Impact of a volume challenge on haemodynamics and prognosis in patients with severe aortic stenosis
Abstract
Aims
A volume challenge can unmask pulmonary hypertension (PH) and its mechanism. We evaluated the impact of a volume challenge on mean pulmonary artery pressure (mPAP) and mean pulmonary artery wedge pressure (mPAWP) and its prognostic implications in patients with severe aortic stenosis (AS) undergoing aortic valve replacement (AVR).
Methods and results
In 285 patients with severe AS (indexed aortic valve area 0.41 ± 0.13 cm2/m2), mPAP and mPAWP were measured before and after administration of 150 ± 58 mL of low-osmolal or iso-osmolal contrast. Following contrast, mPAP and mPAWP rose from 25 ± 10 and 16 ± 8 mmHg by 5 ± 4 and 4 ± 4 mmHg to 30 ± 11 and 20 ± 8 mmHg. There were 112 (39%) patients with pre-contrast PH and 70 (40% of those without pre-contrast PH) patients with post-contrast PH only. Post-contrast PH patients were intermediate between pre-contrast PH and no PH in terms of AS severity, cardiac dysfunction, and haemodynamics. After a median follow-up of 43 months post-AVR, pre-contrast PH patients had numerically the highest mortality driven by those with pre-contrast combined pre-capillary and post-capillary PH (n = 35), while post-contrast changes in mPAP and mPAWP were not related to mortality. Patients with any post-contrast mPAWP > 18 mmHg had significantly higher mortality than those with post-contrast mPAWP ≤ 18 mmHg,
Conclusions
In severe AS, a contrast challenge leads to post-contrast PH in ~40% of patients without pre-contrast PH. However, post-contrast haemodynamic changes do not adversely affect outcomes in patients undergoing AVR. Post-contrast PH represents an intermediate stage of ‘cardiac damage’, which may be attenuated or reversible after AVR.
Introduction
In patients with severe aortic stenosis (AS), pulmonary hypertension (PH) is common and a risk marker.1 Invasive haemodynamic studies have revealed that the presence and type of PH predict mortality in patients with AS undergoing aortic valve replacement (AVR).2-4 However, some aspects of these findings are controversial: in two studies, combined pre-capillary and post-capillary PH (CpcPH) was associated with the worst outcome,2, 4 while in another study, patients with isolated post-capillary PH (IpcPH) and CpcPH had a similar prognosis.3 These discrepancies may be explained by differences in the populations studied as well as differences in the definitions of left atrial pressure elevation [pulmonary artery wedge pressure (PAWP)2 vs. left ventricular end-diastolic pressure (LVEDP)4] and pulmonary vascular involvement [pulmonary vascular resistance (PVR)2 vs. diastolic pressure gradient3, 4] but are potentially also related to differences in the hydration status at the time of the haemodynamic assessment. Fasting prior to cardiac catheterization may result in underestimation of the ‘true’ filling pressures and thereby haemodynamic misclassification. Therefore, provocative testing (i.e. fluid loading or exercise) has been proposed to unmask PH and its mechanisms in patients with a predisposition for post-capillary PH but a borderline haemodynamic constellation.5-8 Fluid loading has been studied in different populations,5, 7, 9-12 but its role in patients with AS is unknown. In the present study, we evaluated the impact of a volume challenge on haemodynamics and its prognostic implications in patients with severe AS prior to AVR. For the purpose of a volume challenge, we used contrast given its proven effect on filling pressures in patients with heart failure13 and the fact that coronary angiography is routinely performed prior to AVR.14
Methods
Study population
This is a retrospective analysis of right heart catheterization data before and after administration of low-osmolal/iso-osmolal contrast in patients with severe AS in a single centre between January 2011 and January 2016 planned for surgical or transcatheter AVR.2 We included all 285 consecutive patients with complete pre-contrast and post-contrast data (Figure S1). The study was approved by the local ethics committee. A waiver of consent was granted. We have previously reported on other aspects of the haemodynamics in this population.2 However, none of the volume challenge data has been published so far.
Cardiac catheterization
Procedures were generally (>95%) performed between 8 and 10 a.m. in the fasting state (fasting overnight, i.e. for ~12 h) and after withholding loop diuretics and renin–angiotensin system inhibitors. Patients underwent coronary angiography using 5 or 6 French catheters via the femoral or radial artery and right heart catheterization using 6 French Swan–Ganz catheters via femoral or brachial access. After arterial and venous was obtained, pre-contrast haemodynamics were measured. Pulmonary artery pressure (PAP) and PAWP were measured. Measurements were obtained at end-expiration, and the mean PAWP (mPAWP) was calculated over the entire cardiac cycle including V waves. Cardiac output was assessed by the indirect Fick method based on blood gases, which were collected in duplicate from the arterial access and pulmonary artery at the time of pre-contrast right heart catheterization. The transpulmonary gradient was calculated as mean PAP (mPAP) − mPAWP. PVR was calculated as transpulmonary gradient/cardiac output. If the aortic valve was crossed, the LVEDP was recorded. Second, coronary angiography with or without ventriculography and/or aortography was performed using low-osmolal or iso-osmolal contrast. Third, measurement of mPAWP and mPAP was repeated immediately after completion of all examinations requiring contrast (post-contrast measurements). Care was taken to always ensure appropriate zeroing before measurements were obtained. All pressure readings were double-checked by the operator by manual review of the pressure tracings before they were entered into the report.
Haemodynamic definitions
In accordance with the 2015 guidelines of the European Society of Cardiology (ESC)/European Respiratory Society (ERS),15 PH was defined as mPAP ≥ 25 mmHg; IpcPH as mPAP ≥ 25 mmHg, mPAWP > 15 mmHg, and PVR ≤ 3 Wood units; CpcPH as mPAP ≥ 25 mmHg, mPAWP > 15 mmHg, and PVR > 3 Wood units; and pre-capillary PH as mPAP ≥ 25 mmHg and mPAWP ≤ 15 mmHg. After contrast administration, the same PH definition (≥25 mmHg) was used, and patients were classified as post-capillary (mPAWP > 15 mmHg) or pre-capillary (mPAWP ≤ 15 mmHg) PH. A differentiation between IpcPH and CpcPH was formally not possible because measurement of cardiac output was not repeated after contrast administration and thus PVR could not be calculated. However, we performed an exploratory analysis using the pre-contrast PVR in combination with post-contrast mPAP and mPAWP to classify patients as IpcPH or CpcPH post-contrast because the PVR unlike PAP and PAWP has been shown to remain unchanged after contrast administration.13 For the main analysis, patients were classified as pre-contrast PH (any PH before contrast), post-contrast PH (any PH only after contrast), and no PH (PH neither before nor after contrast). In addition, we categorized patients according to a post-contrast mPAWP > 18 mmHg vs. ≤18 mmHg because an mPAWP > 18 mmHg after fluid adminstration has been proposed as a clearly abnormal value following a standard fluid challenge.6, 8 In another exploratory analysis, patients were classified according to the proposal of the 2018 6th World Symposium on PH8: IpcPH was defined as mPAP > 20 mmHg, mPAWP > 15 mmHg, and PVR < 3 Wood units; CpcPH as mPAP > 20 mmHg, mPAWP > 15 mmHg, and PVR ≥ 3 Wood units; and pre-capillary PH as mPAP > 20 mmHg, mPAWP ≤ 15 mmHg, and PVR ≥ 3 Wood units.
Follow-up
All patients underwent surgical (72%) or transcatheter (28%) AVR. In patients undergoing surgical AVR, the following additional procedures were performed: surgery of the ascending aorta (n = 19), mitral valve surgery (n = 16), tricuspid valve surgery (n = 2), maze procedure (n = 10), and aortocoronary bypass (n = 72). In 89 patients, one additional procedure was performed; in 14 patients, two procedures were performed; and in one patient, there were three additional procedures. Information on long-term follow-up was obtained from patients, general practitioners, and hospital or practice cardiologists. The median clinical post-AVR follow-up was 43 (31–61) months. The endpoint was all-cause mortality.
Statistical analysis
Categorical data are presented as numbers and percentages, and continuous data are given as mean ± standard deviation or median (inter-quartile range). Clinical characteristics and echocardiographic and haemodynamic data in patients with pre-contrast PH, post-contrast PH, and no PH were compared using χ2 tests, analysis of variance, or Kruskal–Wallis tests. An analogue comparison was performed between patients with different pre-contrast constellations: pre-capillary PH vs. IpcPH vs. CpcPH vs. no PH. Survival of patients with different pre-contrast constellations; patients with pre-contrast PH, post-contrast PH, and no PH; and patients in different haemodynamic categories were compared using Kaplan–Meier plots and log-rank tests. Cox regression (hazard ratios) was used to describe the association between variables of interest and mortality. A P-value < 0.05 was considered statistically significant. Analyses were performed using SPSS statistical package version 20.0 (SPSS Inc., Chicago, IL, USA).
Results
Study population
The mean age of the 285 patients was 74 ± 9 years, and 60% were male. The mean indexed aortic valve area was 0.41 ± 0.13 cm2/m2, and the mean left ventricular ejection fraction (LVEF) was 58 ± 12%.
Pre-contrast haemodynamics
The pre-contrast mPAP, mPAWP, transpulmonary gradient, and PVR in the entire population were 25 ± 10, 16 ± 8 mmHg, 9 ± 5 mmHg, and 2.1 ± 1.4 Wood units. There were 112 (39%) patients with PH: 35 with CpcPH, 63 with IpcPH, and 14 with pre-capillary PH. Clinical characteristics, echocardiographic findings, and invasive haemodynamics of patients with CpcPH, IpcPH, and pre-capillary PH, and no PH are shown in Tables S1 and S2.
Effect of contrast and post-contrast haemodynamics
Patients received 150 ± 58 mL (1.98 ± 0.82 mL/kg body weight) of contrast. In the entire population, there was a post-contrast rise in mPAP and mPAWP by 5 ± 4 and 4 ± 4 mmHg to 30 ± 11 and 20 ± 8 mmHg, respectively, resulting in PH in 70 additional patients [70/285 (25%) of the entire population and 70/173 (40%) of those without pre-contrast PH]: 61 with post-capillary and nine with pre-capillary PH, summarized as post-contrast PH. While there were 29 (10%) patients with a pre-contrast systolic PAP > 60 mmHg, there were 41 (14%) patients with a post-contrast systolic PAP > 60 mmHg.
Clinical characteristics, echocardiographic findings, and invasive haemodynamics of patients with pre-contrast PH, post-contrast PH, and those without any PH are shown in Tables 1 and 2. Details about contrast administration and haemodynamics before and after contrast administration and changes respectively in these three groups are compared in Table 3. Contrast volume was the highest in patients with post-contrast PH. However, the difference in the absolute or body weight-indexed contrast volume between patients with post-contrast PH and those without PH was not statistically significant.
| Pre-contrast PH (n = 112) | Post-contrast PH (n = 70) | No PH (n = 103) | P-value | |
|---|---|---|---|---|
| Age (years) | 76 ± 9 | 76 ± 10 | 72 ± 10**,*** | 0.004 |
| Gender (male) | 66 (59%) | 40 (57%) | 64 (62%) | 0.79 |
| Body mass index (kg/m2) | 29.1 ± 5.16 | 27.4 ± 4.7 | 27.3 ± 4.7** | 0.02 |
| eGFR (mL/min/1.73m2) | 72 ± 31 | 72 ± 31 | 75 ± 24 | 0.65 |
| Haemoglobin (g/L) | 132 ± 19 | 135 ± 16 | 139 ± 15** | 0.006 |
| Diabetes | 26 (23%) | 10 (14%) | 19 (18%) | 0.32 |
| Stroke | 7 (6%) | 1 (1%) | 7(7%) | 0.25 |
| Chronic obstructive lung disease | 18 (16%) | 8 (11%) | 11 (11%) | 0.45 |
| FEV1 (% predicted) | 80 ± 21 | 90 ± 20* | 92 ± 21** | <0.001 |
| Heart rhythm | —** | 0.009 | ||
| Sinus rhythm | 90 (80%) | 66 (95%) | 90 (87%) | |
| Atrial fibrillation | 19 (17%) | 3 (4%) | 6 (6%) | |
| Pacemaker | 3 (3%) | 1 (1%) | 7 (7%) | |
| Heart rate (b.p.m.) | 74 ± 14 | 69 ± 11 | 66 ± 11 | <0.001 |
| Medication | ||||
| Oral anticoagulation | 27 (24%) | 8 (11%) | 12 (12%)** | 0.02 |
| Aspirin | 66 (59%) | 49 (70%) | 63 (61%) | 0.31 |
| Loop diuretics | 75 (67%) | 29 (41%)* | 33 (32%)** | <0.001 |
| Beta-blocker | 58 (52%) | 26 (37%) | 47 (46%) | 0.16 |
| ACEI/ARB | 66 (59%) | 39 (56%) | 57 (55%) | 0.85 |
| Digoxin | 10 (9%) | 3 (4%) | 3 (3%) | 0.14 |
| Spironolactone | 7 (6%) | 3 (4%) | 4 (3%) | 0.70 |
| B-type natriuretic peptide (ng/L) | 386 (180–1003) | 185 (86–306)* | 89 (44–166)**,*** | <0.001 |
| Symptoms | ||||
| Dyspnoea NYHA class | —** | <0.001 | ||
| I | 12 (11%) | 9 (13%) | 34 (33%) | |
| II | 51 (46%) | 45 (64%) | 53 (51%) | |
| III | 41 (36%) | 12 (17%) | 14 (14%) | |
| IV | 8 (7%) | 4 (6%) | 2 (2%) | |
| Logistic Euroscore II | 3.7 (1.8–5.5) | 2.8 (1.6–4.0)* | 2.0 (1.2–3.0)**,*** | <0.001 |
| Mode of AVR | —**,*** | <0.001 | ||
| Surgical AVR | 63 (56%) | 51 (73%) | 90 (87%) | |
| Transcatheter AVR | 49 (44%) | 19 (27%) | 13 (13%) |
- Data are given as numbers and percentages, mean ± standard deviation, or median (inter-quartile range).
- ACEI/ARB, angiotensin converting enzyme inhibitor/angiotensin receptor blocker; AVR, aortic valve replacement; eGFR, estimated glomerular filtration rate; FEV1, forced expiratory volume within the first second; NYHA, New York Heart Association; PH, pulmonary hypertension.
- * Post hoc tests: P < 0.05 for pre-contrast vs. post-contrast PH.
- ** Post hoc tests: P < 0.05 for pre-contrast PH vs. no PH.
- *** Post hoc tests: P < 0.05 for post-contrast vs. no PH.
| Pre-contrast PH (n = 112) | Post-contrast PH (n = 70) | No PH (n = 103) | P-value | |
|---|---|---|---|---|
| Echocardiography | ||||
| Left ventricular end-diastolic diameter (mm) | 46 ± 8 | 48 ± 7 | 46 ± 8 | 0.17 |
| Left ventricular ejection fraction (%) | 53 ± 13 | 59 ± 10* | 62 ± 10** | <0.001 |
| E/e′ | 19.0 ± 10.2 | 16.8 ± 8.2 | 15.2 ± 6.7 | 0.09 |
| Left atrial area (cm2) | 29 ± 8 | 24 ± 5* | 21 ± 4** | <0.001 |
| Indexed left atrial area (cm2/m2) | 15 ± 4 | 13 ± 3* | 10 ± 2**,*** | <0.001 |
| Left atrial volume index (mL/m2) | 49 ± 14 | 43 ± 27 | 39 ± 13 | 0.08 |
| TAPSE (mm) | 20 ± 5 | 22 ± 4 | 23 ± 4** | 0.005 |
| Estimated sPAP (mmHg) | 44 ± 14 | 37 ± 10* | 31 ± 7** | <0.001 |
| Mean aortic valve gradient (mmHg) | 48 ± 17 | 47 ± 21 | 46 ± 15 | 0.80 |
| Aortic valve area (cm2) | 0.75 ± 0.22 | 0.82 ± 0.24 | 0.85 ± 0.26** | 0.009 |
| Indexed aortic valve area (cm2/m2) | 0.38 ± 0.11 | 0.43 ± 0.14* | 0.43 ± 0.13** | 0.001 |
| Aortic regurgitation (at least moderate) | 14 (13%) | 5 (7%) | 7 (7%) | 0.39 |
| Mitral regurgitation | —**,*** | <0.001 | ||
| No | 31 (28%) | 29 (41%) | 78 (76%) | |
| Mild | 62 (55%) | 37(53%) | 21 (20%) | |
| Moderate | 17(15%) | 2 (3%) | 3 (3%) | |
| Severe | 2 (2%) | 2 (3%) | 1 (1%) | |
| Coronary artery disease | 0.66 | |||
| No coronary artery disease | 52 (46%) | 42 (60%) | 49 (48%) | |
| 1-vessel disease | 23 (21%) | 11 (16%) | 23 (22%) | |
| 2-vessel disease | 14 (12%) | 9 (13%) | 13 (13%) | |
| 3-vessel disease | 23 (21%) | 8 (11%) | 18 (17%) | |
| Invasive haemodynamics | ||||
| Mean right atrial pressure (mmHg) | 9 ± 5 | 6 ± 2* | 5 ± 3** | <0.001 |
| Right ventricular end-diastolic pressure (mmHg) | 11 ± 5 | 7 ± 3* | 6 ± 3** | <0.001 |
| mPAP (mmHg) | 35 ± 9 | 21 ± 3* | 17 ± 4**,*** | <0.001 |
| mPAWP (mmHg) | 24 ± 7 | 12 ± 3* | 10 ± 3**,*** | <0.001 |
| Transpulmonary gradient (mmHg) | 12 ± 6 | 8 ± 3* | 8 ± 3** | <0.001 |
| Pulmonary vascular resistance (Wood units) | 2.8 ± 1.8 | 1.8 ± 0.8* | 1.6 ± 0.7** | <0.001 |
| Pulmonary artery compliance (mL/mmHg) | 2.4 ± 1.2 | 3.6 ± 1.1* | 4.6 ± 2.3**,*** | <0.001 |
| Left ventricular end-diastolic pressure (mmHg) | 26 ± 8 | 21 ± 7* | 16 ± 6**,*** | <0.001 |
| Mean aortic pressure (mmHg) | 99 ± 15 | 96 ± 14 | 96 ± 13 | 0.27 |
| Systemic vascular resistance (Wood units) | 20.7 ± 5.6 | 19.3 ± 4.8 | 19.2 ± 4.6 | 0.05 |
| Arterial oxygen saturation (%) | 95 (92–96) | 96 (94–97) | 96 (94–97)** | 0.002 |
| Mixed venous oxygen saturation (%) | 65 (58–70) | 70 (66–73)* | 70 (67–73)** | <0.001 |
| Cardiac output (L/min) | 4.5 ± 1.1 | 4.9 ± 1.1 | 4.9 ± 1.1** | 0.01 |
| Cardiac index (L/min/m2) | 2.3 ± 0.5 | 2.6 ± 0.6* | 2.5 ± 0.6** | 0.001 |
| Stroke volume index (mL/m2) | 32 ± 10 | 38 ± 10* | 39 ± 11** | <0.001 |
- Data are given as numbers and percentages, mean ± standard deviation, and/or median (inter-quartile range).
- E/e′, ratio of peak early mitral inflow velocity to peak early mitral annular velocity; mPAP, mean pulmonary artery pressure; mPAWP, mean pulmonary artery wedge pressure; PH, pulmonary hypertension; sPAP, systolic pulmonary artery pressure; TAPSE, tricuspid annular plane systolic excursion.
- * Post hoc tests: P < 0.05 for pre-contrast vs. post-contrast PH.
- ** Post hoc tests: P < 0.05 for pre-contrast PH vs. no PH.
- *** Post hoc tests: P < 0.05 for post-contrast vs. no PH.
| Pre-contrast PH (n = 112) | Post-contrast PH (n = 70) | No PH (n = 103) | P-value | |
|---|---|---|---|---|
| Pre-contrast | ||||
| mPAP (mmHg) | 35 ± 9 | 21 ± 3* | 17 ± 4**,*** | <0.001 |
| mPAWP (mmHg) | 24 ± 7 | 12 ± 3* | 10 ± 3**,*** | <0.001 |
| Transpulmonary gradient (mmHg) | 12 ± 6 | 8 ± 3* | 8 ± 3** | <0.001 |
| PVR (WU) | 2.8 ± 1.8 | 1.8 ± 0.8 | 1.6 ± 0.7 | <0.001 |
| Post-contrast | ||||
| mPAP (mmHg) | 39 ± 10 | 29 ± 4* | 20 ± 3**,*** | <0.001 |
| mPAWP (mmHg) | 27 ± 8 | 19 ± 4* | 13 ± 4**,*** | <0.001 |
| Transpulmonary gradient (mmHg) | 12 ± 5 | 10 ± 4* | 7 ± 3**,*** | <0.001 |
| Contrast and changes | ||||
| Contrast volume (mL) | 136 ± 47 | 168 ± 73* | 153 ± 53 | 0.001 |
| Contrast volume (mL/kg body weight) | 1.7 ± 0.7 | 2.3 ± 1.0* | 2.1 ± 0.8** | <0.001 |
| Delta mPAP (mmHg) | 4 ± 5 | 8 ± 4* | 3 ± 3*** | <0.001 |
| Delta mPAWP (mmHg) | 4 ± 5 | 7 ± 3* | 3 ± 3*** | <0.001 |
| Delta mPAP/contrast volume (mmHg/mL·kg) | 2.3 ± 3.2 | 4.1 ± 2.2* | 1.6 ± 1.6*** | <0.001 |
| Delta mPAWP/contrast volume (mmHg/mL·kg) | 2.2 ± 2.9 | 3.4 ± 2.3* | 1.7 ± 1.7*** | <0.001 |
- Data are presented as mean ± standard deviation.
- * Post hoc tests: P < 0.05 for pre-contrast vs. post-contrast PH.
- ** Post hoc tests: P < 0.05 for pre-contrast PH vs. no PH.
- *** Post hoc tests: P < 0.05 for post-contrast vs. no PH.
Pre-contrast vs. post-contrast pulmonary hypertension vs. no pulmonary hypertension
Patients with post-contrast PH overall represented an intermediate group between patients with pre-contrast PH and those without PH in terms of heart failure symptoms, AS severity, cardiac dysfunction, haemodynamics, and logistic Euroscore II (Tables 1 and 2). However, PVR, cardiac index, and stroke volume index were similar in patients with post-contrast PH and without PH and thereby significantly lower (PVR) and higher (cardiac index and stroke volume index), respectively, compared with those with pre-contrast PH (Table 2 and Figure S2).
Prognostic impact of pre-contrast haemodynamics
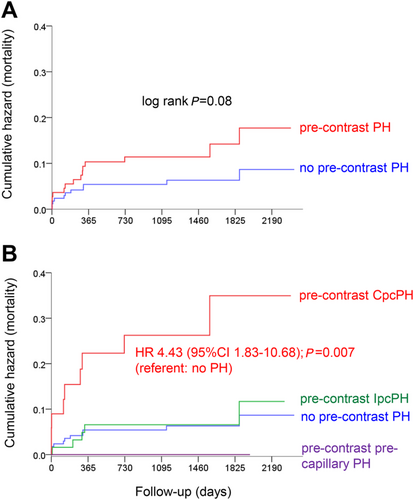
There was a trend towards a mortality difference between patients with any pre-contrast PH and those without pre-contrast PH (i.e. patients without any PH or those with post-contrast PH only; Figure 1). This was driven by patients with pre-contrast CpcPH, who had a more than four-fold mortality than patients without pre-contrast PH (Figure 1).
Prognostic impact of contrast-associated haemodynamic changes
The absolute changes in mPAP [hazard ratio 0.95 (95% confidence interval 0.86–1.04) per 1 mmHg; P = 0.25] and mPAWP [hazard ratio 0.98 (95% confidence interval 0.89–1.08) per 1 mmHg; P = 0.74] were not associated with mortality. Relative changes (per cent change relative to pre-contrast values) and absolute or relative changes indexed to contrast volume per kilogram body weight in mPAP or mPAWP were not associated with mortality either (data not shown).
Prognostic impact of post-contrast haemodynamics
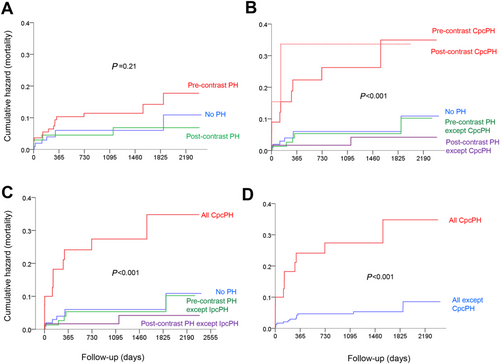
There was no significant mortality difference between patients with pre-contrast PH, with post-contrast PH, and without any PH (Figure 2). However, if patients with pre-contrast CpcPH (n = 35) and patients with CpcPH only after contrast (assuming an unchanged PVR; n = 7) were looked at separately, these patients had significantly higher mortality than all other groups (Figure 2). Patients with pre-contrast CpcPH and post-contrast CpcPH combined (n = 42) had a more than four-fold mortality than patients without any PH (Figure 2) and a more than five-fold mortality than all non-CpcPH patients together (Figure 2).
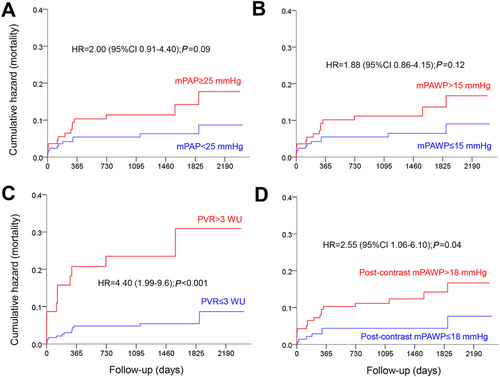
In Figure 3, the prognostic value of a post-contrast mPAWP > 18 mmHg is illustrated in comparison with classical pre-contrast cut-offs for mPAP, mPAWP and PVR. Patients with a post-contrast mPAWP > 18 mmHg had significantly higher mortality than those with post-contrast mPAWP ≤ 18 mmHg (Figure 3), while the comparisons of pre-contrast mPAP ≥ 25 vs. <25 mmHg and pre-contrast mPAWP > 15 vs. ≤15 mmHg failed to reach statistical significance (Figure 3). The highest hazard ratio however was seen for pre-contrast PVR > 3 vs. ≤3 Wood units (Figure 3).
Echocardiography follow-up
Post-AVR echocardiograms were available from 232 patients (81% of the entire population; 90 with pre-contrast PH, 59 with post-contrast PH, and 83 without PH; Table S3). Studies were performed after a median post-AVR follow-up of 15 (13.0–18.0) months. While LVEF and the mean gradient across the aortic valve prosthesis were similar in the three groups, left atrial area was persistently higher in patients with pre-contrast PH vs. no PH, and the estimated systolic PAP was higher in patients with pre-contrast PH than in those with post-contrast PH and no PH.
Application of the 2018 pulmonary hypertension definition
Results were qualitatively similar when using the 2018 PH definition (Supplementary Text, Tables S3–S7, and Figure S3).
Discussion
Main findings
This study represents the first detailed evaluation of the role of a fluid challenge in patients with AS. In a population of patients with severe AS with a 39% prevalence of PH, 40% of patients without pre-contrast PH had PH only after a contrast-based volume challenge. These patients represented an intermediate group in terms of AS severity, symptoms, extent of AS-related ‘cardiac damage’, and haemodynamics. However, patients with post-contrast PH had similar post-AVR mortality as patients without any PH.
A volume challenge is typically used in patients with a constellation of typically mild pre-capillary PH with an mPAWP between 12 and 15 mmHg but risk factors for post-capillary PH.5, 6, 8 Such patients may present with relative hypovolaemia in the cath lab, and despite having a left ventricle characterized by an end-diastolic pressure volume relationship, which is shifted upwards and to the left, the LVEDP may not be high enough to cause an mPAWP > 15 mmHg at that time. Rapid infusion of a volume bolus will put the patient on a steeper part of the end-diastolic pressure volume relationship with a markedly higher LVEDP leading to an mPAWP fulfilling the criterion for post-capillary PH (i.e. >15 mmHg), particularly in presence of the left atrial dysfunction and relevant mitral regurgitation. Identification of patients with ‘occult’ post-capillary PH is clinically important because the 2015 guidelines define pre-capillary PH solely based on mPAWP but without a PVR criterion.15 Misclassification of patients with post-capillary PH as pre-capillary may result in the administration of contraindicated, expensive, and potentially dangerous therapies.16, 17 We found that one-quarter of the population had PH only after the volume challenge, and the number of patients with a systolic PAP > 60 mmHg (a criterion for AVR according to the current ESC guidelines14) increased by >50% after contrast. Given that PH is a prognostic marker in patients with AS1 and is even included as criterion for decision making regarding AVR in current guidelines,14 this is a clinically relevant finding.
Comparison with previous studies
While the relatively small number of available studies evaluating the role of volume challenge in various patient populations used saline,5, 7, 9-11, 18, 19 we used contrast. In three studies,5, 7, 18 infusion of 500 mL of saline (or 7 mL/kg body weight) resulted in an increase in mPAWP by 7 mmHg in patients with occult post-capillary PH and by 2–4 mmHg in patients without. Even healthy subjects were shown to exhibit an increase in mPAWP from 10 to 16 after rapid infusion of 1000 mL of saline.10 In the only contrast study, Denardo et al.13 evaluated haemodynamics in 150 heart failure patients before and after administration of a median volume of 109 mL of low-osmolal or iso-osmolal contrast. The median increase in mPAWP was 4 mmHg for both types of contrast media.13 In the present study, ~50% higher contrast volumes were used, and the overall post-contrast mean rise in mPAWP was also 4 mmHg.
Previous studies have mainly evaluated the effect of fluid loading for diagnostic purposes,5, 7, 11, 18 and only two studies have looked at the prognostic information. Denardo et al.13 reported that in a mixed heart failure with reduced and preserved ejection fraction population, those with both a pre-contrast mPAWP > 15.5 mmHg and a post-contrast decrease in cardiac index had the highest rate of adverse events (death and hospitalizations). In another study in patients with pre-capillary PH, the change in cardiac index after contrast was associated with an increased risk of an event (death, lung transplantation, parenteral therapy, and clinical worsening).9 In the present setting, the changes in mPAWP and mPAP were not associated with post-AVR mortality even when adjusted for the contrast volume.
Clinical implications
Any post-contrast mPAWP > 18 mmHg was associated with increased long-term mortality after AVR. This cut-off was evaluated as it has been suggested as diagnostic for occult post-capillary PH8 because the mPAWP in healthy people has been shown not to exceed this value after ‘normal’ fluid challenge of 500 mL.6 The post-contrast mPAWP is an index of the ability of the aortic valve/left ventricle/left atrium/mitral valve continuum to deal with volume (baseline + acute load). We showed that the fact that the mPAWP exceeded 18 mmHg is prognostically relevant also in the present setting, presumably because it reflects the extent of ‘cardiac damage’ in AS.20 However, occurrence of post-contrast PH (PAP-based definition: mPAP ≥ 25 or >20 mmHg) alone was not an indicator of an increased mortality after AVR. We have to take into account that all patients underwent AVR soon after right heart catheterization resulting in acute3 and presumably also long-term (only echo data available1) changes of haemodynamics, which may have attenuated the prognostic impact of the pre-AVR haemodynamics. Post-contrast PH most often was IpcPH, and we speculate that the underlying substrate was at least partially reversible after AVR, which may explain the lack of a mortality difference between patients with post-contrast PH and no PH. There was also a significant difference in the logistic Euroscore II between patients without PH and those with post-contrast PH (which was not explained by haemodynamics alone), which however did not result in a significant mortality difference between the two groups.
Independently of the definition used, those with pre-contrast PH had numerically the highest mortality, and this was driven by the patients with CpcPH. A PVR > 3 WU was a strong predictor of death and thereby much stronger than a post-contrast mPAWP > 18 mmHg, most likely because pulmonary vascular involvement may persist after AVR. Persistent PH after AVR is a marker of poor prognosis.21 We showed that the pre-contrast PH group had the highest pulmonary pressure several months post-AVR despite similar valve function and normalized LVEF, which supports this concept. The study therefore underscores the importance of right heart catheterization prior to AVR. The role of a volume challenge needs to be defined in a prospective manner using a well-defined protocol.
Strengths and limitations
The present study has limitations. First, the number of patients was relatively low. Still, it is one of the two largest studies (Robbins et al.5: n = 287) evaluating the role of a volume challenge and the first one in patients with AS. Second, we have used the indirect Fick method to assess cardiac output, which is subject to due to errors in the estimation of oxygen consumption.22 However, this technique is routinely used in clinical practice. Third, the amount of contrast was not standardized but varied based on the diagnostic requirements on the one hand and probably also the presumed ability of the patient to tolerate contrast on the other hand. Still, measurement of mPAP and mPAWP post-contrast was systematically performed, which allowed for a unique dataset as a basis for future studies.
Conclusions
In patients with severe AS, a contrast volume challenge leads to post-contrast PH in ~40% of patients without pre-contrast PH. However, post-contrast haemodynamic changes do not adversely affect outcomes in patients undergoing AVR. Post-contrast PH represents an intermediate stage of ‘cardiac damage’, which may be attenuated or reversible after AVR.
Conflict of interest
None.
Funding
None.




