Impact of tricuspid regurgitation on survival in patients with cardiac amyloidosis
Abstract
Aims
Tricuspid regurgitation (TR) is a common finding and has been associated with poorer outcome in patients with heart failure. This study sought to investigate the prognostic value of TR in patients with cardiac amyloidosis (CA).
Methods and results
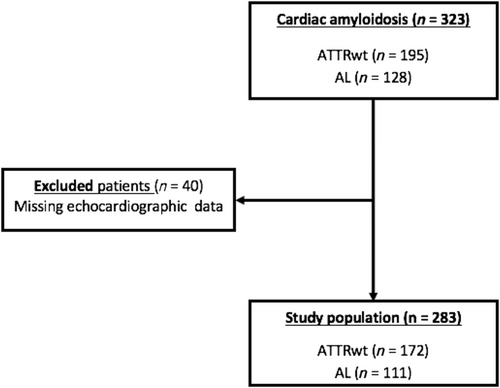
Two-hundred and eighty-three patients with CA—172 (61%) wild-type transthyretin amyloidosis (ATTRwt) and 111 (39%) light-chain amyloidosis (AL)—were consecutively enrolled between December 2010 and September 2019. Transthoracic echocardiographies at time of diagnosis were reviewed to establish the presence and severity of TR and its relationship with all-cause mortality during patients' follow-up. Seventy-four (26%) patients had a moderate-to-severe TR. Moderate-to-severe TR was associated with New York Heart Association status (P < 0.001), atrial fibrillation (P = 0.003), greater levels of natriuretic peptides (P = 0.002), worst renal function (P = 0.03), lower left ventricular ejection fraction (P = 0.02), reduced right ventricular systolic function (P = 0.001), thicker tricuspid leaflets (P = 0.019), greater tricuspid annulus diameter (P = 0.001), greater pulmonary artery pressure (P = 0.001), greater doses of furosemide (P = 0.001), and anti-aldosterone (P = 0.01) and more anticoagulant treatment (P = 0.001). One hundred and thirty-four (47%) patients met the primary endpoint of all-cause mortality. After multivariate Cox analysis, moderate-to-severe TR was significantly associated with mortality [hazard ratio 1.89, 95% confidence interval (1.01–3.51), P = 0.044] in patients with ATTRwt. There was no correlation between TR and death [hazard ratio 0.84, 95% confidence interval (0.46–1.51), P = 0.562] in patients with AL.
Conclusions
Moderate-to-severe TR is frequent in CA, and it is an independent prognosis factor in patients with ATTRwt but not in patients with AL.
Introduction
Mild tricuspid regurgitation (TR) is a common echocardiographic finding, present in 80% to 90% of normal individuals.1 Isolated TR is associated with increased mortality, even in the absence of left (LV) or right ventricular (RV) systolic dysfunction or pulmonary hypertension.2 Despite these findings, tricuspid valve evaluation has long been neglected.
Cardiac amyloidosis (CA) has become a more and more diagnosed condition, especially in the elderly,3 in the last few decades, and is associated with a high mortality. CA is characterized by a restrictive haemodynamic pattern, with elevation of LV filling pressures, which may cause pulmonary hypertension with repercussions on the right ventricle and tricuspid valve.4 Data on the pathophysiology of TR in patients with CA and its prognostic impact are lacking.
The aim of this study was to investigate the epidemiology and prognostic value of TR in CA, in both wild-type transthyretin amyloidosis (ATTRwt) and light-chain amyloidosis (AL) forms of this disease.
Methods
Patients
All consecutive patients diagnosed with CA at the University Hospital of Toulouse were retrospectively included between December 2010 and September 2019.
Cardiac amyloidosis diagnosis was made according to Gillmore's algorithm.5 When typical infiltrative process was highlighted on cardiac magnetic resonance imaging or echocardiography, ATTRwt was confirmed with a Grade 2 or 3 cardiac uptake on a 99mTc-hydroxymethylene diphosphonate scintigraphy and in the absence of monoclonal gammopathy. In other cases—Grade 1 on scintigraphy and/or monoclonal gammopathy—the diagnosis of ATTRwt or AL required a histological confirmation on cardiac or peripheral tissues biopsies. All patients with AL were treated according to national recommendations with Melphalan and Dexamethasone for Mayo Clinic Stages I and II and Velcade with Endoxan and Dexamethasone for Mayo Clinic Stage III as first-line treatment. All patients with ATTRwt underwent genetic testing with no mutation found in the transthyretin gene regardless of age.
Forty patients without echocardiographic data were excluded, resulting in a study population of 284 patients diagnosed with either ATTRwt or AL CA (Figure 1).
Echocardiographic data
All echocardiographic examinations were reviewed by the same operator using GE Healthcare EchoPAC Clinical Workstation software.
Annulus tricuspid diameter was measured in a four-chamber apical view, at end-diastole, in accordance with standard measurements recommended by the European Association of Cardiovascular Imaging (former European Association of Echocardiography).6 Tricuspid valve thickening was measured in a four-chamber apical view, at end-systole, while valve leaflets were coapted. Effective regurgitant orifice area (EROA) and regurgitated volume (RVol) were calculated using proximal isovelocity surface area (PISA) radius and TR jet width.
Right ventricular systolic dysfunction was defined as a tricuspid annulus plane systolic excursion <16 mm and/or a tricuspid annulus S wave <10 cm/s.
Tricuspid regurgitation severity was assessed according to the American Society of Echocardiography criteria,7 thus defining three stages of severity according to the presence of one or more of specific criteria for mild or severe TR and/or direct quantification:
- Mild TR: thin and small central colour jet, vena contracta (VC) width <3 mm, PISA radius <0.4 cm at Nyquist 30–40 cm/s, incomplete or faint continuous wave Doppler jet, systolic dominant hepatic venous flow, normal RV and right atrial dimensions, EROA < 0.2 cm2, and RVol < 30 mL.
- Moderate TR: VC width 0.3–0.69 mm, EROA 0.2–0.4 cm2, and RVol 30–44 mL.
- Severe TR: dilated tricuspid annulus with no valve coaptation or flail leaflet, large central jet >50% of right atrial area, VC width >7 mm, PISA radius >0.9 cm at Nyquist 30–40 cm/s, dense/triangular continuous wave Doppler jet, systolic reversal of hepatic venous flow, dilated RV, EROA > 0.4 cm2, and RVol > 45 mL.
Based on this staging, we separated two groups: no or mild TR and moderate-to-severe TR. Clinical characteristics, biological data, echocardiographic data, and mortality were compared between the two groups.
Clinical endpoint and follow-up
Follow-up was assessed in March 2020 by electronic chart review or by phone interview of patient's general practitioner/cardiologist, patient, or family for the primary endpoint of all-cause mortality.
Ethics
The investigation conforms to the principles outlined in the Declaration of Helsinki. According to French law on ethics, patients were informed that their codified data could be used for the study. According to the French ethic and regulatory law (public health code), retrospective studies based on the exploitation of usual care data have to be declared or covered by reference methodology of the French National Commission for Informatics and Liberties. This study was approved by Toulouse University Hospital with confirmaion that ethic requirements were totally respected in this report.
Statistical analysis
Continuous variables were expressed as means ± standard deviation or as medians with inter-quartile ranges (IQRs) when not normally distributed. Nominal variables were expressed as numbers and percentages. Association between the mean values of continuous variables was assessed using the Mann–Whitney rank-sum test. Nominal variables were investigated by χ2 test or Fisher's exact test when appropriate. Univariate Cox proportional hazards regression analysis was performed to analyse variables associated with all-cause mortality, with results reported as hazard ratios with 95% confidence intervals. Thresholds for N-terminal pro-brain natriuretic peptide (NT-proBNP) and glomerular filtration rate were chosen based on those clinically relevant in previous studies in CA.8 Variables with a P-value <0.05 in the univariate analysis were analysed with a multivariate Cox regression model. Kaplan–Meier curves using the log-rank test were generated to determine the association between moderate-to-severe TR and survival. Patients were censored at the time of death or last registration. A P-value inferior to 0.05 was considered significant. The software SPSS was used for statistical analysis (SPSS Version 20, SPSS Inc., Chicago, IL, USA).
Results
Population baseline characteristics
Two-hundred and eighty-three patients—172 (61%) ATTRwt and 111 (39%) AL—were enrolled in the study. Patients' baseline characteristics and graded by TR severity are summed up in Table 1 for patients with ATTRwt and Table 2 for patients with AL.
| Whole population | Moderate-to-severe TR | No TR or mild TR | P-value | |
|---|---|---|---|---|
| n = 172 | n = 48 | n = 124 | ||
| Clinical characteristics | ||||
| Age at diagnosis (years) | 82 ± 6 | 82 ± 5 | 81 ± 7 | 0.739 |
| Male, n (%) | 153 (89) | 41 (85) | 112 (90) | 0.357 |
| Body mass index (kg/m2) | 25 ± 4 | 25 ± 4 | 25 ± 4 | 0.745 |
| Diabetes mellitus, n (%) | 27 (16) | 8 (17) | 19 (15) | 0.828 |
| Vascular disease, n (%) | 61 (35) | 14 (29) | 47 (38) | 0.283 |
| Hypertension, n (%) | 95 (55) | 25 (52) | 70 (56) | 0.605 |
| Coronary artery disease, n (%) | 44 (26) | 12 (25) | 32 (26) | 0.913 |
| Atrial fibrillation, n (%) | 126 (73) | 42 (88) | 84 (68) | 0.009 |
| Pacemaker, n (%) | 36 (21) | 12 (25) | 24 (19) | 0.414 |
| Moderate-to-severe aortic stenosis, n (%) | 44 (26) | 14 (29) | 30 (24) | 0.503 |
| Moderate-to-severe mitral regurgitation, n (%) | 29 (17) | 8 (17) | 21 (17) | 0.966 |
| NYHA stage, n (%) | ||||
| I, II | 124 (72) | 30 (63) | 94 (76) | 0.067 |
| III, IV | 47 (27) | 18 (38) | 29 (23) | 0.067 |
| Biology | ||||
| Glomerular filtration rate <45 mL/min | 72 (42) | 29 (60) | 43 (35) | 0.001 |
| NT-proBNP > 3000 ng/mL | 93 (54) | 36 (75) | 57 (46) | <0.001 |
| Echocardiography | ||||
| Left ventricular ejection fraction (%) | 49 ± 12 | 46 ± 11 | 51 ± 12 | 0.023 |
| Valve thickness <2 mm | 24 (14) | 2 (4) | 22 (18) | 0.023 |
| Valve thickness ≥2 mm | 106 (62) | 33 (69) | 73 (59) | 0.023 |
| Tricuspid ring diameter (mm) | 34 ± 6 | 38 ± 6 | 32 ± 5 | <0.001 |
| RV thickness (mm) | 9 ± 2 | 9 ± 2 | 9 ± 2 | 0.232 |
| Reduced RV systolic function | 115 (67) | 38 (79) | 77 (62) | 0.025 |
| TR Vmax (m/s) | 2.8 ± 0.5 | 3 ± 0.5 | 2.8 ± 0.6 | 0.050 |
| PASP (mmHg) | 43 ± 12 | 46 ± 12 | 41 ± 11 | 0.011 |
| Medications | ||||
| Diuretics, n (%) | 145 (84) | 41 (85) | 104 (84) | 0.803 |
| Furosemid doses (mg) | 150 [40–125] | 120 [40–438] | 40 [40–120] | 0.006 |
| Anti-aldosterone doses (mg) | 15 [0–25] | 25 [0–25] | 13 [0–25] | 0.425 |
| Beta-blocker, n (%) | 49 (28) | 11 (23) | 38 (31) | 0.314 |
| Amiodarone, n (%) | 42 (24) | 12 (25) | 30 (24) | 0.912 |
| Anticoagulation | 118 (67) | 40 (83) | 78 (63) | 0.010 |
- ATTRwt, wild-type transthyretin amyloidosis; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure; RV, right ventricular; TR, tricuspid regurgitation.
- P-value corresponds to the results of group comparisons using χ2 test or Mann–Whitney U test. In bold are P-values <0.05. The small p values for the EGR and NTPro BNP are offset from the other values.
| Whole population | Moderate-to-severe TR | No TR or mild TR | P-value | |
|---|---|---|---|---|
| n = 111 | n = 26 | n = 85 | ||
| Clinical characteristics | ||||
| Age at diagnosis (years) | 69 ± 10 | 68 ± 8 | 70 ± 11 | 0.528 |
| Male, n (%) | 61 (55) | 15 (58) | 46 (54) | 0.749 |
| Body mass index (kg/m2) | 24 ± 4 | 24 ± 3 | 24 ± 5 | 0.910 |
| Diabetes mellitus, n (%) | 12 (11) | 2 (8) | 10 (12) | 0.587 |
| Vascular disease, n (%) | 24 (22) | 3 (12) | 21 (25) | 0.161 |
| Hypertension, n (%) | 41 (37) | 4 (15) | 37 (44) | 0.011 |
| Coronary artery disease, n (%) | 16 (14) | 3 (12) | 13 (15) | 0.666 |
| Atrial fibrillation, n (%) | 42 (38) | 13 (50) | 29 (34) | 0.156 |
| Pacemaker, n (%) | 13 (12) | 2 (8) | 11 (13) | 0.490 |
| Moderate-to-severe aortic stenosis, n (%) | 8 (7) | 4 (15) | 4 (5) | 0.065 |
| Moderate-to-severe mitral regurgitation, n (%) | 18 (16) | 9 (35) | 9 (11) | 0.004 |
| NYHA stage, n (%) | ||||
| I, II | 64 (58) | 14 (54) | 50 (59) | 0.657 |
| III, IV | 43 (39) | 11 (42) | 32 (38) | 0.657 |
| Biology | ||||
| Glomerular filtration rate <45 mL/min | 38 (34) | 7 (27) | 31 (36) | 0.272 |
| NT-proBNP > 3000 ng/mL | 48 (43) | 12 (46) | 36 (42) | 0.879 |
| Echocardiography | ||||
| Left ventricular ejection fraction (%) | 53 ± 12 | 49 ± 13 | 55 ± 11 | 0.067 |
| Valve thickness <2 mm | 11 (10) | 2 (8) | 9 (11) | 0.403 |
| Valve thickness ≥2 mm | 42 (38) | 13 (50) | 29 (34) | 0.403 |
| Tricuspid ring diameter (mm) | 32 ± 8 | 40 ± 8 | 28 ± 5 | <0.001 |
| RV thickness (mm) | 7 ± 2 | 6 ± 1 | 7 ± 2 | 0.392 |
| Reduced RV systolic function | 63 (57) | 21 (81) | 42 (49) | 0.006 |
| TR Vmax (m/s) | 2,7 ± 0,6 | 3 ± 0,7 | 2.6 ± 0.5 | 0.012 |
| PASP (mmHg) | 39 ± 15 | 50 ± 19 | 35 ± 10 | <0.001 |
| Medications | ||||
| Diuretics, n (%) | 82 (74) | 22 (85) | 60 (71) | 0.079 |
| Furosemid doses (mg) | 149 [5–191] | 80 [40–250] | 70 [0–151] | 0.088 |
| Anti-aldosterone doses (mg) | 9 [0–9] | 0 [0–44] | 0 [0–0] | 0.020 |
| Beta-blocker, n (%) | 30 (27) | 5 (19) | 14 (16) | 0.146 |
| Amiodarone, n (%) | 11 (10) | 3 (12) | 8 (9) | 0.586 |
| Anticoagulation | 40 (36) | 14 (54) | 26 (31) | 0.014 |
- AL, light-chain amyloidosis; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure; RV, right ventricular; TR, tricuspid regurgitation.
- P-value corresponds to the results of group comparisons using χ2 test or Mann–Whitney U test. In bold are P-values <0.05. The small p values for the EGR and NTPro BNP are offset from the other values.
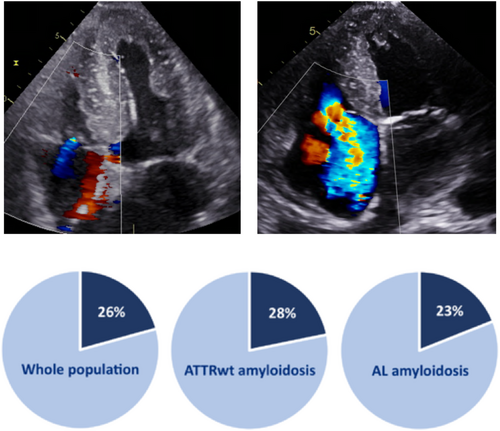
There were 74 (26%) patients with moderate-to-severe TR, respectively: 48 (28%) in the ATTRwt population and 26 (23%) in the AL population.
There was no significant difference in terms of left valve disease in the ATTRwt population, but we found more frequent moderate-to-severe mitral regurgitation in the AL population with moderate-to-severe TR.
In patients with ATTRwt, moderate-to-severe TR was significantly associated with more atrial fibrillation (88% vs. 68%, P = 0.009), greater levels of natriuretic peptides (NT-proBNP > 3000 pg/mL: 75% vs. 46%, P < 0.001), lower LV ejection fraction (46 ± 11% vs. 51 ± 12%, P = 0.023), and more RV dysfunction (79% vs. 62%, P = 0.025), and in patients with AL, moderate-to-severe TR was only associated with RV dysfunction (81% vs. 49%, P = 0.006).
Only one patient benefitted from an interventional treatment of TR, in the AL group, by percutaneous procedure (edge to edge).
Impact of tricuspid regurgitation on outcome
The median follow-up was 14 months (IQR [8–24]): 14 months (IQR [9–24]) and 12 months (IQR [5.7–24.7]) among patients with ATTRwt and AL, respectively. Twenty-nine patients were lost to follow-up, and they were included in the survival analysis according to their last known status.
One hundred and thirty-four (47%) patients died during the follow-up. There were 41 (55%) and 93 (44%) deaths in the moderate-to-severe TR and no TR or mild TR population, respectively (P < 0.0001). There were more deaths in the AL population than in the ATTRwt one (61% vs. 38%, P = 0.0001).
After multivariate analysis among patients with ATTRwt, the hazard ratio (HR) for death in the group with moderate-to-severe TR was 1.89 [95% confidence interval (CI) (1.01–3.51), P = 0.044]. Other variables associated with death were male sex [HR 5.72, 95% CI (1.60–20.39), P = 0.007] and NT-proBNP > 3000 pg/mL [HR 3.33, 95% CI (1.43–7.75), P = 0.005]. Results of survival analysis among patients with ATTRwt are presented in Table 3 and Figures 2 and 3.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Clinical characteristics | ||||
| Age at diagnosis (years) | 1.07 (1.02–1.12) | 0.030 | 1.05 (0.99–1.11) | 0.057 |
| Male | 3.36 (1.05–10.72) | 0.041 | 5.72 (1.60–20.39) | 0.007 |
| Body mass index (kg/m2) | 0.96 (0.90–1.02) | 0.255 | ||
| Diabetes mellitus | 1.58 (0.90–2.78) | 0.109 | ||
| Vascular disease | 1.31 (0.75–2.31) | 0.345 | ||
| Hypertension | 0.86 (0.53–1.41) | 0.328 | ||
| Coronary artery disease | 1.84 (1.10–3.08) | 0.020 | 1.44 (0.79–2.65) | 0.234 |
| Atrial fibrillation | 1.88 (0.98–3.59) | 0.057 | ||
| Pacemaker | 1.05 (0.58–1.18) | 0.870 | ||
| NYHA stage ≥III | 1.88 (1.12–3.16) | 0.017 | 1.13 (0.59–2.16) | 0.704 |
| Biology | ||||
| Glomerular filtration rate <45 mL/min | 2.47 (1.48–4.11) | 0.001 | 1.45 (0.80–2.62) | 0.216 |
| NT-proBNP > 3000 ng/mL | 5.86 (2.75–12.46) | 0.0001 | 3.33 (1.43–7.75) | 0.005 |
| Echocardiography | ||||
| LV ejection fraction | 0.96 (0.00–0.99) | 0.004 | 1.01 (0.98–1.04) | 0.475 |
| Severe or moderate TR vs. no or mild TR | 2.64 (1.60–4.34) | <0.0001 | 1.89 (1.01–3.51) | 0.044 |
| Valve thickness >2 mm vs. valve thickness 0–2 mm | 1.56 (0.71–3.42) | 0.266 | ||
| Reduced RV function (TAPSE < 16 mm or S wave <10 cm/s) | 2.44 (1.27–4.70) | 0.007 | 1.20 (0.59–2.42) | 0.603 |
| PASP (mmHg) | 1.00 (0.98–1.02) | 0.836 | ||
| Medications | ||||
| Diuretics vs. no diuretic | 4.81 (1.5–15.40) | 0.008 | 2.06 (0.61–6.94) | 0.241 |
| Beta-blocker vs. no beta-blocker | 0.89 (0.53–1.51) | 0.686 | ||
| Amiodarone vs. no amiodarone | 1.35 (0.80–2.279) | 0.249 | ||
| Anticoagulation vs. no anticoagulation | 1.60 (0.90–2.85) | 0.108 | ||
- ATTRwt, wild-type transthyretin amyloidosis; CI, confidence interval; HR, hazard ratio; LV, left ventricular; NT-proBNP, N-terminal pro-brain natriuretic peptide; PASP, pulmonary artery systolic pressure; RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
- P-value corresponds to the results of the Wald test. Variables with a P-value <0.05 in the univariate analysis were analysed with a multivariate Cox regression model. In bold are P-values <0.05.
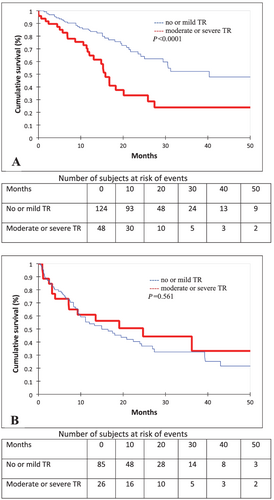
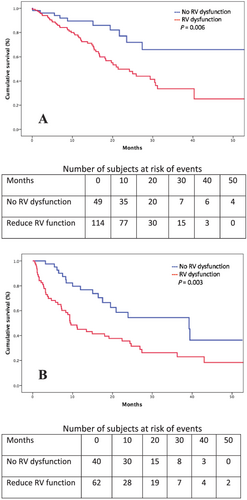
However, among patients with AL, moderate-to-severe TR was not associated with mortality. The unadjusted HR for death in the group with moderate-to-severe TR was 0.84 [95% CI (0.46–1.51), P = 0.562]. The variables associated with mortality in the AL population, in both univariate and multivariate analyses, were NT-proBNP > 3000 pg/mL [HR 2.97, 95% CI (1.43–6.16), P = 0.003, by multivariate analysis] and RV dysfunction [HR 2.43, 95% CI (1.13–5.22), P = 0.022, by multivariate analysis]. Results of survival analysis among patients with AL are presented in Table 4 and Figures 2 and 3.
| Univariate | Multivariate | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Clinical characteristics | ||||
| Age at diagnosis (years) | 0.98 (0.96–1.00) | 0.225 | ||
| Male | 1.12 (0.69–1.83) | 0.623 | ||
| Body mass index (kg/m2) | 0.99 (0.94–1.04) | 0.124 | ||
| Diabetes mellitus | 1.96 (0.96–3.98) | 0.063 | ||
| Vascular disease | 1.22 (0.65–2.30) | 0.522 | ||
| Hypertension | 1.00 (0.61–1.66) | 0.971 | ||
| Coronary artery disease | 1.25 (0.65–2.39) | 0.492 | ||
| Atrial fibrillation | 0.92 (0.56–1.49) | 0.741 | ||
| Pacemaker | 0.65 (0.29–1.43) | 0.288 | ||
| NYHA stage ≥III | 1.17 (1.04–2.74) | 0.035 | 1.28 (0.69–2.36) | 0.439 |
| Biology | ||||
| Glomerular filtration rate <45 mL/min | 1.14 (0.65–1.99) | 0.636 | ||
| NT-proBNP > 3000 ng/mL | 3.86 (1.90–7.84) | 0.0001 | 2.97 (1.43–6.16) | 0.003 |
| Echocardiography | ||||
| Left ventricular ejection fraction | 0.98 (0.96–1.00) | 0.155 | ||
| Severe or moderate TR vs. no or mild TR | 0.84 (0.46–1.51) | 0.562 | 0.77 (0.36–1.64) | 0.498 |
| Valve thickness >2 mm vs. valve thickness 0–2 mm | 0.36 (0.04–2.71) | 0.952 | ||
| Reduced right ventricular function (TAPSE < 16 mm or S wave <10 cm/s) | 2.27 (1.30–3.98) | 0.004 | 2.43 (1.13–5.22) | 0.022 |
| PASP (mmHg) | 1.00 (0.98–1.02) | 0.759 | ||
| Medications | ||||
| Diuretics vs. no diuretic | 0.95 (0.55–1.66) | 0.881 | ||
| Beta-blocker vs. no beta-blocker | 1.21 (0.65–2.28) | 0.538 | ||
| Amiodarone vs. no amiodarone | 1.41 (0.69–2.85) | 0.335 | ||
| Anticoagulation vs. no anticoagulation | 1.09 (0.67–1.80) | 0.710 | ||
- AL, light-chain amyloidosis; CI, confidence interval; HR, hazard ratio; NT-proBNP, N-terminal pro-brain natriuretic peptide; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation
- P-value corresponds to the results of the Wald test. Variables with a P-value <0.05 in the univariate analysis and TR severity were analysed with a multivariate Cox regression model. In bold are P-values <0.05.
Discussion
In this retrospective study investigating the prevalence, the associated factors, and the prognosis of TR in CA, the main results can be summarized as follows: (i) moderate-to-severe TR is a frequent echocardiographic finding in CA, as it was found in about one quarter of our population study as can be seen in Figure 4; (ii) its mechanism seems to be predominantly functional, secondary to other cardiac conditions; and (iii) TR has an independent prognostic impact in patients with ATTRwt but not in patients with AL.
Tricuspid regurgitation appears to be more frequent in CA than in general population, in which the prevalence of moderate-to-severe TR is about 1–5%,1 as well as in systolic heart failure, where it has been encountered in about 19% of patients in a specific cohort study.9 From a pathophysiological point of view, there is evidence of a specific amyloid valvular disease, as valvular infiltration by amyloid deposits has been largely described in autopsy studies,10 affecting more frequently mitral and tricuspid valves11 and causing decreased valvular elasticity (which was showed to be associated with the severity of regurgitation in mitral amyloid valvular disease).12
In this cohort, tricuspid valve thickening was a frequent finding, associated with more severe TR. Nonetheless, most of TR in our study appeared to be functional, secondary to tricuspid annulus dilation and/or atrial fibrillation, with a decrease in LV or RV function and increase in pulmonary artery systolic pressure. This is the main mechanism described for TR in other cardiomyopathies.13
In our study, a moderate-to-severe TR was associated with an increased mortality in patients with ATTRwt but had no impact on survival in patients with AL. This difference is partly explained by the fact that these two types of amyloidosis are very different in terms of pathophysiology and especially in terms of prognosis. Indeed, patients with AL have a higher mortality rate in our study, which can be explained by severe cardiac involvement at the time of management because 39% of patients were New York Heart Association Stage III or IV and 43% of them had an NT-proBNP greater than 3000 ng/mL. These results may suggest that systemic disease progresses more rapidly than TR and its haemodynamic consequences, unlike RV dysfunction, which remains a significant prognostic marker in this population.
Our results are consistent with previous studies from literature. Nath et al. showed in a prospective cohort of 5223 consecutive patients without CA an increased mortality in patients with a moderate-to-severe TR, regardless of LV ejection fraction or pulmonary artery pressures.2 Hung et al. retrospectively studied a population of 117 patients with severe heart failure, screening for echocardiographic evidence of TR, and observed a worse survival in patients with TR, from trace to severe.14 Neuhold et al. observed the same impact of moderate-to-severe TR in heart failure, except in patients with severely depressed LV ejection fraction.9
No patient with a severe TR underwent a surgical valve treatment, even when they fitted European's indications for intervention, with both symptoms and a severe regurgitation, at a time when long-term benefits from tricuspid surgery are being controverted.15
Our study, added to evidence that TR correlates with poorer prognosis in CA, especially in ATTRwt, suggests that an invasive tricuspid treatment could be discussed in patients with CA and severe TR, in the setting of the arising of percutaneous treatments as alternatives to a high-risk surgery, which could improve outcome.16
Study limitations
This study shares all the limitations and bias associated with a retrospective and single-site study. Patients were retrospectively analysed, based on the echocardiography at the time of diagnosis. A prospective study, with evaluation of the severity of TR during follow-up, would help to acknowledge the efficacy of standard medical treatment (mostly loop diuretics and anti-aldosterone) or specific amyloidosis treatment (by chemotherapy for AL or tafamidis for ATTR) on TR, to better understand the impact of TR on symptoms and outcome, and to better define patients who could benefit from an invasive treatment of TR.
Conclusions
Tricuspid regurgitation is common in patients with CA, reaching about one quarter of patients. Moderate-to-severe TR is associated with New York Heart Association status, atrial fibrillation, LV ejection fraction, tricuspid annulus diameter, leaflet thickness, RV systolic dysfunction, pulmonary artery pressure, diuretics, and anticoagulant therapy. Moderate-to-severe TR is an independent prognostic factor associated with mortality in ATTRwt but does not seem to have an impact in AL. Larger-scale studies are needed to better define prevalence, mechanisms, prognostic value, and therapeutic possibilities for TR in patients with CA.
Conflict of interest
There are no conflicts of interest.




