection fraction (LVEF, HFrEF), mid-range LVEF (HFmrEF) and preserved LVEF (HFpEF)—all with different degrees of systolic and diastolic alterations.3 Phenotypically, patients with HFrEF, HFmrEF, and HFpEF suffer from a systemic disease with different comorbidities with great overlaps.4, 5 Classic approaches to categorize these phenotypes include functional classes of symptoms on exertion, for example, by the New York Heart Association (NYHA) classification, global cardiac function assessed by global systolic or diastolic parameters, for example, LVEF or E/e. While the discriminatory effect of LVEF among HF patients has been broadly criticized for decades, especially in distinguishing healthy hearts from HFpEF patients, increasing evidence challenged its meaning because it covers poorly actual cardiac action.6-9 While echocardiographic parameters are extensively used in HF guidelines, they have significant detractions, especially in an era when HFpEF is becoming the dominant presentation.10
Measurements of cardiac contractility and the assessment of myocardial deformation by strain analyses are an emerging and promising tool to better characterize patients.10
Strain imaging provides the opportunity to render the spatial contractility parameters (longitudinal, circumferential, and radial strain) measurable at each voxel level over the cardiac cycle, as the LVEF is limited in discriminating HF patients and both global longitudinal and circumferential strain provide the chance to assess these subjects more accurately—especially by cardiovascular magnetic resonance imaging (CMR).11 Since HFmrEF was just introduced in 2016 by the European Society of Cardiology (ESC) guidelines, assessment of strain imaging in HFmrEF patients has been very limited.
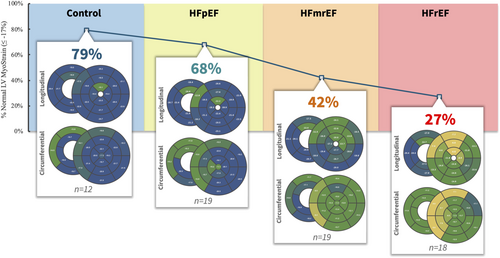
However, usually myocardial deformation is reported as a global value either for the longitudinal axis or the circumferential axis. Recent studies showed the relevance of both myocardial deformation per se and the distribution of its impairment—a comparison between the HF entities regarding their regional differences within the LV myocardium has not been performed. Combining the regional differences and a global view on the affected LV resulted in a summarizing score based on 37 segments of the LV, which has already been introduced.12 The ratio of segments with preserved strain values to all 37 LV segments represents a number reflecting the share of the myocardium that provides a normal strain value of the LV with a global view—allowing for comparisons of how many segments are affected between the HF entities.
While HF entities per se have been characterized regarding strain values, there has not been a comparison of cardiac contractility with regard to the cardiac work or cardiac index.
The aim of this study is to assess the longitudinal and circumferential strain as well as the distribution of the myocardial deformation in an HF population including HFrEF, HFmrEF, and HFpEF patients as well as subjects without HF.
Methods
This study was a prospective study conducted at two centres in Berlin, Germany, the Charité—University Medicine Berlin and the German Heart Centre Berlin, between 2017 and 2018.
Briefly, subjects were screened for diagnosed HF NYHA functional Classes II and III and an age of at least 45 years. The initial diagnosis of HF should have been older than 30 days; the patients were required to be in a stable state with no changes in their HF medication and no HF hospitalization within the previous 7 days. HFrEF was defined as diagnosis of HF, increased N terminal pro brain natriuretic peptide (NT-proBNP) (>220 pg/mL) and LVEF <40%, HFmrEF as the diagnosis of HF, increased NT-proBNP (>220 pg/mL) and 40% ≥ LVEF < 50% as well as HFpEF as diagnosis of HF, increased NT-proBNP (>220 pg/mL) and LVEF ≥50% at the time of study inclusion. We did not distinguish between the causes for HF for recruiting patients.
Additionally, we recruited subjects without HF or major cardiovascular diseases as controls—for this analysis, we excluded those with cardiovascular risk factors, for example, coronary artery disease or diabetes.
All studies included complied with the Declaration of Helsinki, the protocols were approved by the responsible ethics committees, and all patients gave written informed consent. It was registered at the German Clinical Trials Register (DRKS, registration number: DRKS00015615). The detailed inclusion and exclusion criteria are listed on the webpage of the DRKS. Further analyses of this study have already been published.13-16
Cardiac magnetic resonance
All CMR images were acquired using 1.5 T (Achieva, Philips Healthcare, Best, The Netherlands) magnetic resonance imaging (MRI) scanners with a five-channel cardiac surface coil in a supine position. All study participants were scanned using identical comprehensive imaging protocol. The study protocol included initial scouts to determine cardiac imaging planes. Cine images were acquired using a retrospectively gated cine-CMR in cardiac short-axis, vertical long-axis, and horizontal long-axis orientations using a steady-state free precession sequence for volumetry as previously described.13 The calculation of the cardiac indices (CIs) is based on the volumetry of the ventricles. Fast strain-encoded (fast-SENC) MRI was used for strain evaluation, as it has been shown to enable quantification of longitudinal and circumferential strain in free breathing and with high reproducibility.17 Images were blinded to strain analysis, cine, and volumetric measurements, respectively. We waived interobserver analyses based on an analysis that highlighted the robustness of fast-SENC analyses regarding intraoberserver and interobserver variabilities.18
Image analysis
All images were analysed offline using commercially available software (Medis Suite, version 3.1, Leiden, The Netherlands) in accordance to recent consensus document for quantification of LV function using CMR.19 In the analysis were included 2Ch, 3Ch, and 4Ch cine images, and respectively, three preselected mid-ventricle slices from the LV short-axis stack. Image analysis was performed using the software Medis® Suite MR (Medis medical imaging systems, Leiden, The Netherlands, version 3.1) for volume measurements and the software MyoStrain (Myocardial Solutions, Inc., Morrisville, North Carolina, USA, version 5.0) for fast-SENC strain measurements.
Statistical analysis
Statistical analysis was carried out with GraphPad Prism software version 8.4.2 and R version 3.5.1 (2018-07-02) (R Foundation for Statistical Computing, Vienna, Austria).
Normality of variables was assessed by visual assessment of normality curves and the Shapiro–Wilk test. Comparison between groups for continuous variables was performed with a one-way ANOVA for normally distributed data. When a significant P value was obtained using one-way ANOVA, the group means were examined by the Holm–Bonferroni method. Values of P < 0.05 were considered statistically significant.
Results
Study population
There were 68 subjects analysed, including 56 HF patients (HFrEF: n = 19; HFmrEF: n = 19; HFpEF: n = 18) and 12 without HF. We considered the subjects without HF and cardiovascular risk factors as controls in our study. The baseline characteristics are shown in Table 1.
| Controls | HFpEF | HFmrEF | HFrEF | |||
|---|---|---|---|---|---|---|
| N = 12 | N = 19 | N = 19 | N = 18 | |||
| n (%) | n (%) | n (%) | n (%) | |||
| mean ± SD | mean ± SD | mean ± SD | mean ± SD | P value | ||
| Female sex (%) | 5 (41.7) | 9 (47.4) | 6 (31.6) | 3 (16.7) | 0.24 | |
| Age (years) | 58.92 (6.84) | 77.58 ± 8.11 | 67.00 ± 9.64 | 64.22 ± 10.09 | <0.01 | |
| BMI (kg/m2) | 24.84 ± 3.48 | 27.32 ± 3.78 | 26.56 ± 4.16 | 28.83 ± 4.10 | 0.06 | |
| Blood pressure, systolic (mmHg) | 130.5 ± 12.0 | 141.7 ± 17.4 | 131.1 ± 12.5 | 133.7 ± 17.41 | 0.14 | |
| Blood pressure, diastolic (mmHg) | 75.00 ± 7.1 | 76.00 ± 14.62 | 77.16 ± 8.8 | 79.71 ± 13.7 | 0.72 | |
| Heart rate (bpm) | 58.91 ± 7.3 | 64.94 ± 9.5 | 67.26 ± 7.3 | 67.24 ± 12.4 | 0.10 | |
| Presence of CAD (%) | 0 (0.0) | 12 (66.7) | 15 (79.0) | 13 (76.5) | <0.01 for HF only: P = 0.68 | |
| Hypertension (%) | 4 (33.3) | 14 (88.9) | 15 (79.0) | 16 (82.4) | <0.01 for HF only: P = 0.73 | |
| Diabetes mellitus (%) | 0 (0) | 5 (27.8) | 3 (15.8) | 5 (29.4) | 0.1867 for HF only: P = 0.59 | |
| LVEF (%) | 62.00 ± 5.34 | 61.42 ± 5.88 | 44.84 ± 2.93 | 32.89 ± 4.71 | <0.01 for HF only: <0.01 | |
| LVEDVi (mL/m2) | 80.75 ± 12.00 | 69.50 ± 15.02 | 93.63 ± 15.29 | 130.70 ± 24.74 | <0.01 for HF only: <0.01 | |
| SVi (mL/m2) | 49.75 ± 5.79 | 43.79 ± 9.81 | 41.74 ± 5.75 | 42.56 ± 7.28 | 0.03 for HF only: P = 0.72 | |
| Cardiac output (L/min) | 5.73 ± 0.66 | 5.14 ± 1.40 | 5.37 ± 1.01 | 5.70 ± 1.26 | 0.42 | |
| Cardiac index (L/min/m2) | 2.96 ± 0.39 | 2.71 ± 0.58 | 2.79 ± 0.42 | 2.83 ± 0.56 | 0.64 | |
| Septal wall thickness | 8.7 ± 1.2 | 11.0 ± 2.2 | 11.8 ± 2.0 | 11.8 ± 2.7 | <0.01 for HF only: P = 0.51 | |
| NYHA class | II | — | 9 (50) | 16 (84.2) | 12 (70.6) | 0.08 |
| III | — | 8 (44.4) | 3 (15.8) | 5 (29.4) | 0.17 | |
| MLHFQ | 5.3 ± 6.2 | 31.0 ± 23.1 | 27.4 ± 22.5 | 28.5 ± 24.9 | <0.01 for HF only: P = 0.90 | |
| 6MWT (m) | 547.8 ± 130.0 | 344.4 ± 118.3 | 415.5 ± 85.2 | 413.5 ± 125.4 | <0.01 for HF only: P = 0.12 | |
| Concomitant medication | ||||||
| Beta-blocker (%) | 4 (33.3) | 11 (57.9) | 14 (73.7) | 17 (94.4) | <0.01 | |
| ACEi (%) | 1 (8.3) | 4 (21.1) | 7 (36.8) | 9 (52.9) | 0.0481 | |
| MRA (%) | 0 (0.0) | 3 (15.8) | 4 (21.1) | 11 (64.7) | <0.01 | |
| ARNI (%) | 0 (0.0) | 0 (0) | 0 (0) | 5 (27.8) | <0.01 | |
| Loop diuretic (%) | 0 (0.0) | 4 (21.1) | 7 (36.8) | 6 (35.3) | <0.01 for HF only: P = 0.53 | |
| Thiazide diuretic (%) | 1 (8.3) | 4 (21.1) | 2 (10.5) | 1 (0.1) | 0.51 | |
| Blood testing | ||||||
| Haemoglobin (g/dL) | 14.22 ± 0.94 | 13.0 ± 1.3 | 13.7 ± 1.1 | 15.0 ± 1.1 | <0.01 | |
| Haematocrit | 0.41 ± 0.03 | 0.38 ± 0.03 | 0.40 ± 0.03 | 0.44 ± 0.04 | <0.01 | |
| eGFR (mL/min/1.73 m2) | 81.08 ± 10.43 | 69.89 ± 15.13 | 71.21 ± 17.92 | 71.76 ± 20.58 | 0.31 | |
| NT-proBNP (ng/L) | 68.92 ± 28.14 | 554.2 ± 609.3 | 790.2 ± 1,138 | 2,247 ± 3,447 | 0.01 for HF only: P = 0.04 | |
| Troponin T hs (ng/L) | 6.83 ± 3.66 | 19.56 ± 17.72 | 19.22 ± 19.52 | 18.38 ± 11.98 | 0.11 | |
- ACEi, ACE/angiotensin-converting enzyme inhibitor; ARNI, angiotensin receptor neprilysin inhibitor; BMI, body mass index; BSA, body surface area using the Mosteller method; CAD, coronary artery disease; eGFR, estimated glomerular filtration rate using the CKD-EPI formula; HF, heart failure; LVEF, left ventricular ejection fraction; LVEDVi, left ventricular end diastolic volume indexed to BSA; MLHFQ, Minnesota Living with Heart Failure Questionnaire; MRA, mineralocorticoid receptors antagonist; NT-proBNP, N terminal pro brain natriuretic peptide; NYHA class, New York Heart Association Functional Classification; SVi, LV stroke volume indexed to BSA; 6MWT, 6 min walk test.
- Absolute (relative) numbers.
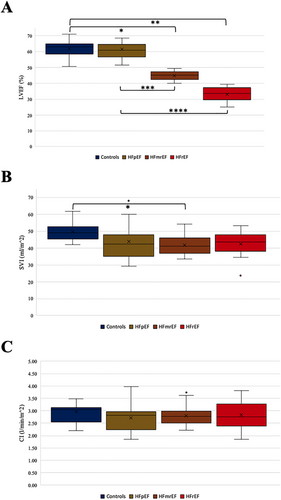
Haemodynamic differences
Figure 1 illustrates the cardiac indices of all four analysed groups, which illustrates that there is no difference in cardiac indices neither between controls and HF groups nor between the HF groups (one-way ANOVA: P = 0.64). In the same manner, the stroke volume indexed to the body surface area was not relevantly different between the groups—while the LVEF and the LV end-diastolic volume index were different among the groups, which underlines der reliability of our data (Table 1).
Strain analyses
While the majority of segments are impaired regarding the contractility measured in strain values in HFrEF patients, the majority is preserved in HFpEF patients (Figure 2). Nonetheless, we see a significant difference in the global, averaged strain value between controls and HFpEF while most segments are intact (Figure 2). Obviously, there are a restricted number of segments in HFpEF patients that are mostly affected to make the global value different. Further analyses showed that these segments are mainly located in the LV septum. The lower the LVEF is, the more segments are affected—this correlates with higher LV septum impairment.
Discussion
In this analysis, we compared for the first time the cardiac indices as well as myocardial deformation pattern changes measured by MRI at rest in a stable but symptomatic outpatient cohort suffering from HFrEF, HFmrEF, and HFpEF as well as a controls cohort without HF. While CI was not different among these groups at rest, strain values showed differences. This finding stayed in contrast to the clinically common perception that a lower LVEF is indicative of a lower cardiac output but was in line with the haemodynamic key rule that in HF, CI is impaired or only maintained at the expense of increased filling pressures.
We explain our findings by the fact that the HF patients investigated in our study were stable and showed symptoms only at exertion, reflected by the NYHA functional Classes II and III—therefore, showing no impairment of CI at rest.
Analysing strain values, we saw differences between the groups in both LV GLS and LV GCS. This finding is also in alignment with previous studies, which showed that both GLS and GCS are robust to LV volume changes, especially more robust compared with LVEF.11, 18, 20 Latest works support the idea that myocardial shortening is a superior parameter for myocardial contraction and function.21 Similar to our analysis, other studies have also shown that septal contraction is primarily impaired—even though in different cardiac diseases leading to HF. Changes of myocardial contraction in HF of different origins require further investigation (Figures 3 and 4).22
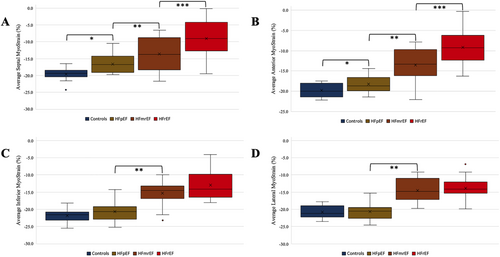
The relationship between LV GCS and LV GLS regarding additional value needs to be further evaluated, especially because there are myocardial compensation mechanisms reported, which showed increase of the one when the other is impaired—which are in contrast to our results, possibly due to different techniques, fast-SENC on the one hand, and tissue tracking on the other.11
The prognostic value of GLS was initially limited, but recent studies showed a significant added value in prognosis with improved techniques, mainly in HFrEF patients—the prognostic aspect of the GCS value has not been conclusively evaluated.23
Our calculations are limited by the moderate number of LVEF values at the extremes and the stable outpatient state of the HF patients (NYHA Classes II and III). An analysis including patients with NYHA Class IV might provide a more elaborate approach to these questions, but it is more challenging to perform a diagnostic CMR in those patients due to their supine position at the time of the exam. Alternatively, a physical stress method at which the patients report symptoms could be added to the protocol to assess the point of time of discomfort—this is not yet an established part of routine MRI diagnostic protocols. Additionally, the optimal stressor is not yet defined. The results of our study are in line and supportive with the recent ESC Heart Failure Association (HFA) position statement for shifting paradigms in the assessment of cardiac function.24 In particular, going away from ejection fraction to myocardial strain evaluation and also from rest to stress assessment of LV function is accompanied by the latest expert panel statement of the HFA regarding HF classification.7 Correspondingly, the newest ESC HFA diagnostic work-up recommendation for HFpEF highlights the relevance of stress imaging to unmask impaired cardiac function in HF.25 We consider our results as a key component in changing the understanding of HF dynamics challenging the current HF classification. In summary, we have to state that the current HF classification of HF patients into LVEF-based entities is only of relevance in HFrEF patients from a treatment point of view. HFrEF is the only entity with an evidence-based treatment while chronic HFmrEF and HFpEF still lack tailored therapy.3 Better characterization of the myocardial action impairment provides both a better pathomechanistic understanding of the underlying disease and better parameters for patient phenotyping as well as trial endpoints.
Limitations
This study was performed at two centres cooperating, one focusing on MRI acquisition and analysis and the other with the remainder of the comprehensive study. The MRI centre used the same scanner for all subjects providing a high level of comparability.
Examinations in an magnetic resonance scanner may cause psychological stress due to the confined space and examination in a hospital setting. We cannot rule out that in some patients, this might have resulted in potentially slightly higher CI values compared with the CI at complete rest at home.
While including more patients than previous studies evaluating the discriminatory effect of strain values across HF groups and a control cohort, our sample size was still small.
We had to exclude patients with implanted ICDs and pacemakers due to MRI contraindications. This limits the generalizability of our study to the general HF population, especially in patients with HFrEF. However, our findings should primarily be considered hypothesis generating.
Conclusions
While cardiac index at rest did not differ significantly between controls and stable HF patients suffering from HFrEF, HFmrEF, or HFpEF, the groups did differ significantly in LV GLS and LV GCS values. Regional strain analysis revealed that the LV septum is the location affected most, with reduced values already visible in HFpEF and further reductions in HFmrEF and HFrEF.
Acknowledgements
We like to express our sincere thanks to all the patients and volunteers who agreed to participate in our study. Likewise, we appreciate the support by the team maintaining the study, especially the image acquisition.
Conflict of interest
S. K. reports grants and other support by the DZHK (German Center for Cardiovascular Research), Partner Site Berlin, Philips Healthcare, BioVentrix, Berlin-Chemie, Merck/Bayer, Novartis, Astra Zeneca, Siemens, and Myocardial Solutions outside of the submitted work. S. K. is also on the advisory board for Merck/Bayer, BioVentrix, and Myocardial Solutions. B. P. has provided steering committee and advisory board services for Bayer Healthcare and MSD and has received steering committee and advisory board/speaker honoraria from Novartis. P. D. is a shareholder of Siemens and Bayer. All other authors declare that they have no relationships relevant to the contents of this paper to disclose.
Funding
This work was supported by the German Centre for Cardiovascular Research (DZHK), funded by the German Federal Ministry of Education and Research, and the Charité—Universitätsmedizin Berlin, Germany, as well as the German Heart Institute Berlin, Germany, Myocardial Solutions, and Novartis.




