Muscle mass, muscle strength, and functional capacity in patients with heart failure of Chagas disease and other aetiologies
Abstract
Aims
Patients with Chagas disease and heart failure (HF) have a poor prognosis similar to that of patients with ischaemic or dilated cardiomyopathy. However, the impact of body composition and muscle strength changes in these aetiologies is still unknown. We aimed to evaluate these parameters across aetiologies in two distinct cohort studies [TESTOsterone-Heart Failure trial (TESTO-HF; Brazil) and Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF; Germany)].
Methods and results
A total of 64 male patients with left ventricular ejection fraction ≤40% were matched for body mass index and New York Heart Association class, including 22 patients with Chagas disease (TESTO-HF; Brazil), and 20 patients with dilated cardiomyopathy and 22 patients with ischaemic heart disease (SICA-HF; Germany). Lean body mass (LBM), appendicular lean mass (ALM), and fat mass were assessed by dual energy X-ray absorptiometry. Sarcopenia was defined as ALM divided by height in metres squared <7.0 kg/m2 (ALM/height2) and handgrip strength cut-off for men according to the European Working Group on Sarcopenia in Older People. All patients performed maximal cardiopulmonary exercise testing. Forearm blood flow (FBF) was measured by venous occlusion plethysmography. Chagasic and ischaemic patients had lower total fat mass (16.3 ± 8.1 vs. 19.3 ± 8.0 vs. 27.6 ± 9.4 kg; P < 0.05) and reduced peak oxygen consumption (VO2) (1.17 ± 0.36 vs. 1.15 ± 0.36 vs. 1.50 ± 0.45 L/min; P < 0.05) than patients with dilated cardiomyopathy, respectively. Chagasic patients showed a trend towards decreased LBM when compared with ischaemic patients (48.3 ± 7.6 vs. 54.2 ± 6.3 kg; P = 0.09). Chagasic patients showed lower handgrip strength (27 ± 8 vs. 37 ± 11 vs. 36 ± 14 kg; P < 0.05) and FBF (1.84 ± 0.54 vs. 2.75 ± 0.76 vs. 3.42 ± 1.21 mL/min/100 mL; P < 0.01) than ischaemic and dilated cardiomyopathy patients, respectively. There was no statistical difference in the distribution of sarcopenia between groups (P = 0.87). In addition, FBF correlated positively with LBM (r = 0.31; P = 0.012), ALM (r = 0.25; P = 0.046), and handgrip strength (r = 0.36; P = 0.004). In a logistic regression model using peak VO2 as the dependent variable, haemoglobin (odds ratio, 1.506; 95% confidence interval, 1.043–2.177; P = 0.029) and ALM (odds ratio, 1.179; 95% confidence interval, 1.011–1.374; P = 0.035) were independent predictors for peak VO2 adjusted by age, left ventricular ejection fraction, New York Heart Association, creatinine, and FBF.
Conclusions
Patients with Chagas disease and HF have decreased fat mass and exhibit reduced peripheral blood flow and impaired muscle strength compared with ischaemic HF patients. In addition, patients with Chagas disease and HF show a tendency to have greater reduction in total LBM, with ALM remaining an independent predictor of reduced functional capacity in these patients. The percentage of patients affected by sarcopenia was equal between groups.
Introduction
Heart failure (HF) is highly prevalent in Western countries, affecting 5.7 million patients in the USA alone and more than 37 million globally.1 The aetiology of HF is multifactorial; guidelines of the European Society of Cardiology2 state that many patients have several different pathologies—cardiovascular and non-cardiovascular—that conspire to cause HF. In general, more than two-thirds of all cases of HF can be attributed to four underlying conditions: ischaemic heart disease, chronic obstructive pulmonary disease, hypertensive heart disease, and rheumatic heart disease.1 Immune-mediated and inflammatory damage, frequently caused by bacteria, spirochaetes, fungi, protozoa, parasites, rickettsiae, and viruses, play a less important role in Western countries. However, parasites like Trypanosoma cruzi have become an extremely important consideration in the differential diagnosis of HF in certain regions of the world. Indeed, the prevalence of the zoonosis Chagas disease among patients with HF has been described to reach 22.7% in endemic regions in Latin America, especially in developing countries such as Brazil.3
Although Chagas disease has been commonly described as a feature of rural and socio-economically deprived communities, the disease has reached an urban and global proportion in recent decades as a result of migration patterns, impacting the epidemiological profile of the disease even in non-endemic regions such as the USA and Europe.4 In light of these alarming facts, it is noteworthy that patients with HF and Chagas disease have shown to have higher rates of hospitalization and mortality as compared with patients with ischaemic and other non-ischaemic causes of HF.3, 5 Despite this, little is known about the metabolic profiles and the co-morbidity burden of patients with HF and Chagas disease. Treatment involves parasite-specific drugs (benznidazole and nifurtimox) during acute infection and times of reactivation and HF-specific drugs and devices. With regard to HF-specific drugs, a scarcity of data is related to the fact that most large-scale HF trials have excluded patients with infectious diseases like Chagas disease. However, beta-blocker therapy has—despite concerns about the appearance of bradyarrhythmias—proven to be safe and efficacious in patients with Chagas disease.6, 7
Some data regarding metabolic and epidemiological features of patients with HF due to Chagas disease have been published. For instance, patients with Chagas disease-associated HF appear to have a lower body mass index (BMI) in comparison with patients with ischaemic or non-ischaemic HF3 and, when only patients with Chagas disease and HF are considered, those who died had lower values of BMI as well.8 Additionally, reduced body weight has also been reported in Chagas disease patients with HF compared with their counterparts with Chagas disease alone.9 Thus, it appears that an overlap is present between Chagas disease and the development/progression of HF that leads to alterations in body weight. Unfortunately, there are no studies assessing body composition features in these patients, neither in a cross-sectional nor longitudinal fashion.
The aim of this study was to evaluate body composition, muscle mass, muscle strength, and functional capacity in patients with Chagas disease enrolled into the Brazilian TESTOsterone-Heart Failure trial (TESTO-HF) and to compare them with data from patients with ischaemic or non-ischaemic HF enrolled into the Studies Investigating Co-morbidities Aggravating Heart Failure (SICA-HF) enrolled in Germany.
Material and methods
Study population
Patients for this analysis were selected from the TESTO-HF trial and the SICA-HF study. Of the total population enrolled in Brazil (169 patients; TESTO-HF) and Germany (327 patients; SICA-HF), 64 male patients were matched by BMI with a tolerance of 2 standard deviation and New York Heart Association (NYHA) functional class II and III. In Germany, patients were enrolled between March 2010 and April 2015 at Charité Medical School in Berlin. In Brazil, patients were enrolled between May 2016 and March 2019 at the Heart Institute of the University of São Paulo Medical School. The inclusion criteria for both studies have been described in detail elsewhere10, 11 (NCT01872299; NCT03463226). For the present analysis, only patients were eligible if they were older than 18 years and had clinical signs and symptoms of chronic HF (NYHA II and III) and a left ventricular ejection fraction (LVEF) ≤40%.
Patients with heart transplantation, muscular dystrophy (i.e. Duchenne muscular dystrophy), stroke, and myocardial infarction with percutaneous coronary intervention or revascularization within at least 6 weeks prior to the study entry were excluded. All patients provided written informed consent before any study-related procedure in both centres. The studies were approved by the local ethics committees in both locations.
Body composition and muscle strength assessment
Dual energy X-ray absorptiometry (DXA) was performed to measure body composition in patients from Brazil (Lunar iDXA; GE Medical Systems Lunar, Madison, WI, USA) and Germany (GE Medical Systems, Madison, WI, USA). An experienced technician in each location performed DXA measurements. The measurements included total and regional fat mass, bone mass, and lean body mass (LBM). Appendicular lean mass (ALM), that is the lean mass of the arms and legs combined, was calculated as the sum of lean mass in the upper and lower limbs. Sarcopenia was defined as ALM divided by height in metres squared <7.0 kg/m2 (ALM/height2) and handgrip strength cut-off for men proposed by the European Working Group on Sarcopenia in Older People.12
Muscle strength was assessed by handgrip dynamometer (Jamar Hydraulic Hand Dynamometer, model J00105 in Brazil and Saehan Corporation Korea Hydraulic Hand Dynamometer, model SH5001 in Germany), and the highest value of three attempts was used.
Cardiopulmonary exercise test
All patients underwent a symptom-limited cardiopulmonary exercise test in both centres, using a ramp protocol. Metabolic parameters, including oxygen consumption (VO2 in mL) and carbon dioxide output (VCO2 in mL), were collected by ventilation gases on a breath-by-breath basis. The test was considered maximal when a respiratory exchange ratio higher than 1.10 was reached.
However, patients in Brazil were assessed on a cycle ergometer while patients in Germany performed the cardiopulmonary exercise test on a treadmill. Moreover, studies have shown a difference of 10–20% in absolute peak VO2 between cycle ergometer and treadmill performance, with the higher values being reported in patients performing treadmill test.13 Thus, to adjust for these differences, we corrected the peak VO2 value using data from the well-designed study by Kim et al., which showed a mean difference of 16% in peak VO2 of patients with HF between these two modalities of exercise, demonstrating a linear increase in peak VO2 with a strong positive correlation (r = 0.92; P < 0.001).14 Other studies have shown similar results between these exercise modalities in patients with HF.15-17
Venous occlusion plethysmography
Forearm blood flow (FBF) was measured by venous occlusion plethysmography. Briefly, this analysis was performed by placing a mercury-filled silastic tube around the forearm with a distance of 5 cm from the humeral-radial joint. The silastic was connected to a transducer that was attached to a plethysmograph. In both locations, the plethysmographs used were manufactured by the same company (Hokanson-AI-6, USA, in Brazil; EC6, Hokanson, Inc., Bellevue, WA, in Germany). The limb was positioned slightly elevated in relation to the heart to enable an adequate venous drainage. Two sphygmomanometer cuffs were place around the wrist and the arm, and the cuff placed on the wrist remained inflated to a suprasystolic pressure throughout the measurement while the upper cuff was inflated and deflated in a 20 s interval fashion characterized by 10 s in each phase.
Laboratory measurements
Blood samples were collected in the morning from an antecubital vein after an overnight fast. Laboratory tests included serum sodium (mEq/L), serum potassium (mEq/L), fasting glucose (mg/dL), creatinine (mg/dL), haemoglobin (g/dL), high-sensitivity C-reactive protein (hs-CRP; mg/L), and urea (mg/dL). B-type natriuretic peptide (BNP; pg/mL) plasma level was measured in Brazilian patients, and N-terminal pro-B-type natriuretic peptide (NT-proBNP; ng/L) was measured in German patients.
Statistical analysis
Descriptive data are presented as mean ± standard deviation, median with percentiles [95% confidence interval (CI)], and frequencies with percentages for categorical variables. One-sample Kolmogorov–Smirnov test was performed to assess the Gaussian distribution of the variables. Serum level of hs-CRP and FBF was not normally distributed, and, thus, Kruskal–Wallis test was used to compare the differences between the three groups (dilated cardiomyopathy, ischaemic, and chagasic patients). Analysis of variance with Scheffe's post hoc analysis was performed for all continuous and parametric variables, whereas the χ2 test was applied to compare categorical variables between groups. Pearson's correlation and logistic regression analyses were used as appropriate. A P-value lower than 0.05 was considered statistically significant in all statistical tests. For statistical analysis, the Statistical Package for the Social Sciences version 23 for Windows (SPSS Inc., Chicago, Illinois, USA) was used.
Results
A sample of 64 male patients with HF was analysed after matching for BMI and NYHA class, 20 of whom had dilated cardiomyopathy, 22 had ischaemic HF (both groups from SICA-HF), and 22 had Chagas disease (from TESTO-HF, Table 1). Fifteen patients were identified to have sarcopenia, of which five patients with dilated cardiomyopathy (ALM/height2 = 6.1 ± 0.4 kg/m2), five ischaemic patients (ALM/height2 = 6.3 ± 0.4 kg/m2), and five chagasic patients (ALM/height2 = 6.3 ± 0.6 kg/m2). There was no statistical difference in the distribution of sarcopenia between groups (P = 0.87).
| Variable | Dilated cardiomyopathy (n = 20) | Ischaemic (n = 22) | Chagasic (n = 22) |
|---|---|---|---|
| Age (years) | 64 ± 14 | 67 ± 9† | 58 ± 6 |
| Weight (kg) | 84.9 ± 18.5 | 77.6 ± 13.2 | 67.8 ± 13.8* |
| Height (m) | 1.71 ± 0.09 | 1.75 ± 0.07† | 1.65 ± 0.06* |
| BMI (kg/m2) | 28.8 ± 5.1 | 25.4 ± 3.9* | 24.7 ± 4.3* |
| NYHA (II/III) | 11/9 | 13/9 | 13/9 |
| LVEF (%) | 31 ± 7 | 24 ± 8* | 28 ± 7 |
| Heart rate (b.p.m.) | 66 ± 11 | 66 ± 11 | 69 ± 9 |
| Sodium (mmol/L) | 141 ± 4 | 141 ± 3 | 139 ± 4 |
| Potassium (mmol/L) | 4.4 ± 0.5 | 4.5 ± 0.5 | 4.5 ± 0.4 |
| Glucose (mg/dL) | 99 ± 17 | 110 ± 48 | 110 ± 17 |
| Creatinine (mg/dL) | 1.26 ± 0.58 | 1.37 ± 0.63 | 1.39 ± 0.45 |
| Haemoglobin (g/dL) | 13.6 ± 2.0 | 13.3 ± 1.7 | 13.6 ± 1.6 |
| hs-CRP (mg/L) | 3.85 (1.06–7.0) | 2.76 (0.95–3.41) | 3.25 (1.59–6.50) |
| Urea (mg/dL) | 57 ± 35 | 59 ± 22 | 60 ± 29 |
| Medication | |||
| Beta-blocker, n (%) | 20 (100) | 21 (95) | 22 (100) |
| ACE-I/ARB, n (%) | 19 (95) | 20 (91) | 22 (100) |
| Diuretics, n (%) | 18 (90) | 17 (77) | 19 (86) |
| Statins, n (%) | 10 (50) | 22 (100)‡ | 10 (45) |
| MRA, n (%) | 12 (60) | 13 (59) | 15 (68) |
| Aspirin, n (%) | 6 (30) | 19 (86)‡ | 3 (14) |
| Calcium channel blocker, n (%) | 2 (10) | 1 (05) | 1 (05) |
| Metformin, n (%) | 4 (20) | 1 (05) | 1 (05) |
- ACE-I, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; hs-CRP, high-sensitivity C-reactive protein; LVEF, left ventricular ejection fraction; MRA, mineralocorticoid receptor antagonist; NYHA, New York Heart Association.
- Data are presented as mean ± SD, median (with lower and upper quartiles), or frequencies and percentages.
- * P < 0.05 vs. dilated cardiomyopathy.
- † P < 0.05 vs. chagasic.
- ‡ P < 0.01 vs. dilated cardiomyopathy and chagasic.
The median value for BNP in patients with Chagas disease was 758 pg/mL (95% CI, 188–1007 pg/mL). The median values for NT-proBNP in dilated cardiomyopathy and ischaemic patients were 976 ng/L (95% CI, 169–3217) and 1354 ng/L (95% CI, 880–3775), respectively. Baseline characteristics are shown in Table 1. Ischaemic patients were older than patients with Chagas disease (67 ± 9 vs. 58 ± 6 years; P = 0.01). Chagasic patients had lower height in comparison with patients with dilated cardiomyopathy and ischaemic HF (1.65 ± 0.06 vs. 1.71 ± 0.09 vs. 1.75 ± 0.07 m, respectively; P < 0.05). Body weight was lower in patients with Chagas disease than in patients with dilated cardiomyopathy (67.8 ± 13.8 vs. 84.9 ± 18.5 kg; P = 0.003), whereas BMI was higher in patients with dilated cardiomyopathy compared with ischaemic and chagasic patients (28.8 ± 5.1 vs. 25.4 ± 3.9 vs. 24.7 ± 4.3 kg/m2, respectively; P = 0.049). LVEF was reduced in ischaemic patients in comparison with dilated cardiomyopathy patients (24 ± 8 vs. 31 ± 7; P = 0.013), whereas there was no difference in LVEF between patients with Chagas disease and the other aetiologies. There was no difference with regard to NYHA class, resting heart rate, serum levels of sodium, potassium, glucose, creatinine, hs-CRP, urea, or haemoglobin between the three groups (Table 1).
Not surprisingly, use of statins and aspirin was higher in ischaemic patients when compared with Chagas disease and dilated cardiomyopathy patients (P = 0.001 and P = 0.0002, respectively), while there was no difference in the use of other medications (Table 1).
Body composition, functional capacity, and handgrip strength
Body composition, functional capacity, and handgrip strength parameters are described in Table 2. Patients with dilated cardiomyopathy presented with higher fat mass in arms, legs, trunk, and total in comparison with those patients with ischaemic HF and Chagas disease (all P < 0.05). Moreover, trunk lean mass was lower in patients with Chagas disease than in ischaemic HF patients (23.9 ± 3.9 vs. 27.4 ± 3.6 kg; P = 0.046), whereas total LBM showed a trend to be lower in chagasic patients when compared with ischaemic HF patients (48.3 ± 7.6 vs. 54.2 ± 6.3 kg; P = 0.09). In addition, bone mineral content in legs and trunk was lower in patients with Chagas disease compared with ischaemic (P = 0.02 and P = 0.02, respectively) and dilated cardiomyopathy patients (P = 0.02 and P = 0.04, respectively). Total bone mineral content was also reduced in patients with Chagas disease in comparison with ischaemic HF patients (2.6 ± 0.6 vs. 3.0 ± 0.4 kg; P = 0.045), and there was a tendency for a similar effect also when compared with dilated cardiomyopathy patients (P = 0.09).
| Variable | Dilated cardiomyopathy (n = 20) | Ischaemic (n = 22) | Chagasic (n = 22) |
|---|---|---|---|
| Fat mass (kg) | |||
| Arms | 2.3 ± 0.9 | 1.6 ± 0.7* | 1.7 ± 1.0* |
| Legs | 8.2 ± 3.1 | 5.5 ± 2.2* | 5.5 ± 3.7* |
| Trunk | 16.3 ± 6.5 | 11.4 ± 5.3* | 9.2 ± 5.8* |
| Total | 27.6 ± 9.4 | 19.3 ± 8.0* | 16.3 ± 8.1* |
| Lean mass (kg) | |||
| Arms | 5.8 ± 1.6 | 5.9 ± 1.0 | 5.8 ± 1.0 |
| Legs | 17.4 ± 3.5 | 16.8 ± 2.6 | 16.0 ± 3.1 |
| Trunk | 26.9 ± 6.5 | 27.4 ± 3.6† | 23.9 ± 3.9 |
| Total | 54.0 ± 11.6 | 54.2 ± 6.3(†) | 48.3 ± 7.6 |
| Bone mass (kg) | |||
| Arms | 0.4 ± 0.1 | 0.4 ± 0.1 | 0.4 ± 0.1 |
| Legs | 1.2 ± 0.3† | 1.2 ± 0.2† | 1.0 ± 0.2 |
| Trunk | 0.9 ± 0.2† | 0.9 ± 0.2† | 0.7 ± 0.2 |
| Total | 2.9 ± 0.6(†) | 3.0 ± 0.4† | 2.6 ± 0.6 |
| Peak VO2 (L/min) | 1.50 ± 0.45 | 1.15 ± 0.36* | 1.17 ± 0.36* |
| Peak VO2 (mL/kg/min) | 17.5 ± 4.5 | 15.4 ± 6.0 | 17.5 ± 4.7 |
| Handgrip strength (kg) | 36 ± 14† | 37 ± 11† | 27 ± 8 |
- VO2, oxygen consumption.
- Data are presented as mean ± SD.
- * P < 0.05 vs. dilated cardiomyopathy.
- † P < 0.05 vs. chagasic. Symbols in brackets denote a trend with P < 0.10.
Absolute peak VO2 was lower in chagasic and ischaemic HF patients when compared with patients with dilated cardiomyopathy (P = 0.03 and P = 0.02, respectively), while there was no difference for relative peak VO2 among the three groups (Table 2). In addition, handgrip strength was reduced in patients with Chagas disease as compared with those with dilated cardiomyopathy (P = 0.03) and ischaemic heart disease (P = 0.02).
Pearson's correlations and logistic regression
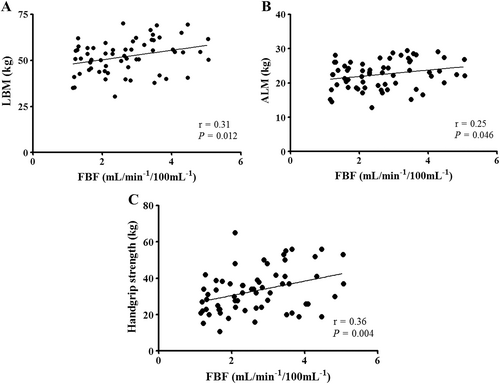
Forearm blood flow was reduced in patients with Chagas disease when compared with patients with ischaemic HF and dilated cardiomyopathy [1.68 (95% CI, 1.37–2.11) vs. 2.75 (95% CI, 2.19–3.25) vs. 3.58 (95% CI, 2.66–4.42) mL/min/100 mL, respectively; P < 0.01; Figure 1]. On the other hand, there was a trend towards higher FBF in patients with dilated cardiomyopathy than in ischaemic HF patients (P = 0.056; Figure 1). In addition, FBF was positively correlated with LBM (r = 0.31; P = 0.012; Figure 2), ALM (r = 0.25; P = 0.046; Figure 2), and handgrip strength (r = 0.36; P = 0.004; Figure 2).

In a univariable logistic regression analysis using absolute peak VO2 (median value >1.15 L/min) as the dependent variable, haemoglobin [odds ratio (OR), 1.598; 95% CI, 1.124–2.273; P = 0.009], ALM (OR, 1.222; 95% CI, 1.059–1.410; P = 0.006), and FBF <2.61 (OR, 0.360; 95% CI, 0.131–0.991; P = 0.048) were associated with higher absolute peak VO2. Furthermore, in a multivariable logistic regression analysis using absolute peak VO2 (median value >1.15 L/min) as the dependent variable, haemoglobin (OR, 1.506; 95% CI, 1.043–2.177; P = 0.029) and ALM (OR, 1.179; 95% CI, 1.011–1.374; P = 0.035) were independently associated with absolute peak VO2 adjusted for age, NYHA, creatinine, LVEF, and FBF (Table 3).
| Variable | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | |
| Age (per year increase) | 0.976 | 0.931–1.023 | 0.307 | |||
| NYHA (per class increase) | 2.493 | 0.899–6.916 | 0.079 | |||
| Creatinine (per 1 mg/dL increase) | 0.710 | 0.284–1.770 | 0.462 | |||
| Haemoglobin (per 1 g/dL increase) | 1.598 | 1.124–2.273 | 0.009 | 1.506 | 1.043–2.177 | 0.029 |
| LVEF (per 1% increase) | 1.053 | 0.984–1.127 | 0.136 | |||
| ALM (per 500 g increase) | 1.222 | 1.059–1.410 | 0.006 | 1.179 | 1.011–1.374 | 0.035 |
| FBF <2.61 mL/min/100 mL (present) | 0.360 | 0.131–0.991 | 0.048 | 0.472 | 0.153–1.459 | 0.192 |
- ALM, appendicular lean mass; CI, confidence interval; FBF, forearm blood flow; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; OR, odds ratio.
Discussion
Our study demonstrates a large overlap in clinical findings among patients with HF due to ischaemic heart disease, idiopathic dilated cardiomyopathy, and Chagas disease. Metabolic patterns, however, differ in patients with Chagas disease who present with lower LBM, bone mineral content, lower peak VO2, lower handgrip strength, and lower FBF. Predictors of peak VO2 include haemoglobin and ALM. The distribution of overt skeletal muscle wasting (sarcopenia) was similar in the three groups.
Chagas disease has been described as a public health issue in endemic countries in Latin America, and migration is thought to cause spreading of the disease outside of its original geographic boundaries leading to a global concern.18, 19 The clinical features of patients with Chagas disease and HF resemble those of patients with ischaemic heart disease and idiopathic dilated cardiomyopathy,20 making the diagnosis and clinical management of these patients more complicated.21 In addition, patients with Chagas disease and HF have been commonly reported to be younger than their counterparts with HF from other aetiologies,5, 22 and such difference in age was also found in our sample.
Additionally, our study is the first to show reductions in compartmental and total bone mineral content in patients with Chagas disease compared with dilated cardiomyopathy and ischaemic HF patients. Moreover, fat mass was reduced in chagasic and ischaemic patients, which may be a sign of an initial state of catabolism in these patients, because loss of fat mass preceding muscle wasting has been shown to be a cachexia phenotype in other populations.23
Although total LBM showed a tendency to be reduced in patients with Chagas disease compared with ischaemic HF patients, these results may be due to the small sample size in our study. Moreover, taking the mean age difference between these groups into consideration, we can also imply that reductions in total LBM may occur in these patients in 10 years of time, because of Chagas disease progression associated with HF and the aging process itself. Interestingly, in an experimental model of animals infected with T. cruzi, the presence of the parasite in the skeletal muscle produced acute and chronic mitochondrial dysfunction that led to permanent skeletal muscle alterations.24 In addition, chagasic patients with cardiomyopathy have shown to have an impairment in oxygen delivery due to abnormal muscle microvasculature.25
Reduced physical functioning and quality of life evaluated by questionnaires have been documented in patients with Chagas disease and HF after only 1 year of follow-up, while pharmacological care improved parameters related to quality of life in these patients.26 In our sample, we found that handgrip strength, a predictor of worse outcomes in patients with HF,27 was reduced in chagasic patients compared with both ischaemic and dilated cardiomyopathy patients. In addition, the impairment of muscle strength has been shown to affect not only appendicular skeletal muscle but also respiratory muscle in patients with Chagas disease and HF,9 suggesting that respiratory muscle weakness may influence ventilatory parameters contributing to reduce peak VO2 as we have demonstrated in chagasic patients in our study.28
Another factor that may lead to diminished functional capacity and reduced LBM is endothelial dysfunction,29 which was shown to be present in Chagas disease patients without HF30 and also in patients with HF regardless of aetiology.31 In our study, patients with Chagas disease and HF showed a reduction in FBF compared with the other two aetiologies (Figure 1), and this decrease in peripheral blood flow was positively correlated with muscle strength, LBM, and ALM (Figure 2).
Furthermore, sympathetic nerve activity, which also controls endothelial function, has been shown to be a hallmark in patients with HF and is also associated with impaired VO2 during exercise in these patients.32 Some studies have shown that patients with Chagas disease present with increased sympathetic outflow33, 34 just like patients with ischaemic HF compared with those with non-ischaemic disease.35 However, other groups have failed to find a difference in sympathetic activation across different aetiologies in patients with HF including Chagas disease.36, 37 Taken together, the pronounced reduction in FBF seen in chagasic patients with HF in our study may be due to the combination of Chagas disease and HF with the involvement of peripheral mechanisms but may also be due to an interplay between sympathetic activation and the immune response of these patients.38
In addition, we found that haemoglobin and ALM were independent predictors of reduced absolute peak VO2. Regardless of HF aetiology, anaemia has been associated with worsening HF39 just like low ALM.40 In univariable and multivariable logistic regression analysis for each group separately, only ALM in patients with dilated cardiomyopathy was independently associated with absolute peak VO2 (median value), implying that the association seen in our results was driven by a higher ALM of these patients (Table 3).
Therapies used in the treatment of HF in patients with Chagas disease include standard HF medications, but also the anti-trypanosomal drugs benznidazole and nifurtimox. These drugs, however, seem to be of limited value in the indeterminate phase of the infection, because clinical trials with these pharmacological agents have shown limited tolerability without significant positive effects on clinical outcomes in patients with Chagas cardiomyopathy.41, 42 In addition, administration of nifurtimox has been associated with side effects that might include anorexia, digestive disorders, and weight loss, but none of the chagasic patients in our study were or had taken any anti-trypanosomal medication for Chagas disease. Additionally, the statistical differences in statin and aspirin use found in our study seem to be founded in the aetiology of HF, once ischaemic patients are more likely to take medication that attenuates ischaemia by improving the progression of atherosclerosis.
Furthermore, exercise training is an important complementary therapy in the treatment of patients with HF so is it for patients with Chagas disease. Cardiovascular rehabilitation programmes have shown to improve cardiac function and respiratory muscle strength in patients with Chagas disease and HF.43 In addition, in an experimental study in rats that performed aerobic exercise training for 9 weeks before inoculation of T. cruzi, exercise training has shown to reduce exposure time to the parasite infection and attenuate cardiac parasitism by diminishing collagen aggregation in cardiac structures, while sedentary animals presented a more severe progression of Chagas disease.44 Moreover, exercise training seems to enhance the host defence and improve immunological and inflammatory markers that attenuate skeletal myositis in these patients.45
Limitations
We have to acknowledge that our study has limitations. First, only male patients with LVEF ≤40% were included, so these findings may be not applied to female patients and to patients with HF with preserved ejection fraction. Second, although we tried to homogenize the sample, the number of patients that were included in the matching analysis was limited, making the sample size small. Third, although both trials have been prospectively conducted, follow-up was not analysed in our study to determine if these body composition and muscle strength alterations would lead to worse outcomes in a long term. Finally, we cannot exclude that there may be differences in the correction of peak VO2 between cycle ergometer and treadmill.
Conclusions
Patients with Chagas disease and HF have decreased fat mass and exhibit reduced peripheral blood flow and impaired muscle strength compared with ischaemic HF patients. In addition, patients with Chagas disease and HF show a tendency to have greater reduction in total LBM, with ALM remaining an independent predictor of reduced functional capacity in these patients. The percentage of patients affected by sarcopenia was equal between groups.
Acknowledgement
Open access funding enabled and organized by Projekt DEAL. [Correction added on 11 November 2020, after first online publication: Projekt Deal funding statement has been added.]
Conflict of interest
None declared.
Funding
This study was financed in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES) - Finance Code 001. G.W.P.D.F. was supported by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; 148758/2016-9); M.R.S. by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP; 2016/24306-0); and C.E.N. by CNPq (303573/2015-5) and FAPESP (2015/22814-5). S.v.H. has been a paid consultant and/or received fees for lectures from Bayer, Boehringer Ingelheim, BRAHMS/Thermo Fisher, Chugai Pharma, Grünenthal, Helsinn, Novartis, Pharmacosmos, Respicardia, Roche, Servier, and Vifor. His research lab is supported by the German Center for Cardiovascular Research (DZHK), the Innovative Medicines Initiative – Joint Undertaking (IMI–JU 115621), and Boehringer Ingelheim and Amgen.




