Characteristics, treatment, and outcomes of newly diagnosed atrial fibrillation patients with heart failure: GARFIELD-AF
Abstract
Aims
Heart failure (HF) and atrial fibrillation (AF) may coexist and influence each other. However, characteristics, anticoagulant treatment, and outcomes of contemporary AF patients with concurrent HF are ill-defined. This study analyses characteristics, treatment, and 2 year outcomes in newly diagnosed Global Anticoagulant Registry in the FIELD-Atrial Fibrillation (GARFIELD-AF) patients with vs. without HF.
Methods and results
GARFIELD-AF is the world's largest observational AF patient study. At enrolment, 11 758 of 52 072 patients (22.6%) had HF; 76.3% were New York Heart Association class II–III. Patients with HF had comparable demographics, blood pressure, and heart rate but more likely had permanent (15.6% vs. 11.9%) or persistent AF (18.9% vs. 13.8%), acute coronary syndromes (16.7% vs. 8.9%), vascular disease (40.8% vs. 20.2%), and moderate-to-severe chronic kidney disease (14.6% vs. 9.0%) than those without. Anticoagulant prescription was similar between the two groups. At 2 year follow-up, patients with HF showed a greater risk of all-cause mortality [hazard ratio (HR), 2.06; 95% confidence interval (CI), 1.91–2.21; P < 0.0001], cardiovascular mortality (HR, 2.91; 95% CI, 2.58–3.29; P < 0.0001), acute coronary syndromes (HR, 1.25; 95% CI, 1.02–1.52; P = 0.03), and stroke/systemic embolism (HR, 1.24; 95% CI, 1.07–1.43; P = 0.0044). Major bleeding rate was comparable (adjusted HR, 1.00; 95% CI, 0.84–1.18; P = 0.968). Among patients without HF at baseline, incidence of new HF was low [0.69 (95% CI, 0.63–0.75) per 100 person-years], whereas propensity to develop worsening HF was higher in those with HF [1.62 (95% CI, 1.45–1.80) per 100 person-years].
Conclusions
Patients with AF and HF have a high risk of all-cause and cardiovascular mortality and stroke/systemic embolism and may develop worsening HF.
Introduction
Atrial fibrillation (AF) and heart failure (HF) may coexist and interact with each other in many patients.1 Considerable evidence points to a high risk for incident AF in HF patients, which may confer a negative prognostic impact.2-8 However, most knowledge of HF in AF has been obtained by focusing on HF patients who have developed AF during the course of their index disease through retrospective analyses of historical randomized clinical trials or registries.3, 4, 9 Therefore, there are inherent limitations due to the use of strict inclusion criteria in clinical studies and outdated management practices with respect to both HF (drugs and device implant) and AF [new oral anticoagulant (NOAC)]. Furthermore, knowledge about AF patients who develop HF is limited, as recently reviewed by Ling et al.10
To gain further insights into the impact of HF on AF patients, we leveraged the Global Anticoagulant Registry in the FIELD-Atrial Fibrillation (GARFIELD-AF). This registry is a large, prospective, multinational registry of over 50 000 AF patients followed for at least 2 years, designed to reflect contemporary clinical practice in a global population. As studies regarding the association of AF and HF have typically focused upon patients with a primary diagnosis of HF, the key interests of this study were to evaluate the clinical characteristics and antithrombotic treatment patterns of patients specifically with newly diagnosed AF who present with or without co-morbid HF. Using this large registry, we aim to establish the impact of concomitant HF upon long-term outcomes in AF patients, particularly all-cause mortality, stroke, and bleeding in AF, in a real-world population.
Methods
Study design
The design of the GARFIELD-AF registry was reported previously.11, 12 Briefly, men and women aged over 18 years with AF were diagnosed according to standard local procedures within the previous 6 weeks, with at least one non-prespecified risk factor for stroke as judged by the local investigator, and no valvular diseases were eligible for inclusion.12 Patients were enrolled prospectively and consecutively at 1317 sites in 35 countries. If random site selection did not generate the required number of sites in a given country, the national lead investigator recommended additional sites (18 of 1317 sites). The sites represent different care settings in each participating country.
Ethics
Independent ethics committee and hospital-based institutional review boards approved the study. The registry was conducted in accordance with the principles of the Declaration of Helsinki, local regulatory requirements, and International Conference on Harmonization–Good Pharmacoepidemiological and Clinical Practice guidelines. All patients provided written informed consent to participate, and their confidentiality and anonymity were maintained.
Procedures and outcome measures
Baseline characteristics were collected at inclusion in the registry.11, 12 Components of the CHA2DS2-VASc13, 14 and HAS-BLED15 risk stratification schemes were documented; the latter score was calculated excluding fluctuations in international normalized ratio. Follow-up data on treatments and outcomes were captured on electronic case report forms (eCRFs) at four-monthly intervals up to 12 months. Submitted data were examined for completeness and accuracy by the coordinating centre (Thrombosis Research Institute London, UK). One-fifth of eCRFs (20%) were monitored against source documentation.12 Data for the present analysis were extracted from the study database in November 2018.
Definitions
Heart failure was defined as left ventricular ejection fraction (LVEF) <40%, history of HF, or physician diagnosis of HF at baseline. Severity of HF was categorized according to the New York Heart Association (NYHA) functional classification. Worsening HF was defined as progressive or acute decompensation of previously stable HF or post-enrolment re-stratification into higher-severity NYHA functional classification, as determined by treating physicians. Vascular disease included coronary artery disease with or without history of acute coronary syndromes (ACS) and/or peripheral artery disease. Chronic kidney disease (CKD) was classified according to National Kidney Foundation guidelines16 into moderate-to-severe (Stages 3–5), mild (Stages 1 and 2), or none.
Statistical analysis
Baseline characteristics are described with frequencies (percentage) for categorical variables and medians (interquartile range) for continuous variables. Incidence rates for the first occurrence of outcome events (per 100 person-years) were estimated by Poisson model with number of events as dependent variable and log of time as offset, that is, a covariate with known coefficient of 1. Hazard ratios (HRs) with 95% confidence intervals (CIs) for outcome events in HF vs. no-HF groups were calculated as unadjusted and adjusted results using Cox proportional hazards models. Adjustment factors were as follows: sex; race/ethnicity; age with spline knots at 48, 65, 79, and 88 years; body mass index (BMI); oral anticoagulant therapy at baseline; type of AF; hypertension; history of bleeding; vascular disease; prior stroke/transient ischaemic attack; moderate-to-severe CKD; diabetes; smoking status; and heavy alcohol intake. Five imputed datasets were used to account for missing data when calculating HRs.
Results
Baseline characteristics
A total of 52 072 AF patients (M/F, 55.8/44.2%; no-HF group, n = 40 314; mean age, 69.5 years; HF group, n = 11 758; mean age, 70.1 years) were followed prospectively for 2 years; their baseline characteristics and medications are summarized in Table 1 and Figure 1. Approximately two-third of cases with and without HF had either paroxysmal or unclassified AF. Numerically, the link with HF was stronger for permanent/persistent than paroxysmal AF. In no-HF and HF groups, median CHA2DS2-VASc score (interquartile range) was 3.0 (2.0–4.0) and 4.0 (3.0–5.0), respectively; HAS-BLED score was 1.0 (1.0–2.0) in both groups. Prior stroke/transient ischaemic attack was documented in 11.6% and 10.9%, respectively; history of bleeding in 2.4% and 3.0%; and type 2 diabetes in 20.5% and 23.4%. Background therapy and implementation of guideline-recommended practice were consistent among all patients (Table 1). Most HF patients (76%) were categorized as NYHA class II–III.
| Parameter | No HF (n = 40 314) | HF (n = 11 758) | P-valuea |
|---|---|---|---|
| Male, n (%) | 22 344 (55.4) | 6720 (57.2) | 0.0009 |
| Age (years), median (IQR) | 71.0 (63.0–78.0) | 71.0 (62.0–79.0) | 0.0002 |
| Ethnicity, n (%) | <0.0001 | ||
| Afro-Caribbean | 186 (0.5) | 57 (0.5) | |
| Asian (not Chinese) | 8926 (22.1) | 2633 (22.4) | |
| Caucasian | 24 692 (61.2) | 7333 (62.4) | |
| Chinese | 2138 (5.3) | 605 (5.1) | |
| Hispanic/Latino | 2689 (6.7) | 707 (6.0) | |
| Other | 594 (1.5) | 233 (2.0) | |
| Unknown | 1089 (2.7) | 190 (1.6) | |
| BMI (kg/m2), median (IQR) | 27.0 (24.0–30.0) | 27.0 (24.0–32.0) | <0.0001 |
| SBP (mmHg), mean (SD) | 133.9 (19.6) | 132.0 (20.4) | <0.0001 |
| DBP (mmHg), mean (SD) | 79.6 (12.7) | 80.1 (13.6) | 0.0009 |
| Heart rate (b.p.m.), mean (SD) | 89.8 (26.7) | 92.5 (26.8) | <0.0001 |
| LVEF (%), median (IQR) | 60.0 (55.0–65.0) | 47.0 (35.0–58.0) | <0.0001 |
| Type of AF, n (%) | <0.0001 | ||
| Permanent | 4798 (11.9) | 1837 (15.6) | |
| Persistent | 5549 (13.8) | 2218 (18.9) | |
| Paroxysmal | 11 894 (29.5) | 2421 (20.6) | |
| New | 18 073 (44.8) | 5282 (44.9) | |
| CHA2DS2-VASc score, median (IQR) | 3.0 (2.0–4.0) | 4.0 (3.0–5.0) | <0.0001 |
| HAS-BLED score, median (IQR) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | <0.0001 |
| Medical history, n (%) | |||
| ACS | 3589 (8.9) | 1956 (16.7) | <0.0001 |
| Vascular disease | 8072 (20.2) | 4761 (40.8) | <0.0001 |
| Moderate-to-severe CKD | 3639 (9.0) | 1721 (14.6) | <0.0001 |
| Stroke/TIA | 4680 (11.6) | 1281 (10.9) | 0.0388 |
| Prior bleeding | 965 (2.4) | 353 (3.0) | 0.0002 |
| Diabetes (type 1 or 2) | 8687 (21.5) | 2868 (24.4) | <0.0001 |
| Stroke prophylaxis | <0.0001 | ||
| VKA | 12 061 (30.3) | 3318 (28.9) | |
| VKA + AP | 3308 (8.3) | 1519 (13.2) | |
| FXA | 6851 (17.2) | 1732 (15.1) | |
| FXA + AP | 1400 (3.5) | 496 (4.3) | |
| DTI | 2410 (6.0) | 612 (5.3) | |
| DTI + AP | 465 (1.2) | 162 (1.4) | |
| AP alone | 8116 (20.4) | 2655 (23.1) | |
| None | 5241 (13.2) | 1000 (8.7) | |
| Cardiac treatmentb | |||
| Angiotensin-converting enzyme inhibitor/angiotensin receptor blocker | 13 433 (50.6) | 5144 (65.4) | <0.0001 |
| Beta-blocker | 14 680 (55.3) | 5168 (65.7) | <0.0001 |
| Diuretic agent | 6864 (25.9) | 4382 (55.7) | <0.0001 |
| Digoxin/digitalis | 1767 (6.7) | 1381 (17.6) | <0.0001 |
| Aldosterone antagonist | 849 (3.2) | 1463 (18.6) | <0.0001 |
- ACS, acute coronary syndromes; AF, atrial fibrillation; AP, antiplatelet; BMI, body mass index; CKD, chronic kidney disease; DTI, direct thrombin inhibitor; FXA, factor Xa antagonist; HF, heart failure; IQR, interquartile range; LVEF, left ventricular ejection fraction; SBP/DBP, systolic/diastolic blood pressure; SD, standard deviation; TIA, transient ischaemic attack; VKA, vitamin K antagonist.
- a For categorical variables, P-values were obtained from a χ2 test or Fisher's exact test, as appropriate. For continuous variables, P-values were obtained from a t-test or a Wilcoxon–Mann–Whitney test, as appropriate.
- b Measured in GARFIELD-AF cohorts 3–5 (no HF, n = 26 526; HF, n = 7867).
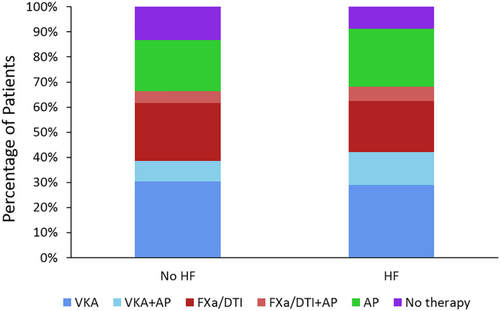
Heart failure was defined according to LVEF, current HF, or a history of HF. Of patients with HF, 73.4% had recorded LVEF measurements, compared with 54.1% of patients without HF. To assess the potential reporting bias introduced by this difference, patients were also checked according to the presence of a new HF diagnosis at baseline or a history of HF only. Under these criteria, 10 347 patients had HF, of which 70% had an LVEF measurement, whereas the remaining no-HF patients had LVEF measure in 55.6% of cases. Although AF patients with and without concomitant HF were comparable in terms of sex, age, ethnicity, BMI, blood pressure, heart rate, and medical history, some of their clinical characteristics were significantly different: HF patients more frequently had history of ACS (16.7% vs. 8.9%), vascular disease (40.8% vs. 20.2%), and moderate-to-severe CKD (14.6% vs. 9.0%; all P < 0.001). Moreover, patients with HF were more likely to have permanent AF (15.6% vs. 11.9%) or persistent AF (18.9% vs. 13.8%).
Antithrombotic and heart failure therapies
A similar proportion of HF and no-HF patients received anticoagulation (68.2% vs. 66.5%; Figure 1). HF patients less frequently received no treatment (8.7% vs. 13.2%) and were more likely prescribed antiplatelet therapy, either alone (23.1% vs. 20.4%) or in combination with anticoagulant (18.9% vs. 13.0%). On the other hand, higher proportions of HF than no-HF patients received cardiovascular agents across all major drug classes (Table 1).
Clinical characteristics and treatment provided across the New York Heart Association functional categorizations
Clinical characteristics of patients stratified by the presence and severity of HF (NYHA I–II or III–IV) are displayed in Table 2. Within the HF group, most clinical characteristics were not markedly different across patients stratified by NYHA functional classification I–II or III–IV, although ACS, CKD, and vascular disease were more frequently observed in those with NYHA functional class III–IV.
| Parameter | No HF (n = 40 314) | NYHA class I–II (n = 6456) | NYHA class III–IV (n = 3057) | P-valuea |
|---|---|---|---|---|
| Sex male, n (%) | 22 344 (55.4) | 3539 (54.8) | 1682 (55.0) | 0.6259 |
| Age (years), median (IQR) | 71.0 (63.0–78.0) | 71.0 (62.0–79.0) | 72.0 (63.0–80.0) | <0.0001 |
| LVEF (%), median (IQR) | 60.0 (55.0–65.0) | 45.0 (43.0–61.0) | 45.0 (35.0–55.0) | <0.0001 |
| Medical history, n (%) | ||||
| Hypertension | 30 519 (75.9) | 5205 (80.7) | 2366 (77.5) | <0.0001 |
| ACS | 3589 (8.9) | 934 (14.6) | 639 (21.0) | <0.0001 |
| Vascular disease | 8072 (20.2) | 2543 (39.6) | 1478 (48.7) | <0.0001 |
| Moderate-to-severe CKD | 3639 (9.0) | 831 (12.9) | 634 (20.7) | <0.0001 |
| CHA2DS2-VASc, median (IQR) | 3.0 (2.0–4.0) | 4.0 (3.0–5.0) | 4.0 (3.0–5.0) | <0.0001 |
| HAS-BLED, median (IQR) | 1.0 (1.0–2.0) | 1.0 (1.0–2.0) | 2.0 (1.0–2.0) | <0.0001 |
- ACS, acute coronary syndromes; CKD, chronic kidney disease; HF, heart failure; IQR, interquartile range; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
- a For categorical variables, P-values were obtained from a χ2 test or Fisher's exact test, as appropriate. For continuous variables, P-values were obtained from a one-way ANOVA or a Kruskal–Wallis test, as appropriate.
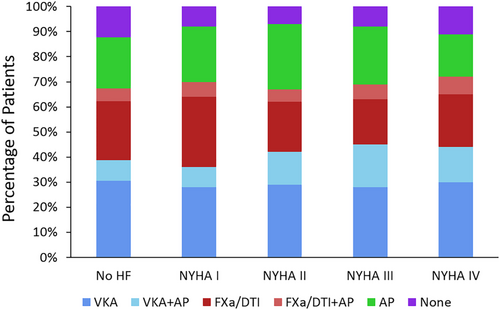
Initial antithrombotic treatment provided to AF patients without HF and those with HF stratified according to NYHA functional class I–IV is shown in Figure 2. Although the number of patients categorized as NYHA functional class IV is rather small (n = 418), these individuals were clearly undertreated; they were less likely to receive anticoagulation and more likely to receive antiplatelet agents only or no antithrombotic treatment than AF patients with less severe or no HF.
Outcomes at 2 year follow-up
Among AF patients without HF at baseline, de novo HF developed in 517 individuals (1.3%) during 2 year follow-up, corresponding to a rate of 0.69 (95% CI, 0.63–0.75) per 100 person-years. However, in the HF cohort, worsening HF was seen in 334 individuals (2.8%), corresponding to a rate of 1.62 (95% CI, 1.45–1.80) per 100 person-years.
In HF vs. no-HF patients, there were significantly (all P < 0.0001) higher incidence rates (95% CI; per 100 person-years) of all-cause death [6.93 (6.59–7.30) vs. 2.95 (2.83–3.08)], cardiovascular death [3.10 (2.87–3.34) vs. 0.87 (0.81–0.94)], non-cardiovascular death [2.08 (1.89–2.28) vs. 1.29 (1.21–1.37)], ACS [0.92 (0.80–1.06) vs. 0.54 (0.49–0.60)], and stroke/systemic embolism (SE) [1.43 (1.28–1.60) vs. 1.02 (0.95–1.09)]. Rates of major bleeding were similar in the two groups [1.03 (0.90–1.18) vs. 0.96 (0.89–1.03); P = 0.4127].
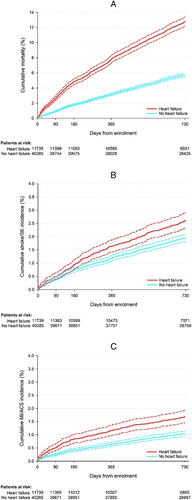
Kaplan–Meier curves depicting the probability of avoiding all-cause death, stroke/SE, and ACS in the HF and no-HF groups over 2 years are shown in Figure 3. In particular, survival curves for all-cause mortality in the two groups immediately began to diverge, with a significantly higher risk of this event noted in HF than no-HF patients throughout the study. Risk of stroke/SE and ACS was also higher in the HF group vs. the no-HF group, albeit delayed, reaching statistical significance after approximately 3 and 6 months, respectively.
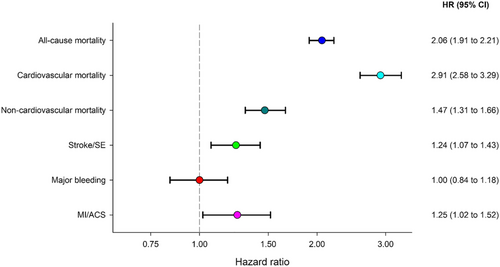
Adjusted HRs for all outcome parameters are presented in Figure 4. Notably, risk of death by any cause was twice, and cardiovascular death thrice, as likely in HF vs. no-HF patients over 2 years.
Two-year outcomes in patients stratified by the presence of HF and NYHA functional class I–IV are shown in Figure 5. A clear correlation was noted between increasingly severe HF and progressively higher incidence rates of adverse outcomes. Especially, the rates of all-cause death, cardiovascular death, and non-cardiovascular death rose exponentially and were almost twice as high in each successive NYHA functional class from I to IV. In patients with no HF who died, cause of death was cardiovascular related in less than one-third of cases and non-cardiovascular in nearly half of cases (others unknown), whereas in patients with NYHA functional class III–IV who died, this trend was reversed (cardiovascular mortality, 47.8%; non-cardiovascular mortality, 27.7%).
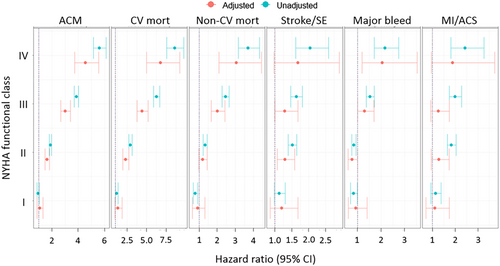
Patients with no HF at baseline were at a low risk of going on to develop HF [incidence rate, 0.69 (95% CI, 0.63–0.75)]; in those with HF, risk of worsening HF increased with higher NYHA functional class from I [rate of HF worsening, 1.16 (95% CI, 0.84–1.58)] to IV [HF worsening, 2.15 (95% CI, 1.27–3.63)].
New heart failure
Of the 40 314 patients with ‘no HF’, 517 (1.3%) developed new HF during the study compared with 39 797 (98.7%) who did not. Patients with new HF were less often male and slightly older than those without (49.5% and 75.0 years vs. 55.5% and 71.0 years). Paroxysmal and persistent AF were more common in patients with new HF than in those without (29.6%; 13.8% vs. 20.9%; 16.1%, respectively), whereas permanent AF was less common in new HF patients (16.1% vs. 11.9%, respectively). Of patients with new HF, 79.6% were Caucasian and 12.2% were Asian, compared with 62.7% and 28.4% of those without new HF. Clinical characteristics differed between these two groups. Patients who developed new HF more often had ACS, vascular disease, diabetes, and moderate-to-severe CKD (13.2%, 28.2%, 26.1%, and 20.7%, respectively), compared with those who did not develop HF (8.9%, 20.1%, 21.5%, and 10.1%, respectively) (Table 3).
| Parameter | No new heart failure (n = 39 797) | New heart failure (n = 517) | P-valuea |
|---|---|---|---|
| Male, n (%) | 22 088 (55.5) | 256 (49.5) | 0.0065 |
| Age (years), median (IQR) | 71.0 (63.0–78.0) | 75.0 (68.0–80.0) | <0.0001 |
| Ethnicity, n (%) | <0.0001 | ||
| Caucasian | 24 301 (62.7) | 391 (79.6) | |
| Hispanic/Latino | 2660 (6.9) | 29 (5.9) | |
| Asian | 11 004 (28.4) | 60 (12.2) | |
| Afro-Caribbean/mixed/other | 769 (2.0) | 11 (2.2) | |
| BMI (kg/m2), median (IQR) | 26.8 (23.9; 30.4) | 28.4 (24.6; 32.9) | <0.0001 |
| SBP (mmHg), mean (SD) | 132.0 (120.0; 145.0) | 135.0 (120.0; 145.0) | 0.4284 |
| DBP (mmHg), mean (SD) | 80.0 (70.0; 88.0) | 80.0 (70.0; 90.0) | 0.8864 |
| Heart rate (b.p.m.), mean (SD) | 83.0 (70.0; 104.0) | 88.0 (72.0; 112.0) | 0.0024 |
| Type of AF, n (%) | <0.0001 | ||
| Permanent | 4715 (11.9) | 83 (16.1) | |
| Persistent | 5492 (13.8) | 57 (11.0) | |
| Paroxysmal | 11 786 (29.6) | 108 (20.9) | |
| New | 17 804 (44.7) | 269 (52.0) | |
| CHA2DS2-VASc score, median (IQR) | 3.0 (2.0–4.0) | 4.0 (3.0–4.0) | <0.0001 |
| HAS-BLED score, median (IQR) | 1.0 (1.0–2.0) | 2.0 (1.0–2.0) | <0.0001 |
| Medical history, n (%) | |||
| ACS | 3521 (8.9) | 68 (13.2) | 0.0007 |
| Vascular disease | 7926 (20.1) | 146 (28.2) | <0.0001 |
| Carotid occlusive disease | 1159 (3.0) | 18 (3.5) | 0.4537 |
| VTE | 1003 (2.5) | 19 (3.7) | 0.0966 |
| Stroke/TIA/SE | 4520 (11.4) | 67 (13.1) | 0.2525 |
| Prior bleeding | 942 (2.4) | 23 (4.5) | 0.0021 |
| Hypertension | 30 107 (75.9) | 412 (79.8) | 0.0361 |
| Hypercholesterolaemia | 15 937 (41.3) | 200 (39.5) | 0.4202 |
| Diabetes | 8552 (21.5) | 135 (26.1) | 0.0111 |
| Cirrhosis | 197 (0.5) | 5 (1.0) | 0.1375 |
| Moderate-to-severe CKD | 3547 (10.1) | 92 (20.7) | <0.0001 |
| Dementia | 488 (1.2) | 5 (1.2) | 0.8938 |
| Treatment | <0.0001 | ||
| VKA ± AP | 11 030 (28.0) | 96 (19.0) | |
| NOAC ± AP | 15 163 (38.5) | 206 (40.7) | |
| AP alone | 7987 (20.3) | 129 (25.5) | |
| None | 5166 (13.1) | 75 (14.8) |
- ACS, acute coronary syndromes; AF, atrial fibrillation; AP, antiplatelet; BMI, body mass index; CKD, chronic kidney disease; IQR, interquartile range; NOAC, new oral anticoagulant; SBP/DBP, systolic/diastolic blood pressure; SD, standard deviation; SE, systemic embolism; TIA, transient ischaemic attack; VKA, vitamin K antagonist; VTE, venous thromboembolism.
- a For categorical variables, P-values were obtained from a χ2 test or Fisher's exact test, as appropriate. For continuous variables, P-values were obtained from a t-test or a Wilcoxon–Mann–Whitney test, as appropriate.
Of patients with no HF, those who developed new HF during follow-up were less likely to receive vitamin K antagonists at baseline than those who did not develop new HF (19.0% vs. 28.0%). Conversely, those who developed new HF were slightly more likely to have received AP therapy alone (25.5% vs. 20.3%). NOAC usage was comparable (Table 3).
Discussion
This analysis of GARFIELD-AF registry on over 50 000 patients provides useful insights into the complex and often challenging condition of de novo AF with co-morbid HF at baseline. GARFIELD-AF is the largest registry to date investigating the impact of concurrent HF in AF patients, providing global observational data in a real-world clinical setting. Several important findings emerged. First, the present data extend previous observations to a more contemporary, multi-country, and multi-setting practice, showing that approximately one quarter of patients with newly diagnosed AF present with HF. Second, this study demonstrates that the rate of anticoagulation was similar, or higher, in AF patients with concurrent HF compared with those without HF and that prescription rates of NOACs were comparable. Third, although most demographics and baseline characteristics were similar in the two groups, classical risk factors were different between HF and no-HF patients. Those with HF had more ACS and CKD and were twice as likely to have history of vascular disease. Fourth, AF patients with HF experienced substantially higher rates of adverse outcomes than those without HF. It is noteworthy that although HF patients received more cardiovascular drugs than no-HF individuals, their prescription rates of beta-blockers and renin-angiotensin-aldosterone system inhibitors were suboptimal, and this may have contributed to worse outcomes. Finally, at odds with common beliefs, among patients with AF and no HF at baseline, incidence of new HF over 2 year follow-up was low. Worsening HF, on the other hand, was more often observed in AF patients in higher NYHA class.
Individually, AF and HF are the two biggest epidemics in cardiology.17 In the USA, AF affects approximately 2% of the population aged under 65 years and 9% of those aged over 65.10, 18 The prevalence rates of these two conditions are expected to increase in future due to aging populations and higher survival rates among people with cardiovascular diseases as better therapies emerge. Importantly, AF and HF are linked aetiologically: many AF patients also have HF, and the likelihood of developing AF increases with severity of HF.18 The Euro Heart Study on AF9 estimated that one-third of AF patients also have HF, a slightly higher prevalence than that observed in the present contemporary patient cohort. In distinction to the important role of HF in AF, a range of further critical risk factors are independently responsible for triggering AF in the absence of HF. Non-exhaustively, examples include vascular disease such as hypertension, atherosclerosis, the presence of pulmonary vein foci, and myocardial infarction. Diabetes, renal dysfunction, and demographic characteristics such as BMI, sex, and age are also closely linked to an increasing rate of incident AF.
Risk factors for AF and HF greatly overlap,19 and AF-induced cardiomyopathy can lead to decline of left ventricular function leading to HF.20 Given these common pathophysiological aspects, it has been postulated ‘AF begets HF, and HF begets AF’.21 Specific pathophysiological changes by which AF and HF contribute to their co-development are complex and not fully understood.19 Cardiac remodelling, activation of neurohormonal compensatory mechanisms, and impairment of left ventricular function have been posited as possible reasons for how AF and HF can cause and exacerbate each other. In our registry, most patients with newly diagnosed AF and HF had preserved LVEF; increased heart rate and loss of atrial contraction in AF may have contributed to development of HF. It is clear, however, that patients with concurrent AF and HF have worse prognosis than those with either condition alone, as confirmed by the present analysis and in a previous report on GARFIELD-AF patients with HF associated with ischaemic cardiomyopathy.22
Both AF and HF are major causes of stroke.23, 24 Indeed, the widely accepted AF-related stroke risk index CHA2DS2-VASc includes 1 point for the presence of HF.13, 14 Subgroup analyses of the landmark trials of NOACs vs. warfarin revealed that these drugs work equally well at preventing stroke/SE in AF patients with and without HF, with similar rates of bleeding.25-28 In the present study, patients with AF and co-morbid HF had an approximately 30% higher risk of stroke/SE compared with no-HF patients, a statistically significant difference (P < 0.001), despite their receiving equivalent anticoagulation regimens. This finding suggests that even in contemporary medicine and after the introduction of NOACs, HF in itself remains a major risk factor for stroke and that patients with concomitant AF and HF should be closely monitored to reduce risk and optimize treatment.
In this analysis, new HF arising during follow-up was seen in only 517 of the 40 314 AF patients (1.3%). This apparently contrasts with reports from the Framingham study, in which 16% of patients developed incident HF after AF diagnosis. It should be noted, however, that the Framingham study followed only 1737 AF patients with no HF at baseline, as opposed to the over 40 000 in GARFIELD-AF. More importantly, whereas GARFIELD-AF patients were followed over 2 years, the 277 cases of incident HF in the Framingham study were accrued over a much longer period of time: between 1980 and 2012. Therefore, incidence rate per person-year was actually quite small. In support of the present findings, similar results were reported by the US-based ORBIT-AF registry of AF patients, in whom de novo HF developed in only a few per cent over 2 years of observation.29 Similar to the ORBIT-AF registry, new HF was associated with a slightly older cohort of patients and was more frequently linked to adverse clinical characteristics, including vascular disease and moderate-to-severe CKD. Age, vascular disease, and renal dysfunction are important independent risk factors for HF, possibly indicating the cause of incident HF in these 517 patients.
The present study also clearly shows that, for patients who already have HF, newly diagnosed AF is indeed an influencing factor associated with clinical deterioration of HF. These findings confirm and extend a recent report from a retrospective analysis of PARADIGM-HF and ATMOSPHERE databases, which shows that new-onset AF was associated with a significantly increased risk of HF rehospitalization.30
This study has limitations. The definition of HF adopted herein (i.e. LVEF <40% or history of HF as adjudged by signs and symptoms) was conceived when the GARFIELD-AF study was designed. However, it tallies well with those suggested by international medical societies; indeed, both the 2013 American College of Cardiology Foundation/American Heart Association guidelines31 (most recently updated 201732) and the 2016 European Society of Cardiology guidelines33 broadly classify HF patients as having preserved or reduced ejection fraction with a cut-off value for the latter ≤40%, on the basis that this was selection criterion used in clinical trials demonstrating efficacy of drug therapies. Importantly, the proportion of patients with a recorded LVEF measurement was notably lower in those without concurrent HF. It is possible that this may introduce a potential reporting bias, whereby those without HF may have been incorrectly assigned to this group simply due to the lack of LVEF measurement. However, LVEF was not the only criterion to allocate patients to the ‘no-HF’ group, as we also recorded history of HF, or current diagnosis of HF: reassessing the assignment of patients into each group according to a history of HF or current HF only shows that a similar number of patients were characterized with HF. Additionally, the proportion of patients in the HF group who had measured LVEF remained substantially higher than in the no-HF patient group. It is therefore likely that the disparity in recorded LVEF measurements may have arisen as patients who presented with no history nor evidence for HF were not considered for measurement of LVEF. A further limitation of this study is that ventricular filling pressures were not collected for this cohort, and thus, we are unable to establish the role it may have played in the prognosis of GARFIELD-AF patients.
Another limitation is outcomes in HF patients with LVEF <40% and ≥40%, who were not analysed separately. This study stratified patients with HF by NYHA functional class I–IV. However, their European Heart Rhythm Association score of AF was not measured; hence, disabilities attributable to these two disease conditions were not differentiated. We were therefore unable to analyse this cohort according to a combination of NYHA and European Heart Rhythm Association scoring. As in all registries, patients were not randomly enrolled to treatments. Also, adverse event recognition was left at investigators' judgement, and not centrally adjudicated—although to minimize errors, a sizable proportion (20%) of eCRFs were audited. Finally, it is possible that, in addition to the extensive collection of ancillary data, other variables that were not measured could have further refined interpretation of the findings.
Conclusions
The present analysis of a very large, contemporary, multi-country registry shows that patients with HF and newly diagnosed AF are treated similarly to those with AF and no HF in terms of anticoagulation; yet they remain a higher-risk group with respect to stroke/SE, all-cause death, and cardiovascular death than no-HF patients. AF confers a relatively low risk of developing HF or worsening HF already present.
Acknowledgements
The authors thank the physicians, caregivers, and patients who participated in this research. Alex Kahney and Rebecca Watkin of Thrombosis Research Institute London, UK, provided editorial assistance.
Conflict of interest
GA has received personal fees from Merck, Menarini, Angelini, Novartis and Behring; AJ Camm has received institutional grants and personal fees from Bayer, Boehringer Ingelheim, Pfizer/BMS and Daiichi Sankyo. JPB has received personal fee from thrombosis research institute; LGM has received grants and personal fees from Bayer AG during the conduct of the study, and grants from Boehringer Ingelheim, grants and personal fees from Pfizer and personal fees from Daiichi Sankyo and has received support by Italian Ministry of Health Ricerca Corrente - IRCCS MultiMedica outside the submitted work; AKK has received grants and personal fees from Bayer AG and Sanofi; personal fees from Bayer AF, Janssen Pharma, Pfizer, Sanofi, Verseon and Anthos Therapeutics. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose. [Correction added on 25 January 2021, after first online publication: The Conflict of Interest has been updated in this version.]
Funding
This work was supported by an unrestricted grant awarded by Bayer Pharma AG (Berlin, Germany) to the Thrombosis Research Institute (London, UK).




