Early rehabilitation after stroke: relationship between the heart rate variability and functional outcome
Abstract
Aims
Impaired autonomic nervous system regulation is frequently observed in patients with stroke. The aim of this prospective study was to evaluate the impact of cardiac autonomic tone on functional outcome after the early post-stroke rehabilitation.
Methods and results
One hundred and three consecutive patients (67 ± 11 years, body mass index (BMI) 27.1 ± 5.4 kg/m2, 64% men) with ischaemic (84% of patients) and haemorrhagic stroke were studied. Depressed heart rate variability (HRV), as a surrogate marker of increased sympathetic tone, was defined by the standard deviation of NN intervals < 100 ms and HRV triangular index ≤ 20 assessed from a 24 h Holter electrocardiogram at admission to rehabilitation (23 ± 16 days after stroke). Twenty-two per cent of patients had depressed HRV at baseline and were comparable with patients with normal HRV with regard to their functional [Barthel Index (BI), modified Rankin Scale (mRS), and Rivermead Motor Assessment (RMA)] and biochemical status. After a 4-week follow-up, 70% of patients with depressed HRV showed a cumulative functional disability, defined by mRS ≥ 4, BI ≤ 70, and RMA ≤ 5, in contrast to patients with normal HRV (35%, P = 0.003). Patients with depressed HRV showed a worse functional status by BI (−16%, P < 0.001), RMA (−12%, P < 0.05), and mRS (+16%, P < 0.01), compared with patients with normal HRV. Cumulative functional disability was associated with depressed HRV (odds ratio 4.25, 95% confidence interval 1.56–11.54, P < 0.005) after adjustment for age, sex, and body mass index (odds ratio 4.6, 95% confidence interval 1.42–14.97, P < 0.05).
Conclusions
The presence of autonomic cardiovascular dysregulation in patients with subacute stroke was associated with adverse functional outcome after the early post-stroke rehabilitation.
Introduction
Stroke is a leading cause of disability in the adult age worldwide.1 Early post-stroke rehabilitation plays an important role in recovery after stroke. A wide range of medical complications, including autonomic imbalance, characterized by decreased vagal modulation and increased sympathetic activation may influence the efficacy of rehabilitation efforts.2, 3 There is an evidence of cardiac dysfunction after clinical and experimental stroke.4-6 A cardiac autonomic dysregulation, manifested by an impaired control of blood pressure and heart rate leading to cerebral hypoperfusion and secondary brain injury, may result in the increased susceptibility for post-stroke complications and contribute to an unfavourable functional outcome.5, 7, 8 Previous studies have described the development of autonomic imbalance in stroke patients using the analysis of heart rate variability (HRV).9
The presence of abnormal HRV in patients with stroke has been associated with adverse neurological outcome, post-stroke disability, and all-cause mortality.10-12 In addition, the adverse impact of autonomic dysfunction, manifested by impaired HRV, on the progression of ventricular remodeling13 and cardiovascular mortality has been reported in chronic heart failure (HF) and myocardial infarction,14 and targeting this parameter by various therapeutic interventions has shown to improve outcomes in these patients.15-17 Furthermore, the neuromodulation therapy, including vagus nerve stimulation, spinal cord stimulation, and baroreflex activation therapy, has recently received an interest to restore sympathovagal balance in patients with chronic HF.18
The effect of cardiovascular autonomic dysfunction on the clinical outcome of stroke patients after administration of the neurological rehabilitation has not been sufficiently investigated. The aim of this prospective study was to evaluate the impact of HRV on the functional outcome of patients with subacute stroke undergoing a hospitalized post-stroke rehabilitation programme.
Methods
Patient population
One hundred and forty-six patients with the diagnosis of acute ischaemic and haemorrhagic stroke admitted to the neurological rehabilitation centre Brandenburgklinik Bernau, Germany from April 2010 to August 2013 were consecutively enrolled into this prospective observational study. Forty-three (29%) patients with permanent atrial fibrillation were preliminary excluded from the analysis. The study patients underwent an individually adjusted early post-stroke rehabilitation programme according to the national standards for rehabilitation procedures after stroke. During the hospitalized rehabilitation, all patients were on individually adjusted standard medical therapy, including anticoagulants, antiplatelet drugs, beta-blockers, Ca channel antagonists, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, diuretics, and statins. Exclusion criteria were the presence of acute and chronic inflammatory disease, acute HF, myocardial infarction, liver cirrhosis, acute renal failure, immunosuppressive therapy, and a history of cancer within the past 5 years. The local ethics committee approved the study protocol, and all participants gave a written informed consent.
Determination of heart rate variability
Baseline two-channel 24 h Holter electrocardiogram (ECG) recordings (CardioDay®, Getemed, Teltow, Germany) were performed at admission to the rehabilitation centre. The HRV was determined using the parameters of time-domain, standard deviation of NN intervals (SDNN), and HRV triangular index. The presence of cardiac autonomic dysfunction in patients with stroke was revealed by depressed HRV,19, 20 which was defined by SDNN < 100 ms and HRV triangular index ≤ 20 according to the Guidelines of the European Society of Cardiology (ESC) and the North American Society of Pacing and Electrophysiology.21
Assessment of the neurological status and functional capacity
The evaluation of neurological status and functional examinations was performed at admission (baseline) and at discharge from the rehabilitation centre after a 4-week follow-up (FU).
Determination of the cumulative functional disability (CFD) included the assessment of functional status by three neurological scales.
The Barthel Index (BI) contains 10 basic activities of daily living related to self-care and mobility with scores ranging from ‘0’ to ‘100’, where the lower scores indicate greater dependency.22
The modified Rankin Scale (mRS) measures physical independence by the assessment of body function, activity, and participation in daily tasks on a scale ranging from ‘0’ (no symptoms) to ‘5’ (bedridden and fully dependent).
The Rivermead Motor Assessment (RMA) gross function subscale consists of scores from ‘1’ to ‘13’, which correspond to a range of physical activities increasing in complexity from ‘turning over in bed’ to ‘hop on the affected leg 5 times’.23
Cumulative functional disability was present, if BI ≤ 70, mRS ≥ 4, and RMA ≤ 5.
Short physical performance battery and handgrip strength
Short physical performance battery (SPPB) included examinations of standing ability with both feet together in the side-by-side, semi-tandem, and tandem positions, time of walking 3 m, and time to rise 5 times from the chair and return to the seated position. The score ranges from 0 (not attended) to 12 points (completed).24 The gait speed of the study patients was determined from the time of walking 3 m.
Isometric muscle strength of the hand was assessed by the handgrip strength test using a handgrip dynamometer (Saehan Corporation, Korea). The highest of three measurements was used for the analysis.
Quality of life and nutritional status
The quality of life was assessed by the European Quality of Life–Five Dimensions (EQ-5D) visual analogue scale, which ranges from ‘0’ to ‘100’, with a higher score indicating better health.25
Appetite was assessed by the visual analogue scale ranging from ‘0’ (no appetite) to ‘10’ (very good appetite).26 The body mass index (BMI) was calculated as a ratio of body weight to height squared (kg/m2).
Blood samples
Venous blood samples were obtained under standardized conditions after overnight fasting. Standard biochemical parameters were determined in a routine laboratory setting.
Statistical analysis
All data were presented as mean ± standard deviation, median [interquartile range (IQR)], or percentage. All variables were tested for normal distribution using the Kolmogorov–Smirnov test. Non-normally distributed data were log-transformed to achieve a normal distribution where indicated. Statistical comparisons were made using unpaired Student's t-tests, analysis of covariance adjusted for baseline, or Mann–Whitney U-test as appropriate. χ2 test was used to assess categorical distribution between the study groups. Logistic regression analyses were used as appropriate. A value of P < 0.05 was considered statistically significant. Statistical analyses were performed with the StatView 5.0 software package (SAS Institute Inc., Cary, NC) and the GraphPad Prism 6.0 software.
Results
Baseline clinical characteristics of the study patients upon admission to the rehabilitation centre (23 ± 17 days after stroke) are presented in Table 1. The mean stroke severity according to the mRS was 3.7 ± 0.8, whereas no differences were observed between patients with ischaemic (81% of patients) and haemorrhagic stroke. Sixteen per cent of all patients (2 women and 14 men) had chronic HF (Table 1).
| Parameter | All patients (N = 103) | Patients with normal HRV (N = 80) | Patients with depressed HRV (N = 23) | P value |
|---|---|---|---|---|
| Age, years | 67 ± 11 | 67 ± 11 | 69 ± 13 | 0.7 |
| Body mass index, kg/m2 | 27.1 ± 5.4 | 26.8 ± 5.3 | 27.9 ± 5.9 | 0.4 |
| Male sex n, % | 88/60 | 73/62 | 15/52 | 0.3 |
| Systolic blood pressure, mmHg | 129 ± 15 | 130 ± 15 | 127 ± 18 | 0.5 |
| Diastolic blood pressure, mmHg | 78 ± 11 | 79 ± 10 | 76 ± 13 | 0.2 |
| Mean blood pressure, mmHg | 95 ± 11 | 96 ± 10 | 93 ± 13 | 0.2 |
| Heart rate, b.p.m. | 72 ± 11 | 69 ± 10 | 80 ± 12 | <0.0001 |
| Stroke severity | ||||
| Time after stroke, days | 23 ± 17 | 22 ± 15 | 26 ± 24 | 0.4 |
| Duration of rehabilitation, days | 26 ± 6 | 27 ± 7 | 26 ± 5 | 0.6 |
| Ischaemic stroke, n (%) | 83 (81) | 65 (81) | 18 (78) | 0.2 |
| Cumulative functional disability, n (%) | 54 (52) | 40 (50) | 14 (61) | 0.4 |
| Barthel Index | 57 ± 23 | 58 ± 22 | 53 ± 26 | 0.3 |
| Modified Rankin Scale | 3.7 ± 0.8 | 3.6 ± 0.8 | 3.9 ± 0.7 | 0.2 |
| Rivermead Motor Assessment | 5.4 ± 2.3 | 5.5 ± 2.3 | 5.1 ± 1.9 | 0.5 |
| Caregiver status | ||||
| Independent at home, n (%) | 70 (69) | 56 (71) | 14 (64) | 0.4 |
| With help at home, n (%) | 30 (30) | 22 (28) | 8 (36) | 0.5 |
| Nursing home, n (%) | 1 (1) | 1 (1) | — | |
| Quality of life, EQ-5D | 49 ± 18 | 51 ± 17 | 48 ± 20 | 0.5 |
| Appetite according to VAS | 6.7 ± 2.0 | 6.8 ± 2.1 | 6.2 ± 1.9 | 0.2 |
| Short physical performance battery | 2.1 ± 3.3 | 2.2. ± 3.4 | 1.8 ± 3.1 | 0.5 |
| Co-morbidities | ||||
| Diabetes mellitus, n (%) | 58 (60) | 48 (41) | 10 (35) | 0.5 |
| Arterial hypertension, n (%) | 91(88) | 71 (89) | 20 (87) | 0.8 |
| Dyslipidaemia, n (%) | 64 (67) | 49 (65) | 15 (71) | 0.7 |
| Coronary artery disease, n (%) | 32 (31) | 24 (30) | 8 (35) | 0.7 |
| Chronic heart failure, n (%) | 16 (16) | 12 (15) | 4 (17) | 0.9 |
| Medication | ||||
| Anticoagulants, n (%) | 73 (71) | 57 (71) | 16 (70) | 0.9 |
| Antiplatelet drugs, % (n) | 70 (67) | 53 (66) | 17 (74) | 0.5 |
| β1-selective blocker, % (n) | 52 (51) | 40 (50) | 12 (52) | 0.9 |
| β1-blocker equivalence dosea | 4.3 ± 2.4 | 4.6 ± 2.6 | 3.3 ± 0.1 | 0.1 |
| Ca channel antagonists, % (n) | 34 (33) | 26 (32) | 8 (35) | 0.8 |
| ACE-I, % (n) | 62 (60) | 46 (58) | 16 (70) | 0.3 |
| Angiotensin receptor blockers, % (n) | 16 (16) | 14 (18) | 2 (9) | 0.3 |
| Diuretics, % (n) | 44 (42) | 32 (40) | 12 (52) | 0.3 |
| Statins, % (n) | 74 (72) | 57 (71) | 17 (74) | 0.8 |
| Biochemistry | ||||
| C-reactive protein, mg/L [IQR] | 5.2 [1.7–8.3] | 5.3 [1.8–8.4] | 4.4 [1.3–8.2] | 0.5 |
| Haemoglobin, mmol/L | 8.2 ± 1.0 | 8.3 ± 1.0 | 8.1 ± 1.0 | 0.2 |
| Creatinine, mg/dL | 1.0 ± 0.8 | 1.1 ± 0.7 | 0.8 ± 0.4 | 0.2 |
- ACE-I, angiotensin-converting enzyme inhibitors; EQ-5D, European Quality of Life–Five Dimensions visual analogue scale; HRV, heart rate variability; IQR, interquartile range; VAS, visual analogue scale.
- a β1-blocker equivalence dose ‘1’ correspond to 23.75 mg metoprolol or 1.25 mg bisoprolol or 1.25 mg nebivilol.
The analyses of 24-hour Holter ECG data revealed 23 (22%) patients with depressed HRV upon admission to the rehabilitation. On the basis of the values of HRV, the entire cohort was divided into two subgroups: patients with normal HRV and patients with depressed HRV. There were no significant differences between the study groups with regard to their baseline demographic, biochemical, and clinical parameters, except for heart rate, physical performance, and the prevalence of co-morbidities (Table 1).
Functional outcome after a 4-week follow-up
The improvement of physical and functional status was observed in all study patients after the hospitalized rehabilitation as manifested by an increase in the BI score by 28%, mRS by 14%, and RMA by 25% (Figure 1A, C, and E).
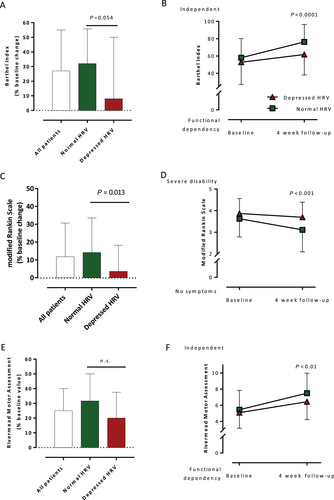
After adjustment for baseline, the functional outcome improved more significantly in patients with normal HRV compared with patients with depressed HRV. This improvement was observed by an increase in the BI score (median change 32% vs. 8%, P = 0.054), mRS (mean change 14% vs. 4%, P = 0.013), and RMA (median change 32% vs. 20%, not significant) in the two groups, respectively (Figure 1A, C, and E). Therefore, patients with depressed HRV remained with a worse physical status compared with patients with normal HRV according to the BI score (62 ± 24 vs. 76 ± 20, P < 0.0001), mRS (3.1 ± 1.0 vs. 3.7 ± 0.7, P < 0.001), and RMA (7.5 ± 2.5 vs. 6.4 ± 2.2, P < 0.01, all respectively) (Figure 1B, D, and F).
Short physical performance battery and handgrip strength
The number of patients who participated in the SPPB after a 4-week FU approximately doubled in the entire cohort compared with baseline (43% vs. 22%, P = 0.001). A significant improvement in the SPPB score was observed in patients with normal HRV in contrast to patients with depressed HRV (Figure 2). No difference in gait speed was observed between the two subgroups after 4 week FU (Figure 2). Although the number of patients who participated in a 3-meter walk test quadrupled compared with baseline, only 31 patients (30%) were able to perform the gait test.
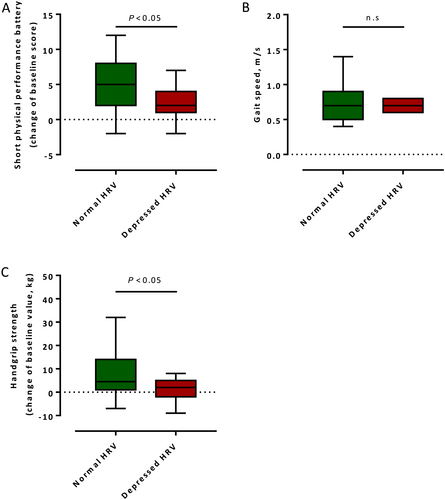
Handgrip strength improved more significantly in patients with normal HRV compared to patients with depressed HRV after a 4-week FU [median change 12.9 (IQR 4.7–30.2) kg vs. 7.1 (IQR −3.8 to 19.2) kg, P < 0.05, respectively] (Figure 2).
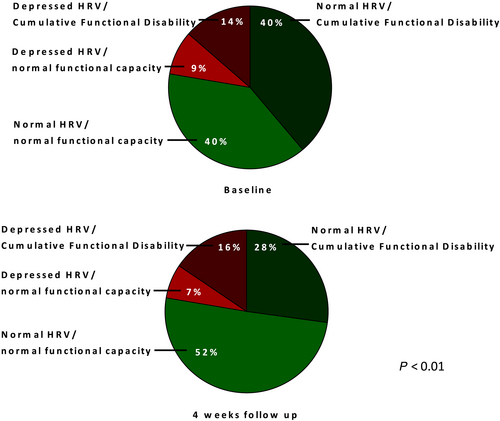
The cumulative functional disability
The CFD reduced from 54% of patients at baseline to 44% of patients after a 4-week FU (P < 0.01). However, a strong trend in the decrease of CFD prevalence was observed only in patients with normal HRV (40% vs. 28%, P = 0.05) (Figure 3).
After a 4-week FU, the CFD was associated with depressed HRV [odds ratio (OR) 4.25, 95% confidence interval (CI) 1.56–11.54, P < 0.005] after adjustment for age, sex, and BMI (OR 4.6, 95% CI 1.42–14.97, P < 0.05). Other baseline factors associated with CFD were age >65 years (OR 2.83, 95% CI 1.24–6.46, P < 0.05), BMI (OR 1.12, 95% CI 1.03–1.21, P < 0.01), the level of haemoglobin (OR 0.61, 95% CI 0.4–0.94, P < 0.05), and quality of life assessed by the European Quality of Life–Five Dimensions visual analogue scale (OR 0.96, 95% CI 0.94–0.99, P < 0.01) (Table 2). No significant associations of CFD with chronic HF (OR 0.77, 95% CI 0.26–3.32, P = 0.7) (Table 2) or other co-morbidities, except for a trend in association with diabetes mellitus (OR 2.08, 95% CI 0.925–4.68, P = 0.08), were observed.
| Parameter | OR [95% CI] | P value | OR [95% CI] | P value |
|---|---|---|---|---|
| Depressed HRV | 4.25 [1.56–11.54] | <0.005 | 4.6 [1.42–14.97] | <0.05 |
| Age >65 years | 2.83 [1.24–6.46] | <0.05 | 3.12 [1.16–8.41] | <0.05 |
| Ischaemic stroke | 2.53 [0.75–8.51] | 0.1 | ||
| Diabetes mellitus | 2.08 [0.925–4.68] | 0.08 | 1.4 [0.51–3.85] | 0.5 |
| Dyslipidaemia | 2.07 [0.85–5.05] | 0.1 | ||
| C-reactive protein, per log (mg/L) | 2.04 [0.98–4.25] | 0.6 | ||
| Arterial hypertension | 1.57 [0.44–5.58] | 0.5 | ||
| β1-selective blocker | 1.34 [0.61–2.97] | 0.5 | ||
| Body mass index, kg/m2 | 1.12 [1.03–1.21] | 0.008 | 1.12 [1.02–1.24] | <0.05 |
| Coronary artery disease | 0.88 [0.38–2.06] | 0.8 | ||
| Age, per year | 1.05 [1.03–1.08] | <0.05 | ||
| Heart rate, per 10 beats | 1.07 [0.75–1.52] | 0.7 | ||
| Quality of life, EQ-5D | 0.96 [0.94–0.99] | 0.004 | 0.96 [0.93–0.99] | <0.01 |
| Duration of rehabilitation (days) | 0.95 [0.89–1.02] | 0.2 | ||
| Appetite according to VAS | 0.90 [0.74–1.1] | 0.3 | ||
| Chronic heart failure | 0.77 [0.26–3.32] | 0.7 | ||
| Haemoglobin, mmol/L | 0.61 [0.4–0.94] | 0.02 | 0.61 [0.36–1.03] | 0.06 |
| Male sex | 0.58 [0.26–1.30] | 0.2 | 1.02 [0.37–2.80] | 1.0 |
- CI, confidence interval; EQ-5D, European Quality of Life–Five Dimensions visual analogue scale; HRV, heart rate variability; OR, odds ratio; VAS, visual analogue scale.
Discussion
The main finding of the current study was the presence of autonomic cardiovascular dysregulation found in every fifth patient 3 weeks after stroke. The cardiac autonomic imbalance evaluated by HRV had a negative impact on the extent of functional performance following the early post-stroke rehabilitation.
Our results are in line with recent clinical trials showing an association between cardiac autonomic control and cognitive performance or rehabilitation outcomes.27, 28 Previously, an association between SDNN and BI was observed after rehabilitation.29 Furthermore, HRV was suggested as a marker of functional recovery after stroke.30 We observed a higher prevalence of CFD in patients with depressed HRV after rehabilitation suggesting an association between cardiac autonomic function and efficacy of rehabilitation after stroke. This association was present even after adjustment for age, sex, and BMI, which confirms the results of previous clinical studies indicating the adverse effect of central autonomic derangement on the level of neurological recovery, physical, and functional outcome of patients with stroke.3, 4, 11, 20 In contrast, neurological deterioration was sought to be a cause of increased sympathetic activity in patients with acute stroke undergoing early mobilization.31 However, in our study, neurological and functional status was similar at the beginning of rehabilitation in both groups, suggesting the unfavourable impact of cardiac autonomic dysregulation on clinical outcome.
A previous study reported significant blood pressure fluctuations in the acute phase of different subtypes of non-cardioembolic ischaemic stroke resulting from autonomic dysfunction, characterized by sympathetic hyperactivity and a decrease in parasympathetic nervous system activity.32 In contrast, the results of the present study did not show any differences in the blood pressure control between patients with normal and depressed HRV. However, our study did not investigate different subtypes of stroke. Vice versa, we aimed to identify relevant clinical parameters equally to patients with all stroke subtypes admitted to the early post-stroke rehabilitation.
In the present study, we assessed the functional outcome using a combined endpoint of CFD. We dichotomized the outcome assessed by all three scales to address an overall functional state, which included the evaluation of body function, physical activities, mobility, self-care, and global functional health of the study patients. The clinical significance of a composite assessment to evaluate the outcome in acute stroke was previously shown.33 In addition, implementation of composite endpoints resulted in an improvement of the clinical trial efficiency and lower sample size for trials investigating minor ischaemic stroke.34
We performed the assessment of SPPB in the current study, which is widely used in geriatric medicine to assess frailty, sarcopenia, and lower extremity function in older persons.35-37 The results have shown that only 22% of all stroke patients at baseline and 43% of patients after a 4-week FU were able to perform the test. Among them, only 30% of patients were able to complete a 3-meter walk test at FU. However, because of the high prevalence of paresis in these patients, the results did not differ between the two groups. The low participation rate of the study population was due to the coordination deficit in patients with paretic limbs, which indicated a relevant floor effect of SPPB in this study cohort.38, 39
Thus, considering that inadequate physical performance observed in patients with depressed HRV after the early rehabilitation is regarded as an indicator of a poor recovery following acute stroke, the findings of the present study particularly underline the clinical significance of targeting cardiovascular autonomic dysfunction to improve the efficacy of treatment, quality of life, and survival of patients with acute stroke. In general, in view of the evidence that autonomic dysfunction also has an important role in the progression of chronic HF, the results of the present study also emphasize the importance of targeting autonomic cardiovascular dysregulation in patients with chronic HF who develop acute stroke, which may lead to acute decompensation of HF, physical disability, and increased mortality. Therefore, treatment of cardiac autonomic derangement by various therapeutic approaches may improve the functional performance and efficacy of HF therapy and decrease the risk of future cerebrovascular events in these patients. In conclusion, this study provides a perspective for future clinical trials evaluating the impact of cardiac autonomic dysregulation on the functional outcome after the early rehabilitation in HF patients with subacute stroke.
Study limitations
The present study is limited by a relatively small sample size. However, in comparison with similar studies22-24, our study remained with the largest patient number. The prescribed medications, such as beta-blockers or angiotensin-converting enzyme inhibitors, may affect the interpretation of autonomic test results40, 41; however, we did not observe a difference in the prescription of these medications between the two patient subgroups. The presence of co-morbidities, such as diabetes mellitus, has been described to influence HRV42. In the current study, this effect was observed as a trend.
Conclusions
The present study has demonstrated an association between autonomic cardiovascular dysregulation with adverse rehabilitation outcome and dependency in patients with subacute stroke. These findings suggest that HRV, as a non-invasive ECG marker, may provide additional information for evaluation of clinical recovery following the hospitalized neurological rehabilitation. Patients with depressed HRV may require an extensive reconditioning treatment with a long-term FU to enhance the physical performance after acute stroke. A wide spectrum of specific training programmes applied in sport and for treatment of psychiatric disorders or metabolic diseases have shown to improve HRV.43, 44 Therefore, randomized clinical trials investigating the effect of a non-pharmacological treatment of cardiovascular autonomic dysfunction on the functional outcome of patients with stroke are warranted.
Conflict of interest
The authors have no conflict of interest to declare.
Funding
A.B. received a research grant from the German Academic Exchange Service (DAAD). W.D. received funding from the German Federal Ministry of Education and Research (BMBF) (grant number EO 0801).




