Heart failure and sleep-disordered breathing: susceptibility to reduced muscle strength and preclinical congestion (SICA-HF cohort)
ABSTRACT
Aims
Increased sympathetic activation in patients with heart failure (HF) and sleep-disordered breathing (SDB) provokes cardiac decompensation and protein degradation and could lead to muscle wasting and muscle weakness. The aim of this study was to investigate the differences in body composition, muscle function, and the susceptibility of preclinical congestion among patients with HF and SDB compared with those without SDB.
Methods and results
We studied 111 outpatients with stable HF who were enrolled into the Studies Investigating Co-morbidities Aggravating Heart Failure. Echocardiography, short physical performance battery (SPPB), cardiopulmonary exercise testing, dual-energy X-ray absorptiometry, bioelectrical impedance analysis (BIA), tests of muscle strength, and polygraphy were performed. SDB was defined as apnoea/hypopnoea index (AHI) >5 per hour of sleep. Central sleep apnoea (CSA) and obstructive sleep apnoea (OSA) were defined as AHI >50% of central or obstructive origin, respectively. A total of 74 patients (66.7%) had any form of SDB [CSA (24 patients, 32.4%), OSA (47 patients, 63.5%)]. Patients with SDB showed increased muscle weakness (chair stand), reduced muscle strength, and lower values of SPPB score (P < 0.05). Patients with SDB did not show overt clinical signs of cardiac decompensation compared with those without SDB (P > 0.05) but had increased amounts of water (total body water, intracellular, and extracellular) measured using BIA (P < 0.05). Increased amounts of total body water were associated with the severity of SDB and inversely with muscle strength and exercise capacity measured by anaerobic threshold (P < 0.05). Altogether, 17 patients had muscle wasting. Of these, 11 (65%) patients had SDB (statistically not significant).
Conclusions
SDB is highly prevalent in patients with HF. Patients with SDB have lower muscle strength and tend to be more susceptible to preclinical congestion.
Introduction
Heart failure (HF) is a major public health issue with steadily increasing incidence and prevalence. The rates of hospitalization and mortality as a result of HF remain high in spite of the advances in its management.1 This signifies the importance of diagnosing and treating modifiable comorbidities such as sleep-disordered breathing (SDB) aiming to reduce morbidity and mortality of HF. SDB is one of the most common comorbidities in patients with HF. Its prevalence ranges between 55% and 80%.2 However, the awareness of its co-existence in these patients is low, and there are unfortunately no routinely used screening programmes for SDB in patients with HF. This could be partially a result of the high expenses spent on polysomnography, the gold standard to establish the diagnosis of SDB, and the limited availability of sleep labs even in developed countries. However, screening for SDB is possible with an ambulatory device that can be used in the patients' home (polygraphy). The analysis of sleep studies leads mainly to distinguish two types of SDB: (i) central sleep apnoea (CSA) and (ii) obstructive sleep apnoea (OSA). However, both types can overlap and present as mixed sleep apnoea.3
Changes in body composition among patients with SDB have been described in the general population in a limited number of smaller studies.4 Two methods are helpful in this regard: dual-energy X-ray absorptiometry (DEXA) and bioelectrical impedance analysis (BIA). Whilst DEXA uses small amounts of radiation, the principle of BIA depends on an electric current that flows at different rates encountering different electrical resistance values in the body due to the different electrical conduction properties of water, fat, or bone.5 The water is localized in two compartments: extracellular water (ECW) and intracellular water (ICW). Fat tissue allows for significantly less conductibility than water, muscle, or bone.5 Body composition in patients with SDB and the differences between CSA and OSA are not well investigated and understood yet. As a result of increased sympathetic activation, SDB may have significant impact on body composition or on the occurrence of muscle wasting. Indeed, there are few, if any, studies, that described the relationship between body composition on one hand and exercise capacity and the severity of HF and SDB in these patients on the other hand.
We sought to investigate the characteristic differences in body composition among patients with SDB of either CSA type or OSA type and to investigate the co-existence of muscle wasting (sarcopenia) in this group of patients. The prevalence of SDB in clinically stable outpatients with HF was evaluated using a portable screening device. Furthermore, we aimed to study the relationship between the total amount of water as a sign of preclinical congestion and exercise capacity and the severity of HF and SDB.
Methods
Study population
Between March 2010 and September 2013, we enrolled patients with HF into the Studies Investigating Co-morbidities Aggravating Heart Failure, a multicentre European observational study that examined the prevalence, incidence, and impact of key comorbidities among outpatients with a clinical diagnosis of chronic HF with either reduced left ventricular ejection fraction (HFrEF) or preserved left ventricular ejection fraction (HFpEF). For the present study, subjects were included from the Department of Cardiology at Charité Medical School, Campus Virchow-Klinikum, Berlin, Germany. All subjects provided written informed consent at enrolment, and the protocol was approved by the local ethic committee. The study was funded by the European Commission's 7th Framework programme (FP7/2007–2013) under grant agreement number 241558 (clinical.trial.gov) and fulfils all principles of the Declaration of Helsinki. The protocol is registered at clinicaltrials.gov under the unique identifier NCT01872299. The inclusion criteria were broad and have been published previously.6 Overall, 111 patients fulfilled the inclusion/exclusion criteria and had polygraphy data available.
Clinical assessments
Polygraphy was used on an outpatient basis using Embletta (Embla, Broomfield, CO, USA) or NOX devices (Nox Medical, Reykjavik, Iceland). The automatically scored tests were validated visually and rescored by two of the authors (TB, CS). SDB was defined as an apnoea/hypopnoea index (AHI) AHI >5 episodes per hour. CSA was defined as AHI being >50% of central origin. OSA was defined as AHI being >50% of obstructive origin. Mixed sleep apnoea was defined when patients have both central events and obstructive events without fulfilling the above-mentioned criteria and still present with AHI >5 epsiodes per hour.
DEXA was used to assess the appendicular lean mass, i.e. muscle mass in both arms and legs and fat masses in the different body compartments (Lunar Prodigy device GE Medical Systems, Madison, WI, USA). Appendicular skeletal muscle mass that includes non-fat and non-bone tissue in both arms and legs combined in grams indexed to body height (in meters squared) was used to evaluate the existence of muscle wasting (sarcopenia). Previously published reference values from the younger Rossetta cohort (age range: 18–40 years) were used as reference standards of muscle mass and yielded cut-off values 2 standard deviations below the mean of the reference values of 7.26 kg/m2 in men and 5.45 kg/m2 in women. These cut-off values, previously used to identify patients with sarcopenia, were used to distinguish patients with muscle wasting from those without.7 Body composition was additionally analysed using BIA (QuadScan 4000, Bodystat Limited, UK). However, this analysis was restricted due to safety issues to patients without an implanted cardiac device such as a pacemaker, an implantable cardioverter defibrillator or cardiac resynchronization therapy (CRT). Altogether, 23 patients with OSA, 18 with CSA, and 17 with no SDB had implanted cardiac devices and as a result, were excluded from the BIA analysis.
Muscle strength was assessed as handgrip strength using a handgrip dynamometer (Saehan Corporation Korea Hydraulic Hand Dynamometer, model SH5001). Knee extension strength (quadriceps strength) was measured in both legs in a sitting position with the patient's legs hanging freely, the ankle fixed by a pressure transducer (kilograms). The best of three measurements was used as defined in the protocol.6 The maximum uptake of oxygen (peak VO2, given in mL/kg/min) was measured using cardiopulmonary exercise testing on a treadmill following the modified Bruce protocol.8 In selected patients, a modified Naughton protocol was used.9 In addition, a 6 min walk test (6-MWT) was performed. The short physical performance battery (SPPB) test was performed according to standard protocol.10 The SPPB test represents a highly standardized geriatric test, consisting of tests for balance, gait, strength, and endurance. It evaluates the ability to stand with both feet juxtaposed, in semi-tandem, and tandem positions, the time to walk 4 m, and the time to rise from a chair and return to the seated position five times in a row. SPPB scores range from 0 to 12 points, and lower SPPB scores indicate impaired mobility. As an estimation of inflammatory activation, high-sensitivity C-reactive protein (hsCRP) was measured.
Statistical analysis
Data are presented as mean ± standard deviation (SD) or median with percentiles. The Statistical Package for the Social Sciences (SPSS version 24) was used for statistical analyses. ANOVA, Student's unpaired t-test, Fisher's exact test, Pearson's simple regression, and logistic regression were used as appropriate. A two-tailed P value <0.05 indicates statistical significance.
Results
The prevalence of different types of SDB in HFpEF and HFrEF patients
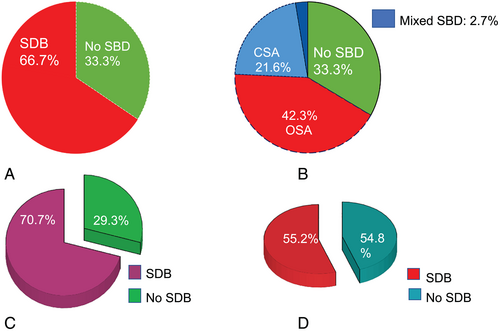
We enrolled 111 patients with HF, 29 (26%) had HFpEF and 82 (74%) HFrEF. Patients' baseline characteristics are summarized in Table 1. In total, 74 (66.7%) patients had any form of SDB. Of these, 24 (32.4%) patients had CSA, 47 (63.5%) patients had OSA, and 3 patients (4.1%) presented with mixed SDB. The percentage of different forms of SDB in the whole cohort was as follows: CSA (21.6%), OSA (42.3%), and mixed SDB (2.7%) (Figure 1). Overall, 16 patients with HFpEF (55.2%) and 58 patients with HFrEF (70.7%) presented with SDB (Figure 1C,D).
| Variable | All patients 111 | SDB 74 | No SDB 37 | P value |
|---|---|---|---|---|
| Age (year) | 67.6 ± 10.2 | 68.2 ± 9.5 | 66.5 ± 11.4 | 0.4 |
| Sex (m/f%) | 73/27 | 71/57 | 29/43 | 0.0001 |
| BMI (kg/m2) | 27.9 ± 4.4 | 28.1 ± 4.4 | 27.5 ± 4.6 | 0.5 |
| NYHA | 2.3 ± 0.7 | 2.3 ± 0.8 | 2.1 ± 0.5 | 0.1 |
| LVEF (%) | 39.5 ± 14.2 | 37.2 ± 14.3 | 44.1 ± 12.6 | 0.02 |
| LVEDD (mm) | 57.4 ± 9.6 | 58.8 ± 9.8 | 54.7 ± 8.5 | 0.03 |
| VE/VCO2 slope | 36.5 ± 7.2 | 37.6 ± 6.8 | 34.1 ± 7.8 | 0.06 |
| Chair stand (point) | 3.0 ± 1.2 | 2.8 ± 1.4 | 3.5 ± 0.8 | 0.006 |
| (GFR) (mL/min) | 59.3 ± 14.3 | 57.5 ± 14.5 | 63.2 ± 13.1 | <0.05 |
| hsCRP (mg/L) | 3.0 ± 2.9 | 3.4 ± 3.3 | 2.2 ± 1.6 | 0.04 |
| Total amount of water (L) | 42.7 ± 8.5 | 45.0 ± 8.1 | 38.9 ± 8.0 | 0.02 |
| Total amount of water/BMI (L/m2/kg) | 1.5 ± 0.05 | 1.6 ± 0.3 | 1.4 ± 0.3 | <0.05 |
| Intracellular water (L) | 23.3 ± 5.2 | 24.8 ± 4.5 | 20.7 ± 5.3 | 0.007 |
| Intracellular water/BMI (L/m2/kg) | 0.8 ± 0.03 | 0.89 ± 0.2 | 0.75 ± 0.2 | 0.01 |
| Extracellular water (L) | 19.1 ± 3.2 | 19.8 ± 3.0 | 17.8 ± 3.2 | 0.04 |
| Extracellular water/BMI (L/m2/kg) | 0.7 ± 0.02 | 0.70 ± 0.1 | 0.65 ± 0.1 | 0.1 |
| Body cell mass (kg) | 33.3 ± 7.4 | 35.5 ± 6.5 | 29.6 ± 7.6 | 0.007 |
| Body cell mass/BMI (m2) | 1.2 ± 0.04 | 1.3 ± 0.2 | 1.1 ± 0.3 | 0.01 |
| Basic metabolic rate (kcal) | 1651.6 ± 305.1 | 1737.7 ± 285.6 | 1508.0 ± 288.7 | 0.01 |
| Basic metabolic rate/BMI (kcal/m2/kg) | 59.8 ± 11.8 | 62.5 ± 12.1 | 55.2 ± 10.2 | 0.04 |
| Minutes of oxygen saturation <90% | 62.6 ± 89.5 | 75.3 ± 99.1 | 33.0 ± 51.9 | 0.03 |
| % of oxygen saturation < 90% | 16.1 ± 22.3 | 19.2 ± 24.2 | 8.9 ± 15.1 | 0.03 |
- BMI, body mass index; hsCRP, high-sensitivity C-reactive protein; GFR, glomerular filtration rate; LVEDD, left ventricle end-diastolic diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; SDB, sleep-disordered breathing.
Exercise capacity, muscle, and cardiac function in patients with HF and SDB.
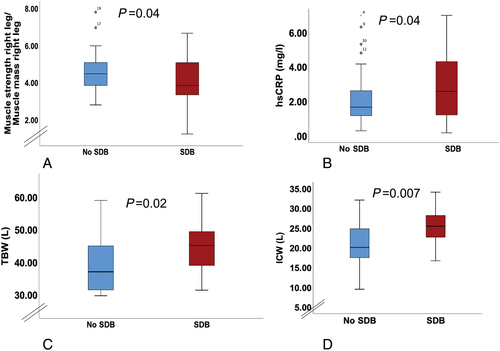
Patients with SDB vs. without SDB showed worse functional parameters [increased proximal muscle weakness (chair stand: 2.8 ± 1.4 vs. 3.5 ± 0.8, P = 0.006), worse SPPB (10.4 ± 1.9 vs. 11.11 ± 1.4, P = 0.04), worse muscle strength (muscle strength right leg/muscle mass right leg: 4.1 ± 1.2 vs. 4.6 ± 1.1, P = 0.04) (Figure 2), and a trend for worse values in VE/VCO2 slope on cardiopulmonary exercise testing (37.6 ± 6.8 vs. 34.1 ± 7.8, P = 0.06)]. Cardiac function was similarly worse in patients with SDB compared with those without SDB [(left ventricle ejection fraction (LVEF): 37.2 ± 14.3 vs. 44.1 ± 12.6%, P = 0.02), diameters of the left ventricular at end-diastole (58.8 ± 9.8 vs. 54.7 ± 8.5 mm, P = 0.03), diameter of the left atrium (49.1 ± 7.0 vs. 43.1 ± 6.6 mm, P = 0.0001)]. Furthermore, hsCRP was more elevated in patients with SDB (3.4 ± 3.3 vs. 2.2 ± 1.6 mg/L, P = 0.04) (Figure 2B). There was no difference in HF therapy between patients with or without SDB (P > 0.05).
A total of 17 patients presented with muscle wasting (sarcopenia). 11 of these patients (65%) had SDB (six patients with OSA and five patients with CSA) vs. six patients (35%) without SDB. However, there was no difference statistically (P > 0.05).
Preclinical signs of congestion in patients with HF and SDB
Comparing patients with vs. without SDB there was no difference between the two groups regarding cardiac congestion signs such as ankle oedema or elevated jugular venous pressure (P = 0.2). However, using BIA analysis we found that patients with SDB showed increased amounts of total body water (TBW: 45.0 ± 8.1 l vs. 38.9 ± 8.0 L, P = 0.02), intracellular (ICW: 24.8 ± 4.5 l vs. 20.7 ± 5.3 L, P = 0.007) (Figure 2C,D), and ECW (19.8 ± 3.0 l vs. 17.8 ± 3.2 L, P = 0.04), elevated body cell mass (35.5 ± 6.5 vs. 29.6 ± 7.6 kg, P = 0.007), basic metabolic rate (1737.7 ± 285.6 vs. 1508.0 ± 288.7 kcal, P = 0.01), and daily expected metabolic rate (2752.7 ± 472.5 vs. 2412.7 ± 427.4 kcal, P = 0.02) compared with those without SDB (Table 1). Similar results were found comparing OSA (24 patients) with patients without SDB (20 patients) as shown in Table 2. Comparing patients with CSA (six patients) with patients without SDB showed increased metabolic rates in patients with CSA [metabolic rate/body mass index (BMI): 67.2 ± 13.2 vs. 55.2 ± 10.2 kcal.m2/kg, P = 0.03] and a trend regarding increased ICW (ICW/BMI: vs. 0.75 ± 0.2 L.m2/kg, P = 0.07). There was no significant difference among patients with CSA and OSA regarding amounts of water or metabolic rates (P > 0.3).
| Variable | OSA 47 | CSA 24 | No SDB 37 | P value |
| Age (year) | 69 ± 8 | 67 ± 12 | 66 ± 11 | 0.6 |
| Sex (m%) | 34 (72%) | 21 (88%) | 23 (62%) | 0.06 |
| BMI (kg/m2) | 28.7 ± 4.8 | 26.9 ± 3.3 | 27.5 ± 4.6 | 0.3 |
| NYHA | 2.3 ± 0.8 | 2.4 ± 0.7 | 2.1 ± 0.5 | 0.2 |
| GFR (mL/min) | 60.1 ± 13.8‡ | 52.8 ± 15.3# | 63.2 ± 13.0 | 0.02 |
| LVEF (%) | 42 ± 15‡ | 28 ± 9# | 44 ± 13 | <0.0001 |
| LVEDd (mm) | 56.9 ± 8.6‡ | 63.6 ± 10.7# | 54.7 ± 8.5 | <0.0001 |
| Total amounts of water (L) | 45.4 ± 8.5* | 42.6 ± 7.4 | 38.9 ± 8.0 | <0.05 |
| Total amount of water/BMI (L/m2/kg) | 1.6 ± 0.3 | 1.7 ± 0.3 | 1.4 ± 0.3 | 0.2 |
| Intracellular water (L) | 25.0 ± 4.6* | 23.7 ± 4.7 | 20.7 ± 5.3 | 0.03 |
| Intracellular water/BMI (L/m2/kg) | 0.9 ± 0.2* | 0.9 ± 0.2 | 0.8 ± 0.2 | 0.05 |
| Extracellular water (L) | 20.0 ± 3.1* | 18.7 ± 2.4 | 17.8 ± 3.2 | 0.1 |
| Extracellular water/BMI (L/m2/kg) | 0.70 ± 0.1 | 0.72 ± 0.8 | 0.65 ± 0.1 | 0.3 |
| Body cell mass (kg) | 35.7 ± 6.6* | 33.8 ± 6.7 | 29.6 ± 7.6 | 0.03 |
| Body cell mass/BMI (m2) | 1.3 ± 0.2* | 1.3 ± 0.2 | 1.1 ± 0.3 | 0.05 |
| Basic metabolic rate (kcal) | 1,734 ± 293* | 1,723 ± 299 | 1,508 ± 289 | <0.05 |
| Basic metabolic rate/BMI (kcal/m2/kg) | 61.2 ± 12.0 | 67.2 ± 13.2# | 55.2 ± 10.2 | 0.07 |
- BMI, body mass index; CSA, central sleep apnoea; GFR, glomerular filtration rate; LVEDD, left ventricle end-diastolic diameter; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; OSA, obstructive sleep apnoea; SDB, sleep-disordered breathing.
- * P < 0.05 in comparison between OSA and no SDB.
- ‡ P < 0.05 in comparison between OSA and CSA.
- # P < 0.05 in comparison between CSA and no SDB.
The relationship between body composition, severity of sleep apnoea, muscle function and exercise capacity
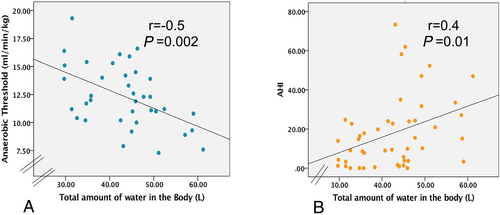
Using simple regression analysis, we found that increasing amounts of total water were significantly associated with an elevated basic metabolic rate (r = 0.97, P < 0.0001), with reduced anaerobic threshold (r = −0.5, P = 0.002), muscle strength of right leg (r = −0.54, P = 0.003), and an increased AHI in the supine position (r = 0.4, P = 0.01) (Figure 3). Increased amounts of ICW was associated similarly with reduced muscle strength of the right leg (r = −0.7, P < 0.0001). Higher values of AHI were associated with reduced muscle strength in the legs (r = −0.22, P = 0.03), with reduced PVO2 (r = −0.25, P = 0.04) and with increased metabolic rate (r = 0.4, P = 0.003). There was no relationship between fat tissue mass and AHI (P > 0.1).
Discussion
This is the first study to examine the differences in body composition in a cohort of patients with HF between those with and without SDB. We found significantly higher amounts of TBW, ICW, and ECW in patients with SDB as a sign of preclinical congestion. Increased amounts of water were associated with an elevated AHI, reduced muscle strength, and exercise capacity as well as with an elevated basic metabolic rate. Altogether, patients with SDB showed worse cardiac function, reduced exercise capacity, and reduced muscle strength compared with those without SDB.
SDB remains underdiagnosed and often unrecognized because of its chronic and insidious incidence.11 Patients may report non-specific symptoms such excessive daytime somnolence, poor sleep quality, recurrent arrhythmias, nocturnal angina, and refractory HF symptoms.12 However, such symptoms may be missing in patients with HF or strongly overlap with the symptoms deemed specific for the HF syndrome itself. The main two types of SDB are the OSA, which is the most common form and presents with fewer symptoms, and the CSA, which is more often associated with more advanced HF and atrial fibrillation. Cheyne–Stokes respiration is typically seen in CSA. Both CSA and OSA are independent risk factors for ventricular arrhythmias that require cardioverter–defibrillator therapy.13
In the present study, we used ambulatory nocturnal screening devices and rescored these tests visually to classify patients into no SDB, SDB with CSA, OSA, or mixed SDB accordingly. We found similar prevalence rates of SDB like those described in other studies.2, 3, 14 About 67% of the whole cohort had any form of SDB. Overall, about 42% had OSA and 22% CSA.3
It is known that the combination of HF and SDB leads to higher sympathetic nervous activity during wakefulness.15 This will lead in turn to increase the metabolic rate and the catecholamine surge and may accelerate the progression of HF.16 Another adverse effect of increased sympathetic activation is provoking the catabolic cascade and the protein degradation in skeletal muscle that could eventually lead to skeletal muscle wasting (sarcopenia) and reduced muscle function.17 We found that 17 of 111 patients presented with skeletal muscle wasting. Of these 11 (65%) patients had SDB vs. 6 patients (35%) without SDB. This was statistically not significant, which is likely a consequence of the small sample volume. Our previous results in both patients with HFpEF and HFrEF signified the clinical importance of muscle wasting showing its association with reduced exercise capacity, muscle strength, and quality of life.18, 19 The prevalence of muscle wasting and its clinical significance in patients with HF and SDB need to be confirmed in further and larger studies.
The pathophysiology and the relationship between HF and SDB, especially CSA, have been recently suggested.3 It is known that patients with HF tend to hyperventilate.20 This could be related to interstitial pulmonary congestion as a result of the fluid redistribution in the supine position and could also lead to the activation of pulmonary stretch receptors and to the stimulation of ventilation yielding relative hypocapnia.21 Each episode of apnoea and arousal results in hypoxia, norepinephrine release, and wide oscillations in carbon dioxide.22 Hypoxia results in cardiac ischemia, and elevated norepinephrine causes atrial and ventricular arrhythmias23 and activates the renin–angiotensin system resulting in sodium retention and neurohormonal activation.16 Neurohormonal activation and ischemia further activate central and peripheral chemoreceptors and baroreceptors, both of which destabilize breathing and trigger CSA.24 This causes additional pump stress leading to adverse myocardial remodelling and downward progression of HF.25
By showing increased amounts of water in patients with SDB and HF, our results underscore an important preclinical finding. Indeed, there was no clinical difference regarding the signs of congestive HF such as elevated jugular vein pressure or ankle oedema between patients with and without SDB. Because therapies for OSA26 and CSA27 are available and because of the high prevalence of this HF-comorbidity, an active screening program for SDB in patients with HF is highly recommended.3 Such screening should also include BIA assessment or other similar techniques to detect preclinical congestion in patients with HF especially in those who additionally have further comorbidities such as SDB.
Previous studies showed the lack of an association between central fat mass effect and OSA in elderly patients.28 This finding was confirmed by our results. Other studies using BIA demonstrated a relationship between the severity of neck fluid volume of water and the severity of SDB. This could be explained by the physiology and pathophysiology of both HF and SDB.21, 29 Overnight rostral fluid displacement from the legs could play a role in the development of OSA independent of body weight.30 Therefore, screening for preclinical congestion in HF should be recommended, particularly in those with SDB or otherwise more advanced disease. Further non-invasive and invasive monitoring in patients with HF and SDB could be necessary. The TIM-HF2 suggested recently that a structured remote patient management intervention in patients with HF could reduce the percentage of days lost due to unplanned cardiovascular hospital admissions and all-cause mortality.31 Invasive procedures like implantation of CardioMems devices seem to be promising and could be necessary for achieving a successful monitoring in this group of patients. In a single study, CardioMems™ implantation resulted in an 80.4% reduction in HF admissions and a 69% reduction in all-cause admissions.32
In conclusion, using ambulatory polygraphies we have shown comparative prevalence rates of SDB, OSA, and CSA in stable outpatients with HF. More than two thirds of stable HF-outpatients presented with any form of SDB. Furthermore, we demonstrated the clinical importance of figuring out the preclinical signs of cardiac congestion in patients with stable HF and SDB. Increased amounts of TBW, ICW, and ECW as a sign of preclinical congestion were associated with reduced muscle strength, reduced exercise capacity, and increased severity of SDB. Therefore, regular screening for SDB in HF is highly recommended. Further and larger randomized studies are needed to confirm our results and to test the necessity for invasive monitoring in patients with both HF and SDB to prevent frequent cardiac decompensations.
Our main limitation is our relatively small number of patients and the non-invasive evaluation of fluid status in our patients. Not all patients underwent complete BIA assessment because of safety concerns in those with implanted cardiac devices.
Conflict of interest
TB, Ch. S, Ch. P, MV, NE, and CS do not have conflict of interest. WD reports speaker fees and advisory honoraria from Aimediq, Bayer, Boehringer Ingelheim, Medtronic, Pfizer, Sanofi-Aventis, Sphingotec, and Vifor Pharma. WD also reports research support from EU (Horizon2020), the German Ministry of Education and Research, German Center for Cardiovascular Research, Vifor Pharma, and ZS Pharma. UL or his institution has received fees for lectures from Amgen, AstraZeneca, Bayer, Berlin-Chemie, Boehringer, Daiichi-Sankyo, Medtronik, Novartis, Sanofi, Servier. SDA has received research support from Vifor International & Abbott Vascular, and fees for consultancy and/or speaking from Astra Zeneca, Bayer, Boehringer Ingelheim, Respicardia, Impulse Dynamics, Janssen, Novartis, Servier and Vifor International. SvH has been a paid consultant for and/or received honoraria payments from Bayer, Boehringer Ingelheim, BRAHMS, Chugai, Grünenthal, Helsinn, Hexal, Novartis, Respicardia, Roche, Sorin, and Vifor. SvH owns shares in Actimed. SvH reports research support from IMI and the German Center for Cardiovascular Research (DZHK).




