Plasma growth differentiation factor 15: a novel tool to detect early changes of hereditary transthyretin amyloidosis
Abstract
Aims
Hereditary transthyretin (ATTRv) amyloidosis is the most frequent and representative form of autosomal dominant hereditary systemic amyloidosis. Disease-modifying treatments of the disease are more effective during the early stages, and we require biomarkers to detect early pathological changes for prompt diagnosis. This study aimed to investigate whether plasma growth differentiation factor 15 (GDF-15) levels could aid detection of early pathological changes in ATTRv amyloidosis.
Methods and results
We retrospectively studied 32 patients with ATTRv amyloidosis, eight asymptomatic TTR mutation carriers, and eight healthy volunteers. We evaluated plasma GDF-15 levels in these subjects as related to levels of brain natriuretic peptide and high-sensitivity troponin T, echocardiographic features, 99mTc-pyrophosphate (PYP) scans, and cardiac magnetic resonance imaging findings. Plasma GDF-15 levels significantly increased even in asymptomatic TTR mutation carriers compared with healthy volunteers (P < 0.01). Plasma GDF-15 levels were significantly correlated with plasma brain natriuretic peptide values (P < 0.01), serum high-sensitivity troponin T values (P < 0.05), and interventricular septal thickness at end-diastole (P < 0.01) in patients with ATTRv amyloidosis. Plasma GDF-15 levels in patients with PYP-positive ATTRv amyloidosis were significantly higher than those in patients with PYP-negative ATTRv amyloidosis (P < 0.01). Plasma GDF-15 levels in patients with late gadolinium enhancement-positive ATTRv amyloidosis were significantly higher than those in patients with late gadolinium enhancement-negative ATTRv amyloidosis (P < 0.01). Groups of patients with different TTR genotypes manifested different plasma GDF-15 levels.
Conclusions
Growth differentiation factor 15 may reflect early pathological changes of ATTRv amyloidosis.
Introduction
Hereditary transthyretin (ATTRv) amyloidosis, which is a fatal autosomal dominant disease, causes failure of systemic organs including the heart and dysfunction of peripheral nerves.1, 2 In the bloodstream, transthyretin (TTR), which is synthesized mainly in the liver, forms a homotetramer with a dimer-of-dimers configuration and causes two types of systemic amyloidosis, ATTRv amyloidosis and wild-type TTR (ATTRwt) amyloidosis.3, 4 More than 150 different mutations in the TTR gene have been identified, and most of them reportedly enhance dissociation of TTR tetramers to monomers followed by TTR amyloid formation in the extracellular spaces of tissues.5 To inhibit TTR amyloid formation, novel disease-modifying therapies, including stabilizers of tetrameric TTR such as tafamidis, have been developed. Clinical studies revealed that earlier treatment with tafamidis was related to a better clinical outcome in patients with ATTRv amyloidosis.6 In addition, recent clinical trials successfully demonstrated that gene-silencing therapies, such as small interfering RNA and antisense oligonucleotide, have beneficial therapeutic effects on the clinical outcomes of patients with ATTRv amyloidosis.7, 8 Prompt interventions with those therapies require early diagnosis of ATTRv amyloidosis, which is becoming of great interest to physicians.
Cardiac involvement is one of the initial pathological changes in patients with ATTRv amyloidosis. Plasma brain natriuretic peptide (BNP) and serum troponin T (TnT) levels also reportedly increased in patients with ATTRv amyloidosis.9-11 In addition, echocardiography, cardiac magnetic resonance imaging (MRI), and bone scintigraphy reportedly detected cardiac involvements in patients with ATTRv amyloidosis.12 However, whether those clinical markers change during early stages of the disease remains to be clarified. To detect early changes in the disease, we require novel clinical markers so that we can accomplish prompt interventions.
Growth differentiation factor 15 (GDF-15), which is a member of the transforming growth factor-β superfamily and a stress-responsive cytokine, is associated with cardiovascular stress, fibrosis, inflammation, and remodelling.13 In patients with cardiovascular disease, GDF-15 was reportedly a useful marker of disease development, progression, and prognosis.14, 15 GDF-15 also reportedly showed prognostic significance in patients with amyloid light-chain amyloidosis.16 In this study, we investigated whether plasma GDF-15 levels, in addition to plasma BNP and serum high-sensitivity TnT (hs-TnT) levels, increased in during the early stages of the disease. The objective of this study was to investigate whether plasma GDF-15 levels could detect early pathological changes in ATTRv amyloidosis.
Methods
Study population
We retrospectively studied 32 patients with ATTRv amyloidosis (mean age: 51.1 ± 2.4 years, male: 53%, twenty-seven V30M, two Y114C, one G47R, one T49S, and one G61L), eight asymptomatic TTR mutation carriers (mean age: 33.6 ± 2.3 years, male: 25%, five V30M, two S50I, and one G47R), and eight healthy volunteers (mean age: 32.0 ± 3.4 years, male: 63%) who visited Kumamoto University Hospital from October 2016 to May 2017. Table 1 provides the clinical characteristics of the study subjects.
| Healthy volunteers (n = 8) | Asymptomatic carriers (n = 8) | Patients with ATTRv amyloidosis (n = 32) | |
|---|---|---|---|
| Age (years) | 32.0 ± 3.4 | 33.6 ± 2.3 | 51.1 ± 2.4 |
| Men, n (%) | 5 (63) | 2 (25) | 17 (53) |
| V30M, n (%) | NA | 5 (63) | 27 (84) |
| Non-V30M, n (%) | NA | 3 (38) | 5 (16) |
| GDF-15 (pg/mL) | 1196.5 ± 373.2 | 2662.8 ± 162.5 | 6481.5 ± 739.7 |
| BNP (pg/mL) | 6.4 ± 0.5 | 8.3 ± 1.1 | 61.3 ± 113.1 |
| hs-TnT (ng/mL) | 0.003 ± 0.0001 | 0.003 ± 0.0003 | 0.0196 ± 0.004 |
| TTR (mg/dL) | NA | 20.0 ± 2.7 | 20.4 ± 2.1 |
| Cr (mg/dL) | NA | 0.64 ± 0.06 | 0.89 ± 0.14 |
| eGFR (mL/min/1.73 m2) | NA | 86.0 ± 2.0 | 74.6 ± 3.1 |
| CRP (mg/dL) | NA | 0.04 ± 0.01 | 0.05 ± 0.01 |
| IVSTd (mm) | NA | NA | 11.7 ± 0.63 |
| LAD (mm) | NA | NA | 31.9 ± 1.1 |
| EF (%) | NA | NA | 63.1 ± 1.0 |
| E/e′ | NA | NA | 12.8 ± 1.1 |
| Kumamoto clinical score | NA | 0 | 14.6 ± 2.2 |
| Ocular symptoms, n (%) | NA | 0 (0) | 11 (34) |
| Gastrointestinal symptoms, n (%) | NA | 0 (0) | 21 (66) |
- ATTRv, hereditary transthyretin; BNP, brain natriuretic peptide; Cr, creatinine; CRP, C-reactive protein; EF, ejection fraction; eGFR, estimated glomerular filtration rate; GDF-15, growth differentiation factor 15; hs-TnT, high-sensitivity troponin T; IVSTd, interventricular septal thickness at end-diastole; LAD, left atrial diameter; NA, not available; TTR, transthyretin.
- Data are shown as means ± standard deviation or as n (%).
Measurement of growth differentiation factor 15 and cardiac biomarkers
We evaluated plasma levels of GDF-15 and BNP and serum levels of hs-TnT in the study subjects. We collected blood samples in collection tubes and centrifuged them at 2000 g for 15 min. Tubes were stored at −80°C before measuring the plasma GDF-15, plasma BNP, and serum hs-TnT levels. Plasma GDF-15 levels were measured by using a sandwich enzyme immunoassay technique (Quantikine; R&D Systems, Minneapolis, MN, USA).
Statistical analysis
We evaluated the data via Student's t-test, the Pearson correlation coefficient, and the Spearman rank correlation coefficient. Because of the abnormal distribution of plasma BNP levels and serum hs-TnT levels, we performed the Pearson correlation test after logarithmic transformation. All analyses were performed with JMP Version 5.1 (SAS Institute Japan, Tokyo, Japan). P values of <0.05 were considered to be statistically significant.
Study protocol approvals and patient consent
The study protocol was approved by the Human Ethics Review Committee of Kumamoto University. Signed consent forms were obtained from all patients or family members.
Results
Plasma GDF-15 and BNP levels and serum hs-TnT levels in patients with ATTRv amyloidosis, asymptomatic TTR mutation carriers, and healthy volunteers
Levels of plasma GDF-15, plasma BNP, and serum hs-TnT increased significantly in patients with ATTRv amyloidosis compared with levels in asymptomatic TTR mutation carriers and healthy volunteers (Figure 1). Although plasma BNP and serum hs-TnT levels did not increase in asymptomatic TTR mutation carriers compared with levels in healthy volunteers (Figure 1 and 1), plasma GDF-15 levels increased significantly in asymptomatic TTR mutation carriers (2662.8 ± 162.5 pg/mL, P < 0.01) compared with levels in healthy volunteers (1196.5 ± 373.2 pg/mL) (Figure 1).
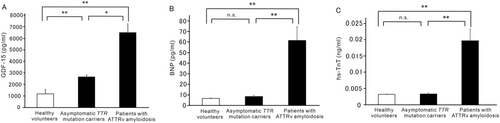
Correlations between plasma GDF-15 levels and other biomarkers in patients with ATTRv amyloidosis
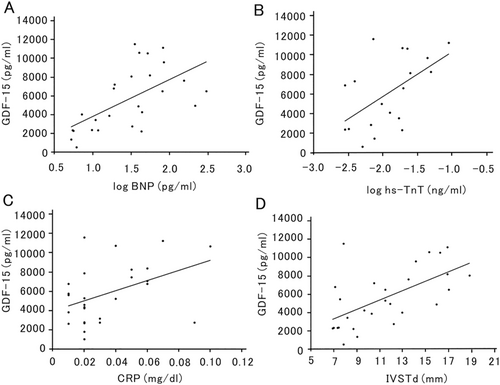
Plasma GDF-15 levels were significantly correlated with plasma BNP (r = 0.5986, P = 0.0003) and serum hs-TnT (r = 0.5468, P = 0.0171) values (Figure 2 and 2). Plasma GDF-15 levels were also significantly correlated with serum C-reactive protein values (ρ = 0.4434, P = 0.0181) (Figure 2). Echocardiographic analyses revealed that plasma GDF-15 levels were significantly correlated with interventricular septal thickness at end-diastole (IVSTd) (ρ = 0.6002, P = 0.0007) (Figure 2). Other biomarkers, including serum TTR and creatinine values, estimated glomerular filtration rate, left atrial diameter, left ventricular ejection fraction, and E/e′, were not correlated with plasma GDF-15 levels in patients with ATTRv amyloidosis (Table 2).
| Clinical data | Spearman rank correlation coefficient (ρ) | P value |
|---|---|---|
| BNP | 0.6525 | 0.0003 |
| IVSTd | 0.6002 | 0.0007 |
| hs-TnT | 0.5397 | 0.0171 |
| CRP | 0.4434 | 0.0181 |
| E/e′ | 0.3507 | 0.0673 |
| eGFR | −0.2874 | 0.1381 |
| Cr | 0.1478 | 0.4529 |
| LAD | 0.2012 | 0.3046 |
| TTR | −0.1179 | 0.6757 |
| EF | −0.0364 | 0.8540 |
- ATTRv, hereditary transthyretin; BNP, brain natriuretic peptide; Cr, creatinine; CRP, C-reactive protein; EF, ejection fraction; eGFR, estimated glomerular filtration rate; GDF-15, growth differentiation factor 15; hs-TnT, high-sensitivity troponin T; IVSTd, interventricular septal thickness at end-diastole; LAD, left atrial diameter; TTR, transthyretin.
Correlations between plasma GDF-15 levels and 99mTc-pyrophosphate and cardiac MRI scans in patients with ATTRv amyloidosis
Of 32 patients with ATTRv amyloidosis, 16 patients obtained 99mTc-pyrophosphate (PYP) scans and 13 patients obtained cardiac MRI. Plasma GDF-15 levels in patients with PYP-positive ATTRv amyloidosis (8242.3 ± 1288.1 pg/mL, P < 0.01) were significantly higher than those in patients with PYP-negative ATTRv amyloidosis (3360.0 ± 563.1 pg/mL) (Figure 3). In addition, plasma GDF-15 levels in patients with late gadolinium enhancement (LGE)-positive ATTRv amyloidosis (9047.0 ± 1078.3 pg/mL, P < 0.01) were significantly higher than those in patients with LGE-negative ATTRv amyloidosis (3631.9 ± 779.7 pg/mL) (Figure 3). In contrast, plasma BNP and serum hs-TnT values in patients with PYP-positive ATTRv amyloidosis did not increase significantly compared with those in patients with PYP-negative ATTRv amyloidosis (Figure 3 and 3). Although plasma BNP values in patients with LGE-positive ATTRv amyloidosis did not increase significantly compared with those in patients with LGE-negative ATTRv amyloidosis (Figure 3), serum hs-TnT values in patients with LGE-positive ATTRv amyloidosis were significantly higher than those in patients with LGE-negative ATTRv amyloidosis (Figure 3).
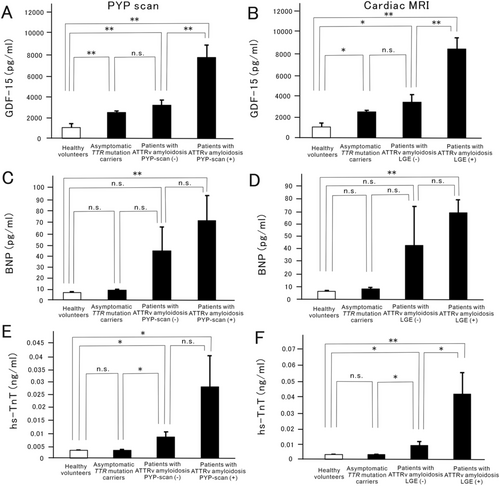
Plasma GDF-15 levels and cardiac imaging in patients with early-onset or late-onset ATTRv amyloidosis who had the TTR V30M mutation or non-V30M mutations
Plasma GDF-15 levels in patients with late-onset ATTRv V30M amyloidosis and patients with ATTRv non-V30M amyloidosis, but not in the patient with the Y114C mutation, were higher than those in patients with early-onset ATTRv V30M amyloidosis. The patients with late-onset ATTRv V30M amyloidosis and those with non-V30M amyloidosis showed 99mTc-PYP uptake and LGE, whereas patients with early-onset ATTRv V30M amyloidosis and the patient with ATTRv Y114C amyloidosis did not (Table 3).
| Early-onset V30M | Late-onset V30M | Non-V30Ma | Y114C | |
|---|---|---|---|---|
| Patients, n | 9 | 3 | 3 | 1 |
| GDF-15 (pg/mL) | 3383.4 ± 629.1 | 7899.0 ± 1573.5* | 8585.7 ± 2388.1* | 3149.0b |
| BNP (pg/mL) | 43.7 ± 23.9 | 90.6 ± 38.2b | 49.6 ± 23.7b | 38.2b |
| hs-TnT (ng/mL) | 0.009 ± 0.002 | 0.018 ± 0.004b | 0.040 ± 0.030b | 0.007b |
| PYP scan positive (%) | 0 (0/9) | 100 (3/3)* | 100 (3/3)* | 0 (0/1)b |
| LGE positive (%) | 0 (0/6) | 100 (3/3)* | 67 (2/3)* | 0 (0/1)b |
- ATTRv, hereditary transthyretin; BNP, brain natriuretic peptide; GDF-15, growth differentiation factor 15; hs-TnT, high-sensitivity troponin T; LGE, late gadolinium enhancement; PYP, 99mTc-pyrophosphate.
- a Except for Y114C.
- b Not significantly different (compared with early-onset V30M).
- * P < 0.05.
Cardiac imaging findings and clinical characteristics of two asymptomatic patients with TTR mutations
We analysed cardiac imaging results and clinical characteristics of two asymptomatic patients who had TTR mutations. Asymptomatic TTR mutation carriers were defined as those who had TTR mutations but did not have amyloid fibril deposition or symptoms such as neuropathy and cardiomyopathy. Plasma GDF-15 levels increased, but plasma BNP levels, serum hs-TnT levels, IVSTd, PYP scans, and cardiac MRI findings were within normal limits (Table 4).
| Case 1 | Case 2 | |
|---|---|---|
| TTR mutation | Ser50Ile (p. Ser70Ile) | Gly47Arg (p. Gly67Arg) |
| Age (years) | 34 | 27 |
| Sex | M | F |
| GDF-15 (pg/mL) | 3229 | 2309 |
| BNP (pg/mL) | 12.0 | 6.5 |
| hs-TnT (ng/mL) | 0.005 | 0.003 |
| IVSTd (mm) | 8.3 | 6.8 |
| EF (%) | 66.3 | 67.1 |
| PYP scan | (−) | (−) |
| EDV (mL) | 81 | 55 |
| ESV (mL) | 28 | 18 |
| T1 mapping | Normal | Normal |
| LGE | (−) | (−) |
- BNP, brain natriuretic peptide; EDV, end-diastolic volume; EF, ejection fraction; ESV, end-systolic volume; GDF-15, growth differentiation factor 15; hs-TnT, high-sensitivity troponin T; IVSTd, interventricular septal thickness at end-diastole; LGE, late gadolinium enhancement; PYP, 99mTc-pyrophosphate.
Discussion
In the present study, we showed that plasma GDF-15 levels increased even in asymptomatic subjects with TTR mutations and in patients with ATTRv amyloidosis, whereas plasma BNP values and serum hs-TnT values did not increase in asymptomatic subjects with TTR mutations. In patients with ATTRv amyloidosis, plasma GDF-15 levels were significantly correlated with cardiac findings including plasma BNP values, serum hs-TnT values, IVSTd, PYP findings, and cardiac MRI results.
Why did plasma levels of GDF-15 increase even during the asymptomatic stage of the disease? Previous studies reported that expression of GDF-15 was induced by cytokines, growth factors, and tissue damage and that it had cardioprotective roles via anti-hypertrophic and anti-apoptotic effects.17-19 Serum interleukin-6 values were reportedly elevated in asymptomatic TTR mutation carriers and patients with ATTRv amyloidosis.20 In vitro studies also showed that the expression of GDF-15 was up-regulated by interleukin-6.21 In patients who had early stages of the disease when only pre-fibrillar TTR deposits were found in nerves, macrophage colony-stimulating factor (M-CSF) was reportedly highly expressed.22 In vitro studies also showed that M-CSF up-regulated GDF-15 expression.23 The pre-fibrillar TTR deposits may enhance GDF-15 expression via M-CSF even during the pre-symptomatic stage of the disease.
Early diagnosis of ATTR amyloidosis requires non-invasive imaging testing and use of blood biomarkers. Recently, bone scintigraphy scans such as 99mTc-PYP and 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid scans were determined to detect cardiac involvement in ATTR amyloidosis.12 99mTc-PYP uptake was reported even during the asymptomatic stage of the disease.24 99mTc-PYP was thought to be the first measurable manifestation of cardiac ATTR amyloidosis and preceded overt echocardiographic, cardiac biomarker, and clinical signs.25 In our study, plasma GDF-15 levels significantly increased even in 99mTc-PYP-negative patients compared with those in healthy volunteers (Figure 3). In addition, plasma GDF-15 levels increased in two asymptomatic TTR mutation carriers who showed no 99mTc-PYP uptake (Table 4). Further studies are needed to evaluate cardiac image of more asymptomatic TTR mutation carriers in order to investigate whether increased plasma GDF-15 levels precede 99mTc-PYP uptake. We also speculate that plasma GDF-15 levels may aid early detection of the non-hereditary type of ATTR amyloidosis, ATTRwt amyloidosis, in which patients first have carpal tunnel syndrome and/or lumbar canal stenosis and then develop fatal cardiomyopathy. Additional studies are needed to clarify whether plasma GDF-15 levels increase during the early stage of ATTRwt amyloidosis with carpal tunnel syndrome and/or lumbar canal stenosis but without symptomatic cardiomyopathy.
As an interesting finding, GDF-15 levels differed in patients with the different TTR genotypes of ATTRv amyloidosis. Plasma GDF-15 levels in patients with late-onset ATTRv V30M amyloidosis and patients with ATTRv non-V30M amyloidosis, but not the patient with the Y114C mutation, were higher than levels in patients with early-onset ATTRv V30M amyloidosis and the patient with ATTRv Y114C amyloidosis. A previous study reported that types of ATTR amyloid fibrils differed in the two groups: the type A group—late-onset ATTRv V30M and ATTRv non-V30M—and the type B group—early-onset ATTRv V30M and ATTRv Y114C.26 Different types of ATTR amyloid fibrils may affect plasma GDF-15 levels and cardiac involvement in ATTRv amyloidosis.
This study has a few limitations, and given that ATTRv amyloidosis is a rare disease, prospective multicentre studies should be performed to confirm our results. As it has been reported that the increase of GDF-15 levels is reversible after treatment,27 plasma GDF-15 levels should be evaluated after novel disease-modifying treatments such as TTR stabilizers and gene-silencing drugs in such prospective multicentre studies. As GDF-15 has been reported to have protective effects on tissues such as anti-inflammatory, anti-apoptotic, and anti-hypertrophic effects,17, 18 the prognostic values of GDF-15 in ATTRv amyloidosis should be validated in order to clarify the pathological role of GDF-15 in ATTRv amyloidosis. Another interesting point in question to investigate is whether plasma GDF-15 levels increase in the extremely early stages of other hereditary and non-hereditary fatal cardiomyopathies.
In conclusion, plasma GDF-15 may reflect early pathological changes of ATTRv amyloidosis.
Acknowledgement
We are indebted to Ms Judith B. Gandy for providing professional English editing of the manuscript.
Conflict of interest
K.T. has received honoraria from Amgen Astellas BioPharma K.K., Kowa Pharmaceutical Co. Ltd., Daiichi Sankyo Co., Ltd., Takeda Pharmaceutical Co., Ltd., Bayer Yakuhin, Ltd., Pfizer Japan Inc., Bristol-Myers K.K., and MSD K.K.; has received trust research/joint research funds from Bristol-Myers K.K. and Kowa Pharmaceutical Co. Ltd.; and has received grants from ITI Co., Ltd., Abbott Medical Japan L.L.C, Abbott Vascular Japan Co., Ltd., Otsuka Pharmaceutical Co., Ltd., Cardinal Health Japan, Kaneka Medix Co., Ltd., Takeda Pharmaceutical Co., Ltd., Mitsubishi Tanabe Pharma, Chugai Pharmaceutical Co, Ltd., Terumo Co, Ltd., Nipro Corporation, Nihon Kohden Corporation, Medtronic Japan Co., Ltd., Japan Lifeline Co., Ltd., Fides-one, Inc., Fukuda Denshi Co., Ltd., and Boston Scientific Japan K.K. M.U. reports personal fees and non-financial support from Pfizer and Alnylam and grants from Prothena, outside the submitted work.
Funding
This research was supported by Grants-in-Aid for Science Research from the Ministry of Education, Culture, Sports, Science and Technology of Japan (Number 18K07502) and the 2019 Global ASPIRE TTR-FAP Competitive Research Grant Award from Pfizer.




