Renin profiling predicts neurohormonal response to sacubitril/valsartan
Abstract
Aims
Clinical trials and observational cohorts show that beneficial effects of sacubitril/valsartan are less strong in an appreciable proportion of patients with heart failure with reduced ejection fraction (HFrEF). Lower blood pressure and impaired renal function predict suboptimal sacubitril/valsartan titration and a less favourable response. Circulating renin encompasses neurohormonal activation, intravascular volume, and renal function. We hypothesized that renin may predict response to sacubitril/valsartan, assessed by changes in N-terminal fraction of pro-brain natriuretic peptide (NT-proBNP).
Methods and results
We performed a prospective, open-label, real-life cohort study. The study population consisted of 80 consecutive HFrEF patients (age 66 ± 10 years, 83% men) planned to initiate sacubitril/valsartan. Clinical and biohumoral assessment, including a full neurohormonal panel, was performed at baseline and at 1, 3, and 6 month follow-up. Response to sacubitril/valsartan was defined as ≥30% reduction in NT-proBNP levels from baseline to 6 months. Patients in the lower renin tertile had higher blood pressure and plasma sodium concentration (all P < 0.05). At follow-up, 38 patients (48%) were classified as responders. Circulating renin was lower in the responder group compared with non-responders (19.8 mU/L, IQR 3.7–78.0 mU/L vs. 55.0 mU/L, IQR 16.4–483.1 mU/L; P = 0.004). After adjustment for age, renal function, and blood pressure, renin was independently associated to response to sacubitril/valsartan (P = 0.018).
Conclusions
In our preliminary study, we show that circulating renin predicts reduction in NT-proBNP levels after sacubitril/valsartan initiation in HFrEF patients. Renin assessment might be useful to discriminate potential responders from the subgroup with a weaker expected benefit, thus needing a closer, tailored management strategy.
Background
The first-in-class angiotensin receptor/neprilysin inhibitor sacubitril/valsartan has been demonstrated to improve morbidity and mortality in heart failure with reduced ejection fraction (HFrEF).1 Although the beneficial effects of sacubitril/valsartan have been consistently reported across different baseline characteristics, recent evidence from community populations shows that a clinically significant response is observed in a subgroup of patients, ranging 50–80% in different series.2, 3 Hypotensive patients had worse tolerance to sacubitril/valsartan in the PARADIGM-HF (Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure) trial.4, 5 Lower systolic blood pressure and worse renal function have been associated to suboptimal sacubitril/valsartan titration and to a less favourable response.3, 6 Circulating concentration and activity of renin, the upstream effector of the renin–angiotensin–aldosterone system (RAAS), are influenced by intravascular volume, blood pressure, and renal function and hold independent prognostic value in patients with chronic and acute HF.7-9 Although plasma renin levels encompass both the extent of neurohormonal activation and most of the clinical determinants of sacubitril/valsartan tolerability, there is currently lack of data about the interaction between circulating renin and response to sacubitril/valsartan therapy.
Aims
We aimed to test the hypothesis that baseline circulating renin levels may predict response to sacubitril/valsartan therapy, as assessed by changes in N-terminal fraction of pro-brain natriuretic peptide (NT-proBNP), in a consecutive cohort of patients with HFrEF.
Methods
From February 2018 to January 2019, consecutive patients with stable HFrEF planned for shifting from an angiotensin-converting enzyme inhibitor (ACEi) or angiotensin receptor blocker (ARB) to sacubitril/valsartan were enrolled in a 6 month, prospective, open-label, real-life cohort study at a single tertiary centre (Fondazione Toscana Gabriele Monasterio, Pisa, Italy). Exclusion criteria were acute coronary syndrome or HF decompensation, coronary artery revascularization or cardiac resynchronization therapy within 3 months before examination, and contraindication to sacubitril/valsartan. After initiation, sacubitril/valsartan was up-titrated according to clinical indication. The study protocol conforms to the 1975 Declaration of Helsinki and was approved by the institution's human research committee. Written informed consent was obtained from each patient. At baseline and at the end of 6 month treatment, patients underwent a complete clinical assessment including echocardiographic examination. NT-proBNP, high-sensitivity troponin T, and a full neurohormonal panel including renin, aldosterone, and norepinephrine were tested at baseline (on the last day of ACEi/ARB therapy) and at 1, 3, and 6 months after the initiation of sacubitril/valsartan. All the assays were performed according to manufacturer's instructions. Renin was measured using a chemiluminescent immunoassay technology (DiaSorin S.r.l., Saluggia, Italy)10; aldosterone was assayed using a radioimmunoassay method (DiaSorin S.r.l.)11; and norepinephrine was assayed with high-performance liquid chromatography technique using the electrochemical detector CLC 100 (Chromsystems GmbH, Munich, Germany).12
Response to sacubitril/valsartan treatment was defined as a ≥30% reduction in NT-proBNP levels from baseline to 6 months.3, 6 Estimated glomerular filtration rate (eGFR) was calculated with MDRD formula. Statistical analysis was performed using IBM SPSS Statistics (IBM Corp. Released 2017. IBM SPSS Statistics for Windows, Version 25.0. Armonk, NY, USA). Normal distribution was assessed through the Kolmogorov–Smirnov test; variables with normal distribution were presented as mean ± standard deviation, while those with non-normal distribution as median and inter-quartile interval. Differences between groups were evaluated through the analysis of variance with Bonferroni correction, paired sample t-test, or the χ2 test, as appropriate. Cox regression analysis was used to test the association with response to sacubitril/valsartan. Two-tailed P values <0.05 were considered as significant.
Results
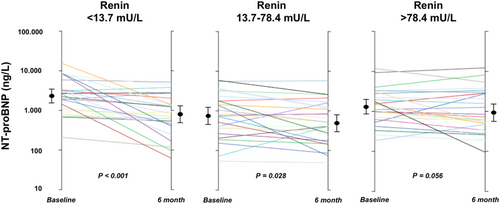
We finally enrolled 80 patients (age 66 ± 10 years, 83% men); baseline characteristics are reported in Table 1. The whole population was divided according to renin tertiles into lower (<13.7 mU/L), middle (13.7–78.4 mU/L), and higher (>78.4 mU/L) tertiles. Patients in the lower renin tertile had higher systolic and diastolic blood pressure, compared with the middle and higher tertiles, as well as higher plasma sodium concentration (all P < 0.05). Although no correlation was observed between LVEF and baseline renin (β = −0.118, P = 0.300), patients in the lower and middle tertiles displayed higher LVEF (both P < 0.05) compared with the higher tertile, as well as a non-significant trend for higher eGFR. No difference could be observed across renin tertiles in terms of background pharmacological therapy, except for a higher use of mineralocorticoid receptor antagonist in the higher tertile. Starting dose of sacubitril/valsartan was also similar.
| Whole population (n = 80) | Lower renin tertile (n = 26) | Middle renin tertile (n = 27) | Higher renin tertile (n = 27) | P for trend | |
|---|---|---|---|---|---|
| Age (years) | 65.8 ± 9.9 | 68.4 ± 8.9 | 65.3 ± 10.9 | 63.9 ± 9.6 | 0.242 |
| Sex (male), n (%) | 66 (83) | 21 (81) | 22 (81) | 23 (85) | 0.899 |
| BMI (kg/m2) | 26.8 ± 4.6 | 27.4 ± 4.6 | 26.4 ± 5.2 | 26.8 ± 4.0 | 0.735 |
| SBP (mmHg) | 120 ± 19 | 129 ± 19 | 123 ± 17 | 111 ± 17 | 0.001 |
| DBP (mmHg) | 73 ± 12 | 77 ± 11 | 73 ± 12 | 69 ± 13 | 0.043 |
| Hypertension, n (%) | 38 (48) | 17 (65) | 17 (37) | 17 (41) | 0.079 |
| Diabetes, n (%) | 20 (25) | 9 (35) | 6 (22) | 5 (19) | 0.377 |
| Ischaemic aetiology, n (%) | 43 (55) | 16 (62) | 13 (48) | 14 (52) | 0.600 |
| Atrial fibrillation, n (%) | 23 (29) | 10 (38) | 8 (30) | 5 (19) | 0.266 |
| NYHA Class III–IV, n (%) | 24 (30) | 9 (35) | 7 (26) | 8 (30) | 0.265 |
| Beta-blocker, n (%) | 78 (98) | 24 (92) | 27 (100) | 27 (100) | 0.100 |
| Beta-blocker dose (%) | 50.0 (25.0–100.0) | 31.3 (25.0–53.1) | 50.0 (25.0–100.0) | 75.0 (50.0–100.0) | 0.008 |
| ACEi/ARBs, n (%) | 73 (91) | 22 (85) | 25 (93) | 26 (96) | 0.311 |
| ACEi/ARBs dose (%) | 50.0 (25.0–96.8) | 50.0 (25.0–90.6) | 50.0 (25.0–100.0) | 37.5 (25.0–50.0) | 0.202 |
| MRA, n (%) | 73 (91) | 20 (77) | 26 (96) | 27 (100) | 0.004 |
| MRA dose (%) | 50.0 (50.0–87.5) | 50.0 (18.8–100.0) | 50.0 (25.0–50.0) | 50.0 (50.0–100.0) | 0.480 |
| Furosemide, n (%) | 55 (69) | 19 (73) | 15 (56) | 21 (78) | 0.184 |
| Furosemide starting dose (mg) | 35 ± 52 | 32 ± 34 | 24 ± 30 | 49 ± 75 | 0.170 |
| Furosemide end dose (mg) | 24 ± 40 | 13 ± 15 | 19 ± 24 | 39 ± 60 | 0.072 |
| Sacubitril/valsartan starting dose, 50/100/200 mg b.i.d., n (%) | 30/34/16 (38/43/20) | 13/11/2 (50/42/8) | 9/13/5 (33/48/19) | 8/10/9 (29/38/33) | 0.159 |
| Sacubitril Sacubitril/valsartan target dose, 0/50/100/200 mg b.i.d., n (%) | 2/24/33/21 (3/30/41/26) | 0/7/11/8 (0/27/42/31) | 0/7/12/8 (0/26/44/30) | 2/10/10/5 (7/37/37/19) | 0.403 |
| Haemoglobin (g/dL) | 13.5 ± 1.5 | 13.4 ± 1.7 | 13.5 ± 1.3 | 13.5 ± 1.6 | 0.968 |
| eGFR (mL/min/1.73 m2) | 68 ± 17 | 70 ± 15 | 70 ± 19 | 63 ± 17 | 0.242 |
| Na (mEq/L) | 138 ± 2 | 140 ± 3 | 138 ± 1 | 137 ± 2 | 0.001 |
| K (mEq/L) | 4.1 ± 0.5 | 4.0 ± 0.4 | 4.1 ± 0.5 | 4.3 ± 0.5 | 0.064 |
| NT-proBNP (ng/L) | 1478 (683–3111) | 2420 (1058–4024) | 789 (367–1783) | 1517 (631–2798) | 0.002 |
| BNP (ng/L) | 233 (122–492) | 324 (139–606) | 177 (99–288) | 305 (184–671) | 0.030 |
| Renin (mU/L) | 42.8 (6.9–121.5) | 4.0 (1.7–6.9) | 41.2 (22.7–54.4) | 298.5 (120.0–500.0) | <0.001 |
| Aldosterone (ng/L) | 92.4 (58.3–136.0) | 74.1 (54.6–109.0) | 76.9 (59.1–143.0) | 110.0 (78.9–165.0) | 0.036 |
| Norepinephrine (ng/L) | 502 (354–777) | 491 (384–821) | 545 (297–794) | 488 (398–751) | 0.721 |
| LVEF (%) | 28 ± 7 | 29 ± 7 | 31 ± 5 | 25 ± 6 | 0.007 |
| LVMI (g/m2) | 136 ± 32 | 145 ± 35 | 127 ± 33 | 136 ± 28 | 0.148 |
| E/E′ | 14.9 ± 8.9 | 16.0 ± 7.3 | 11.6 ± 4.0 | 17.2 ± 12.3 | 0.054 |
| Moderate-to-severe MR, n (%) | 42 (53) | 12 (46) | 14 (52) | 16 (59) | 0.327 |
- ACEi, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; BNP, brain natriuretic peptide; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; LVMI, left ventricular mass index; MR, mitral regurgitation; MRA, mineralocorticoid receptor antagonist; NT-proBNP, N-terminal fraction of pro-brain natriuretic peptide; NYHA, New York Heart Association; SBP, systolic blood pressure.
- Characteristics of the whole study population and of subgroups in the lower (<13.7 mU/L), middle (13.7–78.4 mU/L), and higher (>78.4 mU/L) renin tertiles. Doses of beta-blocker, ACEi/ARBs, and MRA are presented as % of the maximum recommended dose. P values presented in the table refer to between-group comparisons.
At 6 month evaluation, 38 patients (48%) were classified as responders. New York Heart Association class (patients in Class III/IV 11% vs. 30%, P = 0.049) and LVEF (33 ± 8% vs. 28 ± 7%, P < 0.001) improved after 6 months of treatment in the whole population, but no interaction could be detected with renin tertile. Two patients, both in the higher renin tertile, discontinued sacubitril/valsartan because of symptomatic hypotension. Changes in eGFR compared with baseline were similar across renin tertiles. Median per cent reduction in NT-proBNP was −67% (inter-quartile range, IQR −86% to −46%) among responders and 0% (IQR −20% to +32%) among non-responders. Baseline renin levels were lower in the responder group compared with non-responders (19.8 mU/L, IQR 3.7–78.0 mU/L vs. 55.0 mU/L, IQR 16.4–483.1 mU/L; P = 0.004). After sacubitril/valsartan initiation, renin increased in the responder group (P = 0.001, P = 0.001, and P = 0.014 vs. renin levels at 1, 3, and 6 months, respectively), while it remained stable among non-responders (P > 0.05 for all comparisons) (Figure 1).
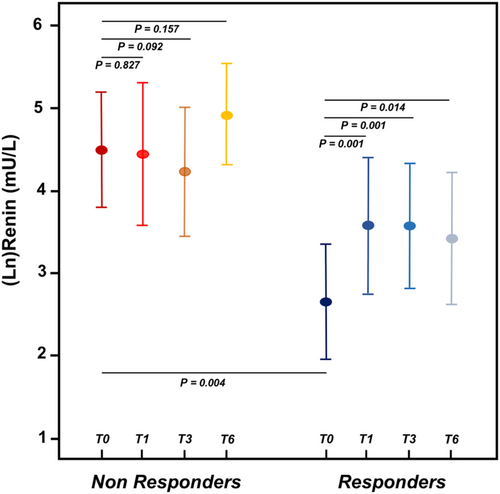
Response to sacubitril/valsartan was observed more frequently among patients in the lower renin tertile compared with the middle and higher tertiles together (17/26, 65% vs. 21/54, 38%; P = 0.033). NT-proBNP decreased from baseline to 6 months to a greater extent in the lower baseline renin tertile compared with the middle and higher tertiles (−41%, IQR −86% to −10%, P < 0.001 vs. −27%, IQR −56% to +4%, P = 0.028 vs. −22%, IQR −49% to +15%, P = 0.056) (Figure 2). At Cox univariate analysis, baseline renin was associated to responder status (odds ratio 0.693, 95% confidence interval 0.534–0.899; P = 0.006). The association between renin and response to sacubitril/valsartan was maintained after adjustment for age, eGFR, and systolic blood pressure (odds ratio 0.676, 95% confidence interval 0.489–0.934; P = 0.018) (Table 2). Renin was independently associated to responder status also when mineralocorticoid receptor antagonist use and beta-blocker dose were included in the multivariable model, while it lost the association when NT-proBNP was added. Similar results were observed when we performed the analysis excluding seven patients who were ACEi/ARB naïve at time of sacubitril/valsartan initiation.
| Variable | OR (95% CI) | P |
|---|---|---|
| Age | 0.998 (0.941–1.058) | 0.945 |
| eGFR | 1.051 (1.014–1.189) | 0.006 |
| Systolic blood pressure | 0.981 (0.953–1.010) | 0.196 |
| Renin | 0.676 (0.489–0.934) | 0.018 |
- CI, confidence interval; eGFR, estimated glomerular filtration rate; OR, odds ratio.
Discussion
Renin–angiotensin–aldosterone system activation is a fundamental player in the neurohormonal imbalance promoting disease progression in HF and constitutes the pathophysiological target of most of the pharmacological strategies improving patients' morbidity and mortality.13, 14 RAAS blockade with ACEi/ARB and mineralocorticoid receptor antagonist is associated to a breakthrough phenomenon, that is, a rebound increase in upstream RAAS effectors15; therefore, a considerable number of patients receiving guideline-recommended treatment for HF displays biohumoral evidence of persistent RAAS activation.16 Further to background therapy, the impairment of kidney function or lower systemic blood pressure, promoting renin release from the juxtaglomerular kidney cells, may represent other clinical correlates of increased renin concentration or activity in modern HF patients.17 Our finding that patients with elevated renin presented with lower systolic and diastolic blood pressure and with slightly worse renal function is further supporting this hypothesis. Moreover, we observed lower sodium concentration in the high renin subset. This is consistent with a stimulatory effect on renin release from the macula densa, sensing a drop in tubular sodium load.18
Our serial biohumoral evaluation has demonstrated an increase in renin levels as early as 1 month after sacubitril/valsartan initiation among responders, while the group of non-responders displayed a persistently high renin along the follow-up. While the expected increase in aldosterone levels, as reported with other RAAS antagonists, is apparently counteracted by neprilysin inhibition achieved by sacubitril/valsartan,19 a renin breakthrough could feature treatment responders. Indeed, while aldosterone levels have been measured serially in a post hoc analysis of the PARADIGM-HF trial,20 circulating renin and its interaction with treatment effectiveness and tolerability have not been tested in a large population of HF patients receiving sacubitril/valsartan. In a pre-specified neurohormonal substudy of the ASTRONAUT (Aliskiren Trial on Acute Heart Failure Outcomes) trial, Vaduganathan and colleagues have recently tested the effects of aliskiren, a direct renin inhibitor, on plasma renin activity and on its prognostic value in hospitalized HF patients. Although renin activity decreased in patients receiving aliskiren, the treatment effects of aliskiren (vs. placebo) on all-cause mortality and the composite of cardiovascular mortality and HF hospitalization did not vary by baseline renin activity.17
Lower blood pressure has been associated to slower and less complete sacubitril/valsartan titration,5 and evidence from community setting is consistently demonstrating that worse renal function is also a constraint to drug up-titration.21, 22 Incomplete dose escalation may be of clinical relevance, as, for example, any dose reduction was associated with a higher risk of major cardiovascular events in the PARADIGM-HF trial.23 Circulating renin concentration encompasses most of the clinical features influencing sacubitril/valsartan tolerability and efficacy. Notably, both patients who discontinued sacubitril/valsartan had renin elevation.
Our findings that renin levels predict response to sacubitril/valsartan need to be consistently confirmed in larger HF populations before they may support the use of renin profiling to tailor up-titration and follow-up strategies in the individual patient.
Acknowledgements
We thank the nurses (Assunta Agazio, CN, and Eleonora Benelli, CN) for collecting the blood samples and for data managing.
Funding
The study has been supported by an unrestricted grant from Roche Diagnostics Italy.
Conflict of interest
None declared.




